
Developing & integrating a mobile application tool into a survivorship clinic for esophageal cancer patients
Introduction
Patients undergoing esophagectomy for esophageal cancer (EC) have significant morbidity and mortality, struggle with symptom management, and require surveillance of the cancer. Thoracic surgeons are trained to perform a variety of procedures and approaches with limited scientific guidance of which technique yields the optimal outcome of extending overall survival while minimizing long-term consequences of technical and physiologic repercussions from their surgery (1,2). Some patients travel long distances to have their surgery performed at high volume hospitals, where the mortality rate is often a third of that at centers performing less than 20 esophagectomies a year (3-6). Unfortunately, when those patients travel back home, their primary providers and caregivers are often unaware of the details of the surgery or how to manage the complex symptoms many patients experience during survivorship care. Local hospitals that do not specialize in these complex surgeries further struggle with high mortality (3-6), failure to rescue (7,8), and a higher morbidity resulting in a lifetime of consequences and decreased quality of life for the patient. Furthermore, in local care settings, communication between surgical teams, primary care providers, patients, and caregivers is often disorganized and results in significant miscommunication and unnecessary suffering (9). The financial, emotional, and physical impact of this breakdown in communication and burden results in extraordinarily high suicide (10) and depression rates (11) among EC patients. Patients continue to struggle even 15 years from surgery, often with severe symptoms (12). As EC patients live longer with immunotherapy (13), enhanced screening and diagnostic tools (14) and more options for advanced disease, these issues are likely to become even more relevant in a larger population.
To address these patients’ needs and create a systematic method for evaluating long-term outcomes after esophagectomy for cancer, our team developed the Upper Digestive Disease Application (UDD App), which is a mobile health (mHealth) application available on the digital store for iOS and Android to the public for measuring and scoring symptoms after foregut (upper digestive) surgery, such as esophagectomy Figure S1 (15). To better support cancer patients through their care continuum, survivorship care, defined as cancer surveillance with symptom management (16), is now mandated within National Comprehensive Cancer Network (NCCN) (17). Coupled with a Survivorship Care Team (SCT) and Clinical Care Charts (CCCs) of algorithmic pathways to guide management, the UDD App facilitates monitoring symptom burden, directing assessment, and quantifying data for patient outcome analysis. The Centers for Medicare and Medicaid Services and The National Quality Forum have also mandated utilizing patient reported outcomes (PROs) for management of patients (18). The U.S. Food and Drug Administration utilizes PRO data to review and approve new medications and devices and to regulate new technology (19). After the first digital version was developed, we continued to collect patient feedback and made appropriate revisions for versions two and three of the digital tool to meet requirements mandated by NCCN, and based on continuous patient feedback.
The purpose of this manuscript is to describe the history, process, and methodology for developing a patient-centric remote monitoring program to enhance survivorship after esophagectomy for cancer. Our unique approach has allowed us to not only measure PROs, but also to develop a systematic and scalable approach to managing patients, guiding choices for surgical techniques, and integrating PRO data to enhance practice. By creating a digital connection with patients for longitudinal care, we have enabled an integration of services to provide comprehensive connectivity throughout the patient experience beyond the acute surgical intervention. For a timeline of the development of the UDD App-guided SCT Timeline, please refer to Figure 1 (12,15,20-28).

Self-management
Patients have indicated that the mere collection of PRO data alone is not acceptable. They want feedback on their scores with guidance and coaching to self-manage symptoms based on score distribution. They find comparison to their peers and provider expert judgement also helpful. Patients and their caregivers indicate they desire a more objective measure and means of determining when their symptoms are severe, when interventions are warranted, and a tool to demonstrate to providers their problems. Having a means to articulate problems has been shown to enhance patient advocacy, making it more difficult for a provider to miss a specific issue and giving the patient a voice.
Survivorship care pathway
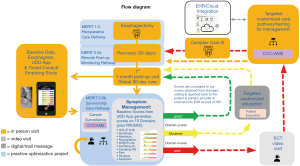
The survivorship care pathway begins after the initial first patient follow-up visits and completion of the global 90-day period, which is typically managed by a surgical team for immediate perioperative care. Patients who have a severe surgical complication, like a complicated leak, may enter the program later once the acute issue has resolved if this is beyond 90 days. Based on results of the Checkmate 577 Trial, patients who have residual active cancer identified in the specimen or nodes at the time of esophagectomy after neoadjuvant treatment are typically now started on adjuvant immunotherapy (29). The survivorship schedule consists of the following: Phase I Survivorship (a total program of five years consisting of video visit + active cancer surveillance with imaging + symptom management every 3 months × 1 year then every 6 months × 1 year then every year × 3 years) followed by Phase II Survivorship (a lifetime of self-symptom management with video visit only if needed + cancer brief assessment) all of which can be remotely monitored by the SCT. Cancer surveillance consists of a video visit, computed tomography (CT) scan of the chest, abdomen, and pelvis and every other year endoscopy. The SCT sees patients by video visit and utilizes the institutional expert-developed CCCs, which are algorithmic care pathways developed by using a specific methodology for a platform at Mayo Clinic called AskMayoExpert (AME) (29), to use evidence-based medicine to guide care when a patient has a severe/worrisome symptom/problem indicated by a severe/poor domain score. The AME team consists of coders, information team specialists, subject matter experts (SMEs), expert review panels, health informaticists, and is currently being migrated over to an Adobe platform for knowledge management. The CCCs indicate if and when a referral is appropriate and lists experts digitally linked within the institution who manage problems that cannot be solved by following the CCCs or require expert management. The goal is to improve symptom scores through utilization of the scores and CCCs, thus also improving overall quality of life. Customized patient education material is automatically sent to patients scoring in a yellow zone with moderate symptoms, Figure 2 (SCT pathway). When patients are identified to have abnormalities on their imaging or signs of recurrence, they are evaluated with a follow-up positron emission tomography (PET)-CT scan or other indicated follow-up, and referrals are generated as needed if recurrent disease is found. The cancer surveillance guide is also guided by a CCC.
Iterative development of the questionnaire
The idea for the tool and the UDD App began when the senior author (SHB) identified a paucity of choices for assessment of symptoms for EC patients after esophagectomy. In her 2007 publication (20) to identify the best thoracic esophageal anastomosis, she discovered the Mellow and Pinkas (30) method for scoring dysphagia, but found the assessment to be inadequate to assess swallowing in a comprehensive manner. As the author began to look at the best means to comprehensively assess all symptoms in EC patients after esophagectomy, she identified a lack of customized tools developed for this special population. Thus, a focus group was formed, and symptom assessment led to a collection of rich patient data about severity, frequency, and interference of symptoms from the support group over time. Newer iterations incorporated the PRO version of Common Terminology Criteria for Adverse Events (CTCAE) format, which focuses on assessing symptom severity, frequency, and interference with daily life.
Patient-oriented symptom management focus groups & support group/stakeholder engagement
We established symptom domains through stakeholder engagement (21). The support group met every month for eight years at Houston Methodist Hospital and included caregivers accompanying the patients. The support group took field trips to meet with chefs to determine best methods for creating bespoke healthy appetizing diets for these patients. Experts were recruited to speak on topics of greatest patient concern. We used a measurement framework to guide the development of a questionnaire to assess the most important symptoms and score them. Each domain was identified through focus group methodology, and then survey items were created for each domain, with constellations of symptoms (e.g., “food gets stuck in throat”) linked to each defined problem (i.e., dysphagia) or (e.g., “I have diarrhea after I eat sugary foods”) linked to each problem (i.e., dumping).
Development of paper version of questionnaire (formerly termed “CONDUIT Tool” and now “UDD App”)
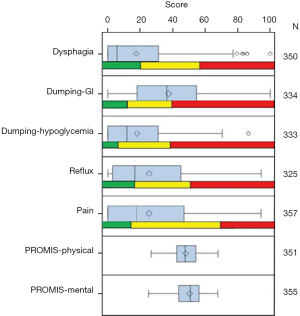
The questionnaire initially adopted the Mayo Clinic Dysphagia and Reflux Scoring Systems (31), Mellows and Pinkas dysphagia assessment (30), and Sigstadt’s Dumping assessment (32) as a conglomeration of already existing scoring tools to comprehensively build a whole questionnaire to evaluate those domains identified by patients to be of highest concern. Utilizing the stakeholder engagement data, we first developed the CONDUIT Tool (Conduit Outcomes Noting Dysphagia/Dumping & Unknown outcomes with Intermittent symptoms over time after esophageal surgery), which was a paper questionnaire that facilitated comparisons of esophageal reconstruction techniques (22). Adding Patient-Reported Outcome Measurement Information System (PROMIS) measures (33), we then demonstrated that the questionnaire had good content validity and psychometric properties (24). The tool covers the major symptoms for patients undergoing esophageal reconstruction (e.g., reflux, dysphagia, and diarrhea), utilizing 5 novel multi-item scales (now, 10 domains with two additional PROMIS measures) which had good reliability, Figure 3A-3C (24). We present the scores to patients by domain and then longitudinally as domain scores change over time.
Testing of questionnaire
Once developed, the paper-based tool was offered to esophagectomy for EC patients from the initial institution (Houston Methodist Hospital), and those scores allowed for a comparison of patients after gastric interposition group to patients after long segment supercharged pedicled jejunal interposition (28).
Testing in a second hospital system
The questionnaire was then brought to Mayo Clinic Rochester, where it was delivered to a small pilot group of patients and determined to be too lengthy and cumbersome for use in routine care. A careful implementation pathway (Figure 2) was developed to stepwise mature the tool for patient assessment, with an eye to standardized scoring and implementation.
Domain development and assessment
Our research team used the tool to determine predictors of domain scores (reflux) (27) and measure domain score change over time with traditional surveillance methods (NCT#02530983). This initial cohort of the 188 patients completed 360 paper questionnaires. Mirroring the known sex distribution of EC in the US, 80% (150 of the 188) were male (24). With ongoing completion of the questionnaire by eligible patients, we now have enrolled 681 patients engaged who have completed at least one questionnaire successfully. Since the initial development of the five domains, we have now revised the questionnaire based on patient feedback and have added additional questions and domains, totalling 10 domains plus two PROMIS scales in the current version.
Standard-setting (setting cut-scores)
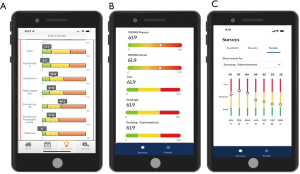
The UDD App prompts intervention for more intense symptoms (red score), low-touch automated intervention for mild to moderate symptoms (yellow score), and/or reassurance with surveillance for no current symptoms of concern (green score). The UDD App was developed to automatically create scores for five symptom domains [pain, dysphagia, reflux, hypoglycemic (HG) dumping, and generalized/gastrointestinal (GI) dumping] that are salient to esophagectomy patient care, accompanied by scoring for general mental health (MH) and general physical health (PH) using two well-established instruments, PROMIS-MH & PROMIS PH. The five domain and two PROMIS scales are automatically scored by color/severity (red-yellow-green) and number (100-0 scale), respectively, with color changes indicative of symptom severity, moving from red to green (termed “Going to Green”) as a patient’s condition improves. Utilizing a modified Angoff Method and expert consensus, cut scores were determined (23). After adding new domains, the lab then conducted additional Delphi rounds to develop new and revised cut scores for each domain, totaling now 10 domains (pain, dumping-generalized, dumping-gastrointestinal, heartburn, regurgitation, aspiration, weight loss, dyspnea, dysphagia and nausea) (23). Color-coded symptom reporting provides immediate visual depiction to patients and the SCT to demonstrate severity of scores in a user-friendly manner (Figure 2A-2C). We described the selection and training of panelists and the processes they were asked to follow in order to support defensibility of cut scores. This study was an essential step in the process of validation of a tool that can be used to manage patients after esophagectomy. The cut scores derived from modified Angoff methods and expert consensus are seen in Tables S1,S2.
Continued development of the UDD App
The original paper questionnaire was relabeled as the UDD tool as it was expanded to address needs of foregut disease patients. Leveraging the knowledge gained from the above referenced studies, we then designed a mobile application, the UDD App to administer the tool and triage patients according to their individual scores, Figure 3A-3C and Figure S2. Three versions of the digital tool have been developed and tested to-date.
Development of coaching and triage tools
The first group of patients completing a paper version were scored through a Medidata Rave database and did not receive dashboards or reports. This allowed the lab to calculate in a natural setting how scores changed over time when patients simply took the questionnaire without access to an immediate dashboard, educational content and without access to a SCT. Later groups were provided real-time access to scoring for self-management and then access to educational tools to improve scores over time. Through expert team meetings with certified patient education specialists and medical illustrators, along with SMEs for each domain, we developed customized educational content for patients who are suffering in a particular domain housed within a knowledge management system termed Health Education & Content Services (HECS) (Figure 2 and Figure S3). The mobile application scores automatically launch targeted education for patients who need coaching (Figures 2,3A-3C) followed by automatic reassessment for improvement. SMEs develop CCC triage algorithms to identify what score phenotypes are concerning for a post-operative problem that could require intervention or assessment by the SCT, including but not limited to stricture requiring a swallow esophagram and possible balloon dilation, reflux requiring medical proton pump inhibitor therapy, poor conduit emptying requiring esophagram and possible revisional surgery (Figure 2).
Normative value analysis
As a prospective study analyzing adult esophagectomy patients using the UDD tool from 2015 to 2018 (NCT02530983), 569 patients were included and assessed for eligibility with 241 patients consented and offered the tool (24). Of these, 188 patients (median age: 65 years; range: 24 to 87 years; 80% male patients) completed the questionnaire and had calculable scores. Of the 188 patients, 50 (26.6%) patients were identified as potential beneficiaries for educational intervention to improve symptoms (received moderate scores for a domain), and 131 (69.7%) patients were identified as needing further testing or provider intervention (received poor scores for a domain) based on the tool. The UDD tool scores, when compared with standardized scales with established preliminary normative scores, could be used to identify and triage patients who need targeted education, further testing, or provider interventions (Figure 4). These score ranges served as the first set of normative standards to aid in the interpretation of conduit performance among providers and patients.
Dashboard development and presentation of contextual scores
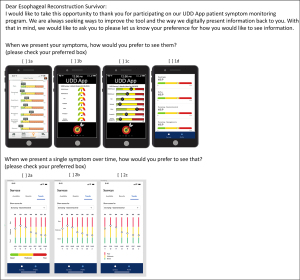
As the various versions of the UDD App were developed, patient selection of preferred dashboard features allowed designers to make easy-to-read metrics that allowed patients to evaluate scores of their domains from an individual time point and then longitudinally as their scores changed over time. Continuous feedback on both the items and the dashboards allowed continuous improvement with a patient-centric development plan. Patients also gave feedback on the initial coaching tool that explains the color-coding system (Figure S4). Plans are currently underway to conduct surveys to determine which contextual items from the survey are relevant and helpful when presenting numerical domain scores and their standardized color-coded severity score. The customized patient dashboard was developed with patients selecting, through customer experience research (Figure 5), those scales easiest to interpret and follow, including for patients who are color-blind. The newest version of the App has been translated into Spanish and a pilot test for Spanish-speaking individuals is planned.
Mixed methods analysis of the UDD App
To assess feasibility and acceptability of the UDD App, we conducted a single arm pilot study of esophagectomy patients who were instructed to download the UDD App. A mixed methods approach, including quantitative and qualitative post-study interviews, was utilized over the year during 2020, including 50 esophagectomy patients who met criteria and consented to participate. Of those consented to use the UDD App, 98% (49/50) successfully completed the entire digital questionnaire establishing feasibility (threshold defined as the lower bound of the exact binomial one-sided 95% confidence interval (CI) is 90.9%, and 98% exceeds the feasibility threshold of 90%). There were 109 questionnaires from the 50 patients (with 62% of patients voluntarily returning during the study duration to re-test without being prompted, demonstrating engagement and retention of patients), and 32 patients were contacted for telephone interviews. Most participants (23, 72%) self-identified as having high computer literacy. Aside from reporting domain scores, all patients reported that using the smart phone UDD App was easier than or equivalent to the traditional paper approach, providing evidence of acceptability for the use of remote electronic patient-reported outcome (ePRO) monitoring. Acceptability (15) was also demonstrated by analysis of structured telephone interviews, where patient reports were assessed with thematically analyzed interviews, which revealed (I) the value of identifying problems through use of the UDD App to raise symptom awareness, (II) enhanced communication with providers and caregivers, (III) the potential for the UDD App to stay connected to the care team, (IV) enhanced convenience for patients, with patients able to be monitored without having to return for costly visits or take time off work to return for evaluation, (V) concerns about privacy, and (VI) a desire for more personalized digital engagement tools. A high compliance rate confirmed the UDD App as a reliable tool for patients to monitor symptoms and function after esophagectomy (15).
Criterion validity assessment of the patient scores compared to provider scores demonstrated variable agreement
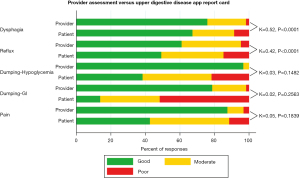
A sample of prescreened patients presenting after esophagectomy were enrolled in a prospective clinical trial (NCT02530983) offering the UDD App just before a thirty-minute visit with a provider to establish criterion validity. Providers were given score sheets to concurrently rate patients in the same five symptom domains based on their clinical evaluation after the visit with the patient. Agreement between PROs using the UDD App and provider responses were assessed using the weighted kappa statistic. The magnitude of agreement between patients and providers was moderate for dysphagia (κ=0.52, P<0.001) and reflux (κ=0.42, P<0.001), but disparate when assessing dumping-HG (κ=0.03, P=0.148), dumping-GI (κ=0.02, P=0.256) and pain (κ=0.05, P<0.184) (Figure 6). While there was satisfactory agreement between PROs and provider evaluation of dysphagia and reflux following esophagectomy, there was discordance for dumping-related symptoms and pain. Clinicians may underestimate the severity of these problems. The UDD App may provide an opportunity for more accurate and remote assessment with monitoring that supersedes an isolated provider assessment (26).
Predictors of patient-reported reflux after esophagectomy
A retrospective study assessed patients who were at least six months after reconstruction with a gastric conduit and who had completed at least one UDD App questionnaire (2015–2018). Of 110 patients with a median age of 65 years, 80% male, 95% with malignant disease, 71% treated with perioperative chemoradiation, a multivariable linear regression analysis revealed patient reported reflux (UDD App score was in the red zone) was significantly worse in specific patient groups. Helping us to better understand why patients are at risk for poor domain scores, we discovered the absence of perioperative chemoradiation therapy (P=0.02), and a shorter gastric conduit length (P=0.02) were the main predictors of UDD App patient reported severe (poor) reflux scores after esophagectomy (27).
Assessment of complex surgical interventions
Specific interventions, like the complex esophageal reconstruction long segment supercharged jejunal interposition (SPJ), have been assessed using the UDD App (26,28). Continuing to monitor and manage symptoms over time utilizing the tool to deliver the patient into the green zone would be the task of a SCT for a variety of patients, extending beyond esophagectomy.
Long-term survivor PRO data analysis after esophagectomy
From 2000 to 2011, EC survivors after esophagectomy were sent the UDD questionnaire via their choice of a paper version or digital enrollment to determine domain scores long-term, and to determine self-management and engagement with the tool. Many of these patients had been lost to follow-up or were no longer followed by their providers. From this analysis of 895 patients, a total of 329 surviving patients were assessed for eligibility (NCT#02708303). Recent contact information was available in 119 patients who were offered enrollment into the trial by mail with consent forms and instructions to complete the questionnaire. Of those, 92/119 (77%) patients consented and completed the UDD App. The most recent version of the questionnaire was answered by 66/92 patients and the majority of participants were males (n=51, 77%) (and in keeping with EC demographics) with a mean age of 57 (±7) years. Of these patients, more than a third completed two sequential questionnaires (39%) without provider initiation or coaching. Almost a third of these respondents continued to suffer from severe reflux and dumping ten years or more from esophagectomy, providing more evidence that patients continue to struggle during long-term survivorship which we will investigate in our future pragmatic trial (Figure S5).
Engagement/retention of patients
Today, more than 701 esophagectomy patients have engaged in completion of either a paper version, web-based version, or digital smartphone/tablet version of the UDD App. Of this number, 556 are currently using the UDD App, with 41 patients who began with a paper version successfully transitioning to a digital version. The majority of patients who continue to engage with the app have symptoms in the yellow or red category and are actively being educated or managed by the SCT to improve scores. Retention of subjects enrolled in the app remains high (90%), demonstrating on-going patient engagement with this meaningful mHealth tool.
Championing domain score management
Mayo Clinic’s AME program, existing within a knowledge management platform administered by the Center of Digital Health, includes a multitude of features, such as CCCs, hyperlinks to resources, expert lists for contact, guidelines and resource lists, links to patient education, and clinical trial lists. This resource provides caregivers (like SCTs) the resources to provide consistent and quality care across an enterprise. Anyone visiting “AskMayoExpert.mayoclinic.org” can view publicly available AME COVID-19 content as of April 17, 2020. This access has been leveraged by Mayo Clinic Laboratories and partners including Epic and Google, furthering Mayo Clinic’s mission to discover, apply, and share knowledge during the COVID-19 pandemic. Launching the UDD App at no charge to the public on the App Store additionally delivers on the mission to serve patients beyond the walls of the hospital.
There are currently 13 Domain Management AME Care Pathways that are either developed or underway to facilitate management by the SCT. These include EC survivorship care in general (linking the CCCs and providers to the UDD App), PROMIS-Health-Related Quality of Life (HRQoL) Physical Health Overall, PROMIS-HRQoL Mental Health Overall (34), Weight Loss/Protein Malnutrition, Dumping (Generalized and GI) (35), Heartburn, Regurgitation, Pain (including specifically a care pathway for Post-thoracotomy Pain Syndrome), Dysphagia, Nausea, Aspiration, and Dyspnea/Breathlessness. The flow diagram for patients going through SCT pathway (Figure 2) includes phases of quality improvement projects. The programs include an initial enhanced recovery after surgery (ERAS) pathway for preoperative and immediate postoperative care Multidisciplinary Esophagectomy Recovery Initiative Team (MERIT 1.0), remote post-discharge monitoring (MERIT 2.0a), and survivorship care (MERIT 2.0b). This manuscript describes the evolution of the MERIT 2.0b program.
Strengths and limitations
The UDD App has many strengths, including delivering to the patient a tool to identify problems, enhance communications with their care team and triage symptoms. This communication and assessment tool enables standardized scoring of symptoms for clear delineation into categories of severity. The tool is available to anyone for free. Results can be exported in a .pdf format by email to other providers. The UDD App currently has limitations: (I) the App has predominately undergone a single institution validation, and it is in the process of being tested on a global scale. As the application is now available to the public, it is accessible to anyone worldwide and this will enable us to acquire an external validation. (II) A patient might be suffering from a problem within a timepoint but does not complete a questionnaire at that instance due to the fact that a questionnaire is not due at that time. Our solution involves a feature of patient-initiated query beyond the regular scheduled interval. (III) The inaccessibility to a mobile device or internet still poses a problem to many patients. We try to either provide a tablet or a paper version to complete a questionnaire during their scheduled clinic visit.
Conclusions
In summary, development of the UDD App ePRO Tool as an integrated part of a survivorship program to facilitate virtual and remote patient access providing longitudinal care to a population that was previously unattended and suffering with significant disease burden is described using User Experience research methodology. PRO data collection should be derived from patient needs and extensive patient-oriented analysis. The same methodology should be employed to create domain dashboards as an iterative process with continuous patient and provider feedback. PRO data collection without reporting back to the patient and offering symptom management or tools for improvement is useless to patients and is a significant additional task burden. Patients deserve to know their scores, have them presented in a meaningful and interpretable manner, and then have guidance of how to improve scores. Programs such as these should be an integral part of comprehensive cancer patient care, as mandated by the NCCN, American College of Surgeons (ACS) Commission on Cancer, and American Society of Clinical Oncology (ASCO). Surgeon and multispecialty involvement in the longitudinal care of patients is critical to enhancing understanding of the impact of techniques, approaches, interventions, and digital technology. Collecting data about PROs enable machine learning algorithms to be employed to facilitate assessment and improvement in care. Sharing lessons learned and best practice in this area can facilitate adoption and patient-centered program development, potentially reaching underprivileged and previously underserved populations.
Acknowledgments
The UDD App is now available on the App store for iOS and Android. Non-Mayo Clinic patients can use the questionnaire for scoring and export the dashboard for tracking their domain scores. Mayo Clinic patients have access to tailored patient education and the survivorship clinic. Institutions wanting to utilize the tool for external validation to monitor patient symptoms can guide patients to register and utilize the UDD App.
Funding: This application was made available for patient benefit and research through the Vicky & Steve Dols Foundation.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1343/coif). SHB has a patent for an anastomotic esophageal stent. She has a clinical trial on lung ablation funded by Medtronic and a clinical trial on cryo ablation mesothelioma funded by Steris. She has been compensated as a consultant for Medtronic. She has released all profit from the UDD AppTM. LLC serves as a committee member in the Society of Critical Care Medicine PADIS Guidelines and the American Thoracic Society Planning and Evaluation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Society of Thoracic Surgeons Composite Score for Evaluating Esophagectomy for Esophageal Cancer. Ann Thorac Surg 2017;103:1661-7. [Crossref] [PubMed]
- Raymond DP, Seder CW, Wright CD, et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann Thorac Surg 2016;102:207-14. [Crossref] [PubMed]
- Clark JM, Boffa DJ, Meguid RA, et al. Regionalization of esophagectomy: where are we now? J Thorac Dis 2019;11:S1633-42. [Crossref] [PubMed]
- Funk LM, Gawande AA, Semel ME, et al. Esophagectomy outcomes at low-volume hospitals: the association between systems characteristics and mortality. Ann Surg 2011;253:912-7. [Crossref] [PubMed]
- Wouters MW, Wijnhoven BP, Karim-Kos HE, et al. High-volume versus low-volume for esophageal resections for cancer: the essential role of case-mix adjustments based on clinical data. Ann Surg Oncol 2008;15:80-7. [Crossref] [PubMed]
- Speicher PJ, Englum BR, Ganapathi AM, et al. Traveling to a High-volume Center is Associated With Improved Survival for Patients With Esophageal Cancer. Ann Surg 2017;265:743-9. [Crossref] [PubMed]
- Abdelsattar ZM, Habermann E, Borah BJ, et al. Understanding Failure to Rescue After Esophagectomy in the United States. Ann Thorac Surg 2020;109:865-71. [Crossref] [PubMed]
- Weledji EP, Verla V. Failure to rescue patients from early critical complications of oesophagogastric cancer surgery. Ann Med Surg (Lond) 2016;7:34-41. [Crossref] [PubMed]
- Acher AW, LeCaire TJ, Hundt AS, et al. Using Human Factors and Systems Engineering to Evaluate Readmission after Complex Surgery. J Am Coll Surg 2015;221:810-20. [Crossref] [PubMed]
- Chen C, Lin H, Xu F, et al. Risk factors associated with suicide among esophageal carcinoma patients from 1975 to 2016. Sci Rep 2021;11:18766. [Crossref] [PubMed]
- Housman B, Flores R, Lee DS. Narrative review of anxiety and depression in patients with esophageal cancer: underappreciated and undertreated. J Thorac Dis 2021;13:3160-70. [Crossref] [PubMed]
- Abou Chaar M, Godin A, Blackmon SB, et al. Patient-reported Outcomes after Esophagectomy for cancer in Long-Term Survivors. Abstract accepted at the Southern Thoracic Surgical Association 59th Annual Meeting 2022.
- Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 2021;384:1191-203. [Crossref] [PubMed]
- Iyer PG, Taylor WR, Johnson ML, et al. Highly Discriminant Methylated DNA Markers for the Non-endoscopic Detection of Barrett's Esophagus. Am J Gastroenterol 2018;113:1156-66. [Crossref] [PubMed]
- Chlan LL, Wzientek C, Pierson KE, et al. Upper Digestive Disease App for Electronic Patient-Reported Outcomes: A Mixed Methods Pilot Study. Ann Thorac Surg 2022;114:1142-51. [Crossref] [PubMed]
- National Cancer Institute. Cancer Terms Dictionary: Definition of Survivorship. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivorship. Accessed on 9/1/2022.
- National Comprehensive Cancer Consortium. Esophageal and Esophagogastric Junction Cancers (Version 3.2022). 2022. Available online: www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed on 8/26/2022.
- National Quality Forum. Patient-Reported Outcomes in Performance Measurement. 2012. Available online: https://www.qualityforum.org/projects/n-r/Patient-Reported_Outcomes/Patient-Reported_Outcomes.aspx. Accessed on 8/26/2022.
- United States Food & Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. Available online: https://www.fda.gov/media/77832/download. Accessed on 9/1/2022.
- Blackmon SH, Correa AM, Wynn B, et al. Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg 2007;83:1805-13; discussion 1813. [Crossref] [PubMed]
- Stephens EH, Gaur P, Hotze KO, et al. Super-Charged Pedicled Jejunal Interposition Performance Compares Favorably With a Gastric Conduit After Esophagectomy. Ann Thorac Surg 2015;100:407-13. [Crossref] [PubMed]
- Lee MK, Yost KJ, Pierson KE, et al. Patient-reported outcome domains for the esophageal CONDUIT report card: a prospective trial to establish domains. Health Qual Life Outcomes 2018;16:197. [Crossref] [PubMed]
- Lee MK, Yost KJ, Pierson KE, et al. Standard setting for a novel esophageal conduit questionnaire: CONDUIT Report Card. J Patient Rep Outcomes 2018;2:51. [Crossref] [PubMed]
- Mahajan NN, Lee MK, Yost KJ, et al. Preliminary Normative Standards of the Mayo Clinic Esophagectomy CONDUIT Tool. Mayo Clin Proc Innov Qual Outcomes 2019;3:429-37. [Crossref] [PubMed]
- Chlan LL, Ruddy K, Pierson KE, et al. Upper digestive disease application tool as a digital health intervention to collect dynamic electronic patient -reported outcomes in esophagectomy patients: A single arm pilot trial. Southern Thoracic Surgical Association 2021 Meeting Program Book, 2021.
- Traynor MD, Chlan LL, Wzientek C, et al. Agreement Between UDD App and Provider Evaluation of Esophagectomy Symptoms in a Mobile App Tool. Ann Thorac Surg 2022; [Crossref] [PubMed]
- Hasan IS, Mahajan N, Viehman J, et al. Predictors of Patient-Reported Reflux After Esophagectomy. Ann Thorac Surg 2020;110:1160-6. [Crossref] [PubMed]
- Mohan AT, Mahajan NN, Mardini S, et al. Outcomes of Standardized Protocols in Supercharged Pedicled Jejunal Esophageal Reconstruction. Ann Thorac Surg 2023;115:210-9. [Crossref] [PubMed]
- Wald JT, Lowery-Schrandt S, Hayes DL, et al. Mayo Clinic Care Network: A Collaborative Health Care Model. J Am Coll Radiol 2018;15:167-72. [Crossref] [PubMed]
- Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction: analysis of technical and functional efficacy. Arch Intern Med 1985;145:1443-6. [Crossref] [PubMed]
- Grudell AB, Alexander JA, Enders FB, et al. Validation of the Mayo Dysphagia Questionnaire. Dis Esophagus 2007;20:202-5. [Crossref] [PubMed]
- Sigstad H. A clinical diagnostic index in the diagnosis of the dumping syndrome. Changes in plasma volume and blood sugar after a test meal. Acta Med Scand 1970;188:479-86. [Crossref] [PubMed]
- PROMIS List of Adult Measures. Available online: http://www.healthmeasures.net. Accessed 08/26/2022.
- Garant A, Whitaker TJ, Spears GM, et al. A Comparison of Patient-Reported Health-Related Quality of Life During Proton Versus Photon Chemoradiation Therapy for Esophageal Cancer. Pract Radiat Oncol 2019;9:410-7. [Crossref] [PubMed]
- van Beek AP, Emous M, Laville M, et al. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev 2017;18:68-85. [Crossref] [PubMed]


