
Innovation in rigid bronchoscopy—past, present, and future
Introduction
Everette M. Rogers described innovation as “an idea, practice, or object that is perceived as new by an individual unit of adoption.” (1). Rigid bronchoscopy (RB) is one such example: Gustav Killian conceived the idea, transformed it into a tool, and dramatically changed the practice of respiratory medicine for the next 100 years. Its impact was profound and widespread over the world. Since then, there have been improvements in the instrument design, optics, illumination, and utility. Many other revolutionary tools have since been developed inspired from this original and revolutionary concept, such as tracheoscopes, laryngoscopes and even flexible bronchoscopes with ever-expanding indications that have been widely adopted by the international community, bringing together multiple and previously independent specialties such as thoracic surgery, otolaryngology, and interventional pulmonology (IP), to optimally manage central airway disorders in a multidisciplinary fashion. In this review, we illustrate some of the most significant innovations related to RB, describing how it started, showing the current advancement and picturing the future.
The beginning
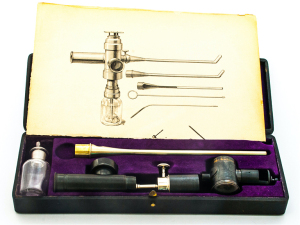
Gustav Killian, a German laryngologist, learned the laryngoscopy technique from Kirstein and had the idea to modify Rosenheim’s esophagoscope to access the lower airways (2,3). He was the first to reach passed the larynx into the trachea and beyond the main carina into the bronchi (4). In 1897, he successfully removed a foreign body from the right main stem bronchus for the first time (4). It was the beginning of a new era, which instantly reduced the mortality rate significantly from foreign body aspiration (5). Later, in the United States, Algernon Coolidge performed the first tracheo-bronchoscopy using an open urethroscope and a head-mirror to remove a hard-rubber cannula from the right main bronchus at the Massachusetts General Hospital in 1898 (6). In 1904, Chevalier Jackson Sr, a US otolaryngologist, developed a safer and simpler bronchoscopic technique by adding distal illumination and designing numerous tools to remove foreign bodies and take biopsies, thus advancing bronchoscopic management of benign and malignant airway diseases (Figure 1) (3,8-12). The Mutter Museum in Philadelphia, Pennsylvania, exhibits the collection of numerous foreign bodies removed by Dr. Jackson over the years.

Illumination
The crucial step in the advancement of endoscopy was endoluminal illumination. The French urologist Antonin Jean Desormeaux (1815–1894) is considered the real founder of practical endoscopy. In 1853, he introduced an illuminated urethroscope to the Academy of Medicine in Paris (12). The design of the instrument was similar to that introduced by Bozzini, but the source of light was “gazogene”, a mixture of alcohol and one part turpentine (Figure 2). Kussmaul used the device to perform the first illuminated rigid esophagoscopy.
A significant milestone was reached in 1887 when the German urologist Maximilian Nitze introduced the first miniature electric bulb without a separate cooling system (12-16). Up to this point, all endobronchial illumination was achieved using an external source. Einhorn from the United States built the first esophagoscope with a distally illuminated tube. Chevalier Jackson Sr used this concept for his laryngeal and endobronchial procedures (12). In 1940, Broyles perfected optical bronchoscopic telescopes and forceps illuminated by a small bulb at the tip, and later, introduced the first fiber illuminated bronchoscope (12).
The invention of the rod-lens system by Harold H. Hopkins and N.S. Kapany in 1959 led Karl Storz, a German physician, to develop the cold light illumination system, which was instrumental in the design of the current rigid telescope and the flexible fiberbronchoscope (17-20).
Anesthesia
Improvement in anesthesia techniques was fundamental to the practice of bronchoscopy. In 1884, Jellinek introduced cocaine as a local anesthetic, blunting pharyngeal and laryngeal reflexes. Cocaine allowed Killian to perform his human procedures using topical anesthesia (3,4). Progressive pharmacological advancement led to the development of general anesthesia (21). In 1967, Sander’s ventilation technique using thiopentone, suxamethonium, and jet ventilation, further improved anesthesia during bronchoscopy (22,23).
Diagnostic and therapeutic tools
Following these achievements in anesthesia techniques, several procedures became possible using the rigid bronchoscope as a diagnostic as well as a therapeutic tool. In 1949, Eduardo Schieppati was the first to perform a transbronchial needle aspiration (TBNA) for subcarinal adenopathy using a RB. The technique was later perfected by Euler in 1955 and Schiessle in 1962 for mediastinal masses (3,24,25). Euler, in 1948 used a similar technique of transbronchial access to perform pulmonary and aortic angiography (3). Howard Anderson from the Mayo Clinic was the first to carry out transbronchial lung biopsy using RB and forceps in 1965 (26).
The rigid bronchoscope was recognized as a tool that kept the airway secure, allowing safer re-canalization of central airways while managing major hemoptysis in expert hands (27-31).
The application of airway electrosurgery through RB was described in 1932 when Francis E. Gilfoy reported a case of a sessile tumor just above the main carina. He fulgurated it successfully several times (32). Then, in 1933, Kernan published a series of ten cases of carcinoma of the lung and bronchus treated with radon implantation and diathermy (33). Afterwards, significant complications decreased its use until new advancements allowed improvements in this technology to regain its usage, especially in flexible bronchoscopy (34,35).
The use of cryotherapy in the tracheobronchial tree started in the US in 1974 (36). Neel, Sanderson, and Carpenter experimented with animals and carried out the first cases on humans (37-40). They developed the technique and a 55 cm. cryosurgical probe (36,37,39). After a few years, this method dropped in favor of the laser. Posteriorly, Maiwand in the United Kingdom and Homasson in France began to use this technique again (41-43).
Strong, in 1974, and Laforet in 1976, were the first to use Carbon Dioxide (CO2) laser in benign and malignant diseases of the trachea and bronchi respectively (44,45). Toty and Dumon from France, and Shapshay and McDougall from the United States were the pioneers in using neodymium-doped yttrium aluminum garnet (Nd:YAG) laser through the rigid bronchoscope (3,27,28,46-52).
The use of endobronchial stents can be traced back to the 19th century (53). However, the development of the Montgomery T-tube and the dedicated Dumon silicone stent revolutionized the management of benign and malignant central airway diseases (54-57). Today, J. F. Dumon is recognized as the father of modern RB and IP for his contribution to managing central airway diseases using laser and silicone stents, improvements in the rigid scope, and RB teaching to physicians worldwide (7,58).
Advancements in the modern era
Education
The introduction of the flexible fiberbronchoscope by Shigeto Ikeda in 1966, resulted in a precipitous drop in the number of RB performed (20,59). However, J. F. Dumon in Marseille, France continued to promote the practice of RB, especially to carry out laser photocoagulation and stent placement. He also designed several devices to efficiently place silicone stents in the endobronchial tree. Thus, Dumon has been rightly credited for the resurgence and instruction of the technique (60).
Today, a number of hands-on courses in RB are routinely offered around the world, and IP has become a well-recognized subspecialty (60,61). More than 50 fellowship programs in IP have emerged in the United States alone, carrying on the torch of RB (62). The American Association for Bronchology and Interventional Pulmonology, founded in 1992, the American College of Chest Physicians and the Association of Interventional Pulmonary Fellowship Directors have worked tirelessly to formalize IP training, establish standards, and develop the curriculum necessary for accreditation of IP fellowships (7,62-66). Similar efforts have also been made in Europe, Australia and New Zealand, India and South America (67-69).
RB is also performed in thoracic surgery as well as otolaryngology, yet not all specialties have adopted a standardized curriculum to demonstrate that necessary competencies have been acquired (70).
The role of simulation training specific to RB using mannequin and cadaver models has continued to improve the educational training of both trainees and physicians seeking to gain knowledge and competency in RB (71).
In an effort to improve skills acquisition and standardization, simulators have been introduced and are now commonly used in many training programs. In a systematic review, Cook et al. showed that simulation-based bronchoscopy training is effective for various tasks, including RB (72). Salud et al. assessed RB performance in a manikin-based simulation with pressure sensors. When experienced and novice operators were evaluated, novices touched more areas and exerted more tissue pressure (73). Hsiung and colleagues designed a pediatric RB model to retrieve airway foreign bodies to train and assess pediatric surgical trainees’ RB skills (74).
Deutsch et al. investigated multiple modalities for RB training and suggested that animal models remain superior for the acquisition of psychomotor skills, with the obvious ethical caveats entailed (75). A study lead by Barrete characterized the forces and torques in RB. This study showed discrepancies between forces and torques applied during manikin and patient intubation, indicating a possible mismatch between the haptic feedback received by clinicians in RB training and its application in real cases (76). These findings could mean that more emphasis should be made on training in cadavers, and simulators with physical resemblance are needed (77). In an attempt to further improve training models, Al-Ramahi et al. created a 3D printed airway simulating the size and mechanical properties of various age group airways for foreign body removal training (78).
The use of real-time feedback on skills as well as utilization of video assessment of the learner allows additional learner self-adjustment in procedural egometrics (71).
Attempts have also been made to standardize skills assessment. Mahmood et al. developed a tool to objectively assess essential competencies in RB: this tool (RIGID-TASC) was shown to be accurate and reproducible to score and classify operators from novice to expert in RB intubation and navigation (79). This tool is may be considered an essential step to assess competency and give task-specific feedback in RB training (79).
In the near future, it will be desirable that the physicians in practice develop skills on realistic training models, complete stepwise progression to more challenging complex tasks, and be assessed objectively to have obtained the necessary competency before performing RB procedures in patients. Basic competency skills and expertise can be achieved after a routine training period but will plateau if a deliberate practice model is not adopted (71).
The primary goal of training is to establish basic competency through deliberate practice with specific rehearsal and editing of specific tasks under supervision and constructive feedback which can lead to mastery learning (71). Mastery in procedural skills remains a dynamic ongoing process beyond a dedicated year of IP fellowship training. Adaptation to evolving technology beyond postgraduate training requires the same deliberate skills practice (71).
Instrumentation
A few notable changes have taken place in the scope design and instrumentation since the end of the last century. The most widely used RB devices at this time are those built by Karl Storz, Richard Wolf, and Dumon (58).
The Efer-Dumon® rigid bronchoscope (EFER Endoscopy, La Ciotat, France) was designed in 1985. Enhancements were made in 2000 and then in 2014 with improvements in intubation tubes, stent placement instrumentation, accessories, and video bronchoscopy system. One of the most prominent characteristics of the Efer-Dumon® RB is its modular connector. This locking system has a 360° rotating ventilation port and an airtight union between the tubes and the universal base. Its video system has autoclavable optics with digital technology and a variable focal length coupler allowing loss-less image size adjustment (80).
Additional advancements have been made by Richard Wolf GmbH, a company based in Germany. It developed the TEXAS bronchoscope, which has an integrated semi-flexible telescope in a separate scope channel, allowing for a larger inner lumen for instrumentation. It has a distal lens irrigation system which allows intraprocedural cleaning if the lens gets obscured with secretion or blood. Additionally, there are other accessories like endobronchial shaver blades and tip-controlled instruments (scissors and forceps) with articulation in the distal tip and a 360-degree rotatable shaft. Images advances have been achieved integrating the 4K technology (ENDOCAM logic 4K®) which provides four times improvement in the resolution of a regular HD camera (81).
Stenting
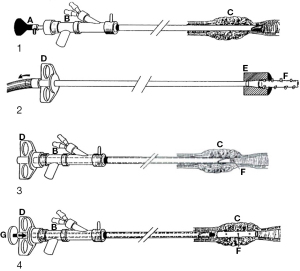
In 1987, Dumon created a studded cylindrical prosthesis made of silicone to maintain patency in patients with extrinsic or mixed airway obstruction (56,57). The use of RB is essential in silicone stent placement and removal (57,82). Additionally to the indispensable proceduralist expertise, the rigid scope and several rigid tools are crucial for the stenting technique (57) (Figure 3).
In the mid-1980s, Gianturco used a self-expandable metallic stent (SEMS) for the first time in patients with cancer (83). They were placed easily using an endotracheal tube, a Teflon catheter, and fluoroscopic guidance (83). Hence, metallic stents became more extensively used because of their properties, such as easy deployment or radiopacity (83). However, severe complications such as granulation tissue or tumor in-growth, difficulty to be removed and airway or vascular perforation discouraged their use, especially in patients with benign airway diseases, leading to a black box warning from the FDA (84,85). Since then, significant advances in metal stent technology have been achieved, leveraging the advantage of self-expandable metal stents now fully or partially covered to mitigate these complications (86,87). Despite all those improvements, some experts still recommend RB for central airway stent placement and removal irrespective of the type of stent used because it is a safe and effective method (82). A 2020 tracheobronchial stenting survey with data from 47 countries showed that respondents used RB (either alone or in combination with flexible bronchoscopy) in 84% of stent placement and 93% of removal despite SEMS being the most commonly used stents (88).
A recent study performed by the ESCODULE study group compared two sets of subjects from a retrospective cohort of patients with malignant central airway obstruction (CAO) treated with either Dumon silicone stent or third-generation SEMS (Aerstent® Leufen Medical GmbH, Germany). It showed that both stents successfully restored airway patency, but there was a significantly increase in complications with Aerstent® (89). This study used RB for all patients, and despite the improved operating characteristics in metal stents it shows that RB continues to have a value (89).
We anticipate that stents will continue to evolve with some recent promising innovations worth discussing here. Stents with biodegradable (BD) material that could maintain patency in an airway for a limited time are the subject of extensive research (90). They might be helpful in benign airway diseases when temporal stenting is desirable (84). Several synthetic degradable polymers have been studied (90-94). Research in animal models and patients have demonstrated a good safety profile, biocompatibility, and stent degradation-time depending on the polymer used (93,95-99).
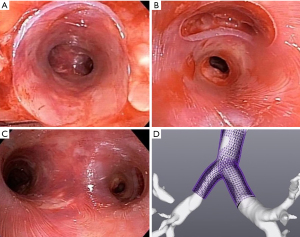
3D technology is also being used to create stents. Patients with complex benign airway diseases require frequent interventions and stenting to restore airways patency (100). Thomas R. Gildea, trying to improve management in patients with complex non-malignant central airway diseases, developed a patient-specific silicone airway stent (3DPSS) using computed tomography imaging and 3D printing technology (101,102). A retrospective evaluation at the Cleveland Clinic under FDA expanded access compared 3DPSS to commercially available stents (CAS), showed no difference in stent loading, placement, or removal, and the mean duration between bronchoscopies was significantly longer with 3DPSS when compared to CAS (100,103). Common adverse events observed with CAS were less frequent with 3DPSS, and a significant increase in the lifespan of the patient-specific stents was observed (100,104) (Figure 4). Moreover, RB remains an important and relevant tool for these recent stent innovations (100,105,106).
In high-risk RB stenting for central airway obstruction and imminent ventilation failure, ECMO has been used as ventilatory support in patients with malignant and benign diseases. Several case reports suggest favorable outcomes and a low complication rate (107-113).
Future in RB
Robotic endoscopic technology was recently introduced in pulmonary medicine, attempting to improve the performance of flexible bronchoscopy in peripheral pulmonary lesions (114-119). Safety and feasibility studies have been performed (120). A study designed to evaluate the clinical safety and diagnostic accuracy of the robotic-assisted bronchoscopy with biopsy in pulmonary lesions is currently recruiting (ClinicalTrials.gov Identifier: NCT04182815). However, this technology has been developed for diagnosis in the lung periphery and could potentially deliver needle-based therapy.
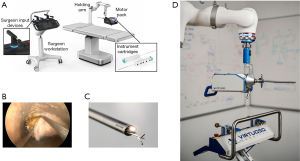
Recent research and development have focused on therapeutic RB for treatment of CAO. Gafford et al. developed a new robotic system based on thin robotic manipulators embedded in a standard rigid bronchoscope. These articulated tools bend and elongate to provide maneuverability at the level of the target, without scope movement (121). They used an ex-vivo model to demonstrate the ability to remove tissue and restore airway patency efficiently. Pre- and post-CT scans showed a reduction of the airway obstruction from 75% to 14% (average) for five CAO resections performed (121). They also established the robotic system’s potential to significantly reduce the forces applied to the patient’s head and neck (from 80.6 to 4.1 N) (121).
This feasibility study opens avenues to explore endoscopic airway surgery further (Figure 5). Endoscopic tracheal repair is intended only when the patient is unsuitable for surgery and relies on secondary healing after stent deployment (122). Endoscopic airway surgery remains anecdotal (123).
An RB robotic system in the future could have the potential to revolutionize respiratory medicine again and treat endoscopically airway dehiscence, subglottic or tracheal stenosis, tracheomalacia, and perform thoracic natural orifice transluminal endoscopic surgery (NOTES) (124-126).
In the future, we envision the development of highly maneuverable articulated arm bronchoscopic robots that can work in small, angled areas, combining different tools that have the potential to make a precise cut, dissection, suture, resection, ablation, or coagulation to solve luminal, airway wall, or extraluminal problems.
Conclusions
We have witnessed over one hundred years of innovation in RB. Significant improvements in instruments, optics, techniques, and procedures have been achieved. Evolving diagnostic and therapeutic tools are likely to cement the important role of RB further. We envision developments in RB robotic technology to have the potential to continue to revolutionize respiratory medicine.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-779/coif). Cleveland Clinic and Cleveland Clinic Institutional Officials/Leaders have an equity interest in Visionair and are entitled to royalty payments from the company for technology developed at Cleveland Clinic. Visionair is the manufacturer of the stents mentioned in this review. TRG is the inventor and may be entitled to royalty payments from the company in accordance with Cleveland Clinic policy. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rogers EM. Diffusion of Innovations. 5th edition. Free Press. 2003.
- Killian G. Ueber directe bronchoskopie. Münchener Med Wochenschrift 1898;27:844-7.
- Ernst A, Herth FJF. Principles and practice of interventional pulmonology Principles and Practice of Interventional Pulmonology. 2013. Available online: https://www.springer.com/gp/book/9781461442912
- Zöllner F. Gustav Killian, father of bronchoscopy. Arch Otolaryngol 1965;82:656-9. [Crossref] [PubMed]
- Rafanan AL, Mehta AC. Adult airway foreign body removal. What's new? Clin Chest Med 2001;22:319-30. [Crossref] [PubMed]
- Jackson C. Bronchoscopy; Past, Present and Future. N Engl J Med 1928;199:759-63. [Crossref]
- Panchabhai TS, Mehta AC. Historical perspectives of bronchoscopy: Connecting the dots. Ann Am Thorac Soc 2015;12:631-41. [Crossref] [PubMed]
- Boyd AD. Chevalier Jackson: the father of American bronchoesophagoscopy. Ann Thorac Surg 1994;57:502-5. [Crossref] [PubMed]
- Jackson CL, Konzelmann FW. Bronchoscopic Aspects of Bronchial Tumors. J Thorac Surg 1937;6:312-35. [Crossref]
- Jackson C. Endothelioma of the Right Bronchus Removed By Peroral Bronchoscopy. Am J Med Sci 1917;153:371-5. [Crossref]
- Jackson C. Malignant Growths of the Lung: Bronchoscopic Diagnosis. Arch Otolaryngol - Head Neck Surg 1930;12:747-52. [Crossref]
- Marsh BR. Historic development of bronchoesophagology. Otolaryngol Head Neck Surg 1996;114:689-716. [PubMed]
- Ward PH, Berci G, Calcaterra TC. Advances in Endoscopic Examination of the Respiratory System. Ann Otol Rhinol Laryngol 1974;83:754-60. [Crossref]
- Herr HW. Max Nitze, the cystoscope and urology. J Urol 2006;176:1313-6. [Crossref] [PubMed]
- Moran ME. The light bulb, cystoscopy, and Thomas Alva Edison. J Endourol 2010;24:1395-7. [Crossref] [PubMed]
- Reuter MA, Reuter HJ. The development of the cystoscope. J Urol 1998;159:638-40. [Crossref] [PubMed]
- Ellis H. The Hopkins rod-lens system. J Perioper Pract 2007;17:272, 274.
- Linder TE, Simmen D, Stool SE. Revolutionary Inventions in the 20th century the history of endoscopy. Arch Otolaryngol - Head Neck Surg 2021;123:1161-3. [Crossref] [PubMed]
- Cockett WS, Cockett AT. The Hopkins rod-lens system and the Storz cold light illumination system. Urology 1998;51:1-2. [Crossref] [PubMed]
- Ikeda S. Flexible bronchofiberscope. Ann Otol Rhinol Laryngol 1970;79:916-23. [Crossref] [PubMed]
- Matioc AA. An Anesthesiologist's Perspective on the History of Basic Airway Management: The "Progressive" Era, 1904 to 1960. Anesthesiology 2018;128:254-71. [Crossref] [PubMed]
- Spoerel WE, Grant PA. Ventilation during bronchoscopy. Can Anaesth Soc J 1971;18:178-88. [Crossref] [PubMed]
- Hart SM. A further modification of a simple apparatus for pulmonary ventilation during bronchoscopy. Br J Anaesth 1970;42:78-80. [Crossref] [PubMed]
- Schieppati E. Mediastinal puncture thru the tracheal carina. Rev Asoc Med Argent 1949;63:497-9. [PubMed]
- Schiessle W. Transbronchial and transtracheal puncture in peritracheobronchial adenopathies. J Fr Med Chir Thorac 1962;16:551-69. [PubMed]
- Andersen HA, Fontana RS, Harrison EG Jr. Transbronchoscopic lung biopsy in diffuse pulmonary disease. Dis Chest 1965;48:187-92. [Crossref] [PubMed]
- Cavaliere S, Foccoli P, Farina PL. Nd:YAG laser bronchoscopy. A five-year experience with 1,396 applications in 1,000 patients. Chest 1988;94:15-21. [Crossref] [PubMed]
- Hetzel MR, Smith SG. Endoscopic palliation of tracheobronchial malignancies. Thorax 1991;46:325-33. [Crossref] [PubMed]
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1985;87:712-4. [Crossref] [PubMed]
- Gerasin VA, Shafirovsky BB. Endobronchial electrosurgery. Chest 1988;93:270-4. [Crossref] [PubMed]
- Mitchell PD, Kennedy MP. Bronchoscopic management of malignant airway obstruction. Adv Ther 2014;31:512-38. [Crossref] [PubMed]
- Gilfoy FE. Primary Malignant Tumors of the Lower Third of the Trachea: Report of a Case With Successful Treatment By Electrofulguration and Deep X-Rays. Arch Otolaryngol - Head Neck Surg 1932;16:182-7. [Crossref]
- Kernan JD. Carcinoma of the Lung and Bronchus: Treatment With Radon Implantations and Diathermy. Arch Otolaryngol - Head Neck Surg 1933;17:457-75. [Crossref]
- Takizawa N, Oho K, Amemiya R, et al. Electrosurgery via the Fiberoptic Bronchoscope BT - Bronchology: Research, Diagnostic, and Therapeutic Aspects: Proceedings of the Second World Congress for Bronchology, held at Düsseldorf, FRG, 2–4 June 1980. In: Nakhosteen JA, Maassen W, editors. Bronchology: Research, Diagnostic, and Therapeutic Aspects Dordrecht: Springer Netherlands, 1981:559-61.
- Taguchi H, Nagata T, Kawai H, et al. High-Frequency Electrosurgical Treatment of Tracheal Obstruction Using the Flexible Bronchofiberscope BT - Bronchology: Research, Diagnostic, and Therapeutic Aspects: Proceedings of the Second World Congress for Bronchology, held at Düsseldorf, FRG, 2–4 J. In: Nakhosteen JA, Maassen W, editors. Bronchology: Research, Diagnostic, and Therapeutic Aspects Dordrecht: Springer Netherlands, 1981:563-5.
- Neel HB 3rd, Farrell KH, Payne WS, et al. Cryosurgery of respiratory structures. II. Cryonecrosis of the lung. Laryngoscope 1974;84:417-26. [Crossref] [PubMed]
- Neel HB 3rd. Cryosurgery for the treatment of cancer. Laryngoscope 1980;90:1-48. [Crossref] [PubMed]
- Sanderson DR, Neel HB, Payne WS, et al. Cryotherapy for bronchogenic carcinoma: report of a case. Mayo Clin Proc 1975;50:435-7. [PubMed]
- Sanderson DR, Neel HB 3rd, Fontana RS. Bronchoscopic cryotherapy. Ann Otol Rhinol Laryngol 1981;90:354-8. [Crossref] [PubMed]
- Carpenter RJ 3rd, Neel HB 3rd, Sanderson DR. Comparison of endoscopic cryosurgery and electrocoagulation of bronchi. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol 1977;84:313-23. [PubMed]
- Maiwand MO. Cryotherapy for advanced carcinoma of the trachea and bronchi. Br Med J (Clin Res Ed) 1986;293:181-2. [Crossref] [PubMed]
- Homasson JP, Renault P, Angebault M, et al. Bronchoscopic cryotherapy for airway strictures caused by tumors. Chest 1986;90:159-64. [Crossref] [PubMed]
- Homasson JP, Bell NJ. History of tracheobronchial cryotherapy. In: Cryotherapy in chest medicine. 1992:27-33.
- Strong MS, Vaughan CW, Polanyi T, et al. Bronchoscopic Carbon Dioxide Laser Surgery. Ann Otol Rhinol Laryngol 1974;83:769-76. [Crossref]
- Laforet EG, Berger RL, Vaughan CW. Carcinoma obstructing the trachea. Treatment by laser resection. N Engl J Med 1976;294:941. [Crossref] [PubMed]
- Toty L, Personne C, Colchen A, et al. Bronchoscopic management of tracheal lesions using the neodynium yttrium aluminium garnet laser. Thorax 1981;36:175-8. [Crossref] [PubMed]
- Toty L, Personne CL, Hertzog P, et al. Use of a laser beam (YAG) with a flexible fiber for endoscopic treatment of some broncho-tracheal lesions (author's transl). Rev Fr Mal Respir 1979;7:475-82. [PubMed]
- Dumon JF, Reboud E, Auconte F, et al. Treatment of tracheobronchial lesions with Laser Yag. Minerva Med 1981;72:2593-600. [PubMed]
- Shapshay SM, Strong MS. Tracheobronchial obstruction from metastatic distant malignancies. Ann Otol Rhinol Laryngol 1982;91:648-51. [Crossref] [PubMed]
- Hetzel MR, Millard FJ, Ayesh R, et al. Laser treatment for carcinoma of the bronchus. Br Med J (Clin Res Ed) 1983;286:12-6. [Crossref] [PubMed]
- Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium-YAG laser in bronchology. Chest 1984;86:163-8. [Crossref] [PubMed]
- McDougall JC, Cortese DA. Neodymium-YAG laser therapy of malignant airway obstruction. A preliminary report. Mayo Clin Proc 1983;58:35-9. [PubMed]
- Bond CJ. Note on the Treatment of Tracheal Stenosis By a New T-Shaped Tracheotomy Tube. Lancet 1891;137:539. [Crossref]
- Montgomery WW. I: Reconstruction of the cervical trachea. Ann Otol Rhinol Laryngol 1964;73:5-15. [Crossref] [PubMed]
- Montgomery WW. T-tube tracheal stent. Arch Otolaryngol 1965;82:320-1. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Dumon JF, Cavaliere S, Diaz-Jimenez JP, et al. Seven-year experience with the dumon prosthesis. J Bronchol 1996;3:6-10. [Crossref]
- Flannery A, Daneshvar C, Dutau H, et al. The Art of Rigid Bronchoscopy and Airway Stenting. Clin Chest Med 2018;39:149-67. [Crossref] [PubMed]
- Ikeda S, Yanai N, Ishikawa S. Flexible bronchofiberscope. Keio J Med 1968;17:1-16. [Crossref] [PubMed]
- Beamis JF, Mathur PM. Interventional Pulmonology: Current Status and Future Direction. In: Interventional Bronchoscopy Totowa, NJ: Humana Press, 2013:3-14.
- Lee HJ, Sachdeva A. Training program of interventional pulmonology fellowships: USA. J Thorac Dis 2015;7:S415-7. [PubMed]
- Pastis NJ, Nietert PJ, Silvestri GA. Variation in training for interventional pulmonary procedures among US pulmonary/critical care fellowships: A survey of fellowship directors. Chest 2005;127:1614-21. [Crossref] [PubMed]
- Mullon JJ, Burkart KM, Silvestri G, et al. Interventional Pulmonology Fellowship Accreditation Standards: Executive Summary of the Multisociety Interventional Pulmonology Fellowship Accreditation Committee. Chest 2017;151:1114-21. [Crossref] [PubMed]
- Lee HJ, Feller-Kopman D, Shepherd RW, et al. Validation of an interventional pulmonary examination. Chest 2013;143:1667-70. [Crossref] [PubMed]
- Ernst A, Silvestri GA, Johnstone DAmerican College of Chest Physicians. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- Lamb CR, Feller-Kopman D, Ernst A, et al. An approach to interventional pulmonary fellowship training. Chest 2010;137:195-9. [Crossref] [PubMed]
- Colt HG, Williamson JP. Training in interventional pulmonology: What we have learned and a way forward. Respirology 2020;25:997-1007. [Crossref] [PubMed]
- Galluccio G, Tramaglino LM, Marchese R, et al. Competence in operative bronchoscopy. Panminerva Med 2019;61:298-325. [Crossref] [PubMed]
- Corbetta L, Patelli M. Executive Summary of Training and Competence Standards for the Interventional Pulmonology Master Program in Italy. J Bronchology Interv Pulmonol 2018;25:6-8. [Crossref] [PubMed]
- Ernst A, Wahidi MM, Read CA, et al. Adult Bronchoscopy Training: Current State and Suggestions for the Future: CHEST Expert Panel Report. Chest 2015;148:321-32. [Crossref] [PubMed]
- Aslam W, Lee HJ, Lamb CR. Standardizing education in interventional pulmonology in the midst of technological change. J Thorac Dis 2020;12:3331-40. [Crossref] [PubMed]
- Kennedy CC, Maldonado F, Cook DA. Simulation-based bronchoscopy training: systematic review and meta-analysis. Chest 2013;144:183-92. [Crossref] [PubMed]
- Salud LH, Peniche AR, Salud JC, et al. Toward a simulation and assessment method for the practice of camera-guided rigid bronchoscopy. Stud Health Technol Inform 2011;163:535-41. [PubMed]
- Hsiung GE, Schwab B, O'Brien EK, et al. Preliminary Evaluation of a Novel Rigid Bronchoscopy Simulator. J Laparoendosc Adv Surg Tech A 2017;27:737-43. [Crossref] [PubMed]
- Deutsch ES, Christenson T, Curry J, et al. Multimodality education for airway endoscopy skill development. Ann Otol Rhinol Laryngol 2009;118:81-6. [Crossref] [PubMed]
- Barrette LX, Turkseven M, De S, et al. Characterization of Applied Forces and Torques During Rigid Bronchoscopy Intubation. J Bronchology Interv Pulmonol 2020;27:246-52. [Crossref] [PubMed]
- Mahmood K. You Can't Improve What You Can't Measure: Smart Learning Meets Rigid Bronchoscopy. J Bronchology Interv Pulmonol 2020;27:227-8. [Crossref] [PubMed]
- Al-Ramahi J, Luo H, Fang R, et al. Development of an Innovative 3D Printed Rigid Bronchoscopy Training Model. Ann Otol Rhinol Laryngol 2016;125:965-9. [Crossref] [PubMed]
- Mahmood K, Wahidi MM, Osann KE, et al. Development of a Tool to Assess Basic Competency in the Performance of Rigid Bronchoscopy. Ann Am Thorac Soc 2016;13:502-11. [Crossref] [PubMed]
- Efer Medical - Bronchoscope Efer-Dumon Series Iii [cited 2021 Mar 7]. Available online: http://www.efermedical.com/spip.php?article34
- Pulmonology / thoracic surgery - Richard Wolf [cited 2021 Mar 7]. Available online: https://www.richard-wolf.com/en-us/disciplines/pulmonology-thoracic-surgery/
- Dutau H, Dumon JF. Airway Stenting Revisited: 30 Years, the Age of Reason? J Bronchology Interv Pulmonol 2017;24:257-9. [Crossref] [PubMed]
- Wallace MJ, Charnsangavej C, Ogawa K, et al. Tracheobronchial tree: expandable metallic stents used in experimental and clinical applications. Work in progress. Radiology 1986;158:309-12. [Crossref] [PubMed]
- Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg 2018;7:273-83. [Crossref] [PubMed]
- Lund ME, Force S. Airway stenting for patients with benign airway disease and the Food and Drug Administration advisory: a call for restraint. Chest 2007;132:1107-8. [Crossref] [PubMed]
- Avasarala SK, Freitag L, Mehta AC. Metallic Endobronchial Stents: A Contemporary Resurrection. Chest 2019;155:1246-59. [Crossref] [PubMed]
- Guibert N, Saka H, Dutau H. Airway stenting: Technological advancements and its role in interventional pulmonology. Respirology 2020;25:953-62. [Crossref] [PubMed]
- Mathew R, Hibare K, Dalar L, et al. Tracheobronchial stent sizing and deployment practices airway stenting practices around the world: a survey study. J Thorac Dis 2020;12:5495-504. [Crossref] [PubMed]
- Ortiz-Comino RM, Morales A, López-Lisbona R, et al. Silicone Stent Versus Fully Covered Metallic Stent in Malignant Central Airway Stenosis. Ann Thorac Surg 2021;111:283-9. [Crossref] [PubMed]
- Dutau H, Musani AI, Laroumagne S, et al. Biodegradable Airway Stents - Bench to Bedside: A Comprehensive Review. Respiration 2015;90:512-21. [Crossref] [PubMed]
- Korpela A, Aarnio P, Sariola H, et al. Comparison of tissue reactions in the tracheal mucosa surrounding a bioabsorbable and silicone airway stents. Ann Thorac Surg 1998;66:1772-6. [Crossref] [PubMed]
- Korpela A, Aarnio P, Sariola H, et al. Bioabsorbable self-reinforced poly-L-lactide, metallic, and silicone stents in the management of experimental tracheal stenosis. Chest 1999;115:490-5. [Crossref] [PubMed]
- Robey TC, Välimaa T, Murphy HS, et al. Use of internal bioabsorbable PLGA "finger-type" stents in a rabbit tracheal reconstruction model. Arch Otolaryngol Head Neck Surg 2000;126:985-91. [Crossref] [PubMed]
- Zopf DA, Flanagan CL, Wheeler M, et al. Treatment of severe porcine tracheomalacia with a 3-dimensionally printed, bioresorbable, external airway splint. JAMA Otolaryngol Head Neck Surg 2014;140:66-71. [Crossref] [PubMed]
- Saito Y, Minami K, Kobayashi M, et al. New tubular bioabsorbable knitted airway stent: biocompatibility and mechanical strength. J Thorac Cardiovasc Surg 2002;123:161-7. [Crossref] [PubMed]
- Liu KS, Liu YH, Peng YJ, et al. Experimental absorbable stent permits airway remodeling. J Thorac Cardiovasc Surg 2011;141:463-8. [Crossref] [PubMed]
- Lischke R, Pozniak J, Vondrys D, et al. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg 2011;40:619-24. [Crossref] [PubMed]
- Stehlik L, Hytych V, Letackova J, et al. Biodegradable polydioxanone stents in the treatment of adult patients with tracheal narrowing. BMC Pulm Med 2015;15:164. [Crossref] [PubMed]
- Vondrys D, Elliott MJ, McLaren CA, et al. First experience with biodegradable airway stents in children. Ann Thorac Surg 2011;92:1870-4. [Crossref] [PubMed]
- Aravena Leon C, Inaty H, Urbas A, et al. Early Outcomes With 3D Printing and Airway Stents. Chest 2019;156:A199-200. [Crossref]
- Young BP, Machuzak MS, Gildea TR. Initial Clinical Experience Using 3d Printing And Patient-Specific Airway Stents: Compassionate Use Of 3d Printed Patient-Specific Airway Stents. Am J Respir Crit Care Med 2017;Conference:195-A1711.
- Gildea TR, Young BP, Machuzak MS. Application of 3D Printing for Patient-Specific Silicone Stents: 1-Year Follow-Up on 2 Patients. Respiration 2018;96:488-94. [Crossref] [PubMed]
- Aravena C, Gildea TR. Patient-specific airway stent using three-dimensional printing: a review. Ann Transl Med 2022 Jan;0(0):0–0.
- Aravena Leon C, Inaty H, Urbas A, et al. Clinical Outcomes With 3D Patient Specific Airway Stents Compared To Commercially Available Airway Stents. 21st World Congr Bronchol Interv Pulmonol 2020;PO-397:397.
- Zając A, Krysta M, Kiszka A, et al. Biodegradable airway stents: Novel treatment of airway obstruction in children. Adv Clin Exp Med 2019;28:961-5. [Crossref] [PubMed]
- Alraiyes AH, Avasarala SK, Machuzak MS, et al. 3D printing for airway disease. AME Med J 2019;4:14. [Crossref] [PubMed]
- Ignacio RC Jr, Falcone RA Jr, Brown RL. A case report of severe tracheal obstruction requiring extracorporeal membrane oxygenation. J Pediatr Surg 2006;41:E1-4. [Crossref] [PubMed]
- Chen A. ECMO-assisted Rigid Bronchoscopy for Tracheal Obstruction. J Bronchology Interv Pulmonol 2009;16:296-7. [Crossref] [PubMed]
- Hong Y, Jo KW, Lyu J, et al. Use of venovenous extracorporeal membrane oxygenation in central airway obstruction to facilitate interventions leading to definitive airway security. J Crit Care 2013;28:669-74. [Crossref] [PubMed]
- Martinod E, Portela AM, Uzunhan Y, et al. Elective extra corporeal membrane oxygenation for high-risk rigid bronchoscopy. Thorax 2020;75:994-7. [Crossref] [PubMed]
- Dunkman WJ, Nicoara A, Schroder J, et al. Elective Venovenous Extracorporeal Membrane Oxygenation for Resection of Endotracheal Tumor: A Case Report. A A Case Rep 2017;9:97-100. [Crossref] [PubMed]
- George TJ, Knudsen KP, Sodha NR, et al. Respiratory support with venovenous extracorporeal membrane oxygenation during stenting of tracheobronchomalacia. Ann Thorac Surg 2012;94:1736-7. [Crossref] [PubMed]
- Meyer S, Dincq AS, Pirard L, et al. Bronchotracheal Stenting Management by Rigid Bronchoscopy under Extracorporeal Membrane Oxygenation (ECMO) Support: 10 Years of Experience in a Tertiary Center. Can Respir J 2021;2021:8822591. [Crossref] [PubMed]
- Chen AC, Gillespie CT. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann Thorac Surg 2018;106:293-7. [Crossref] [PubMed]
- Chen AC, Pastis NJ, Machuzak MS, et al. Accuracy of a Robotic Endoscopic System in Cadaver Models with Simulated Tumor Targets: ACCESS Study. Respiration 2020;99:56-61. [Crossref] [PubMed]
- Yarmus L, Akulian J, Wahidi M, et al. A Prospective Randomized Comparative Study of Three Guided Bronchoscopic Approaches for Investigating Pulmonary Nodules: The PRECISION-1 Study. Chest 2020;157:694-701. [Crossref] [PubMed]
- Fortin M, Yarmus L, Rendina EA, et al. Multi-institutional retrospective analysis of adverse events following rigid tracheobronchoscopy. Respirology 2021;26:87-91. [Crossref] [PubMed]
- Benn BS, Romero AO, Lum M, et al. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021;199:177-86. [Crossref] [PubMed]
- Pritchett M, Muller L, Ost D, et al. Integration of Shape-Sensing Robotic-Assisted Bronchoscopy and Cone-Beam Ct for the Biopsy of Pulmonary Nodules. Chest 2021;160:A1622-4. [Crossref]
- Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic Bronchoscopy for Peripheral Pulmonary Lesions: A Multicenter Pilot and Feasibility Study (BENEFIT). Chest 2021;159:845-52. [Crossref] [PubMed]
- Gafford JB, Webster S, Dillon N, et al. A Concentric Tube Robot System for Rigid Bronchoscopy: A Feasibility Study on Central Airway Obstruction Removal. Ann Biomed Eng 2020;48:181-91. [Crossref] [PubMed]
- Chaaban S, Simoff M, Ray C, et al. Posterior Tracheal Laceration Treated with a Stent. Ann ATS 2017 [cited 2021 Mar 14];14:1224. Available online: www.atsjournals.org
- Mozer AB, Michel E, Gillespie C, et al. Bronchoendoscopic Repair of Tracheoesophageal Fistula. Am J Respir Crit Care Med 2019;200:774-5. [Crossref] [PubMed]
- Makris KI, Rieder E, Swanstrom LL. Natural orifice trans-luminal endoscopic surgery (NOTES) in thoracic surgery. Semin Thorac Cardiovasc Surg 2010;22:302-9. [Crossref] [PubMed]
- Nouraei SA, Kapoor KV, Nouraei SM, et al. Results of endoscopic tracheoplasty for treating tracheostomy-related airway stenosis. Clin Otolaryngol 2007;32:471-5. [Crossref] [PubMed]
- Aravena C, Almeida FA, Mukhopadhyay S, et al. Idiopathic subglottic stenosis: a review. J Thorac Dis 2020;12:1100-11. [Crossref] [PubMed]


