
Efficiency and toxicity of nab-paclitaxel and camrelizumab in the second or above line treatment of advanced non-small cell lung cancer: a retrospective cohort study
Highlight box
Key findings
• This is the first study to report the promising efficiency of nab-ptx and camrelizumab with tolerated toxicity in the second or above treatment line of advanced NSCLC through depleting Treg ratio.
What is known and what is new?
• Chemotherapy drugs such as nab-ptx have the increasingly recognized potential to combine with PD-1/PD-L1 inhibitors in NSCLC.
• Camrelizumab and nab-ptx could play a synergistic effect in the second or above treatment line of advanced NSCLC.
What is the implication, and what should change now?
• This regimen may have the potential to become an effective treatment for NSCLC. however, the real value of this regimen needs to be further confirmed.
Introduction
Immuno-oncology (IO) targeting programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) has become one of the main treatments for advanced non-small cell lung cancer (NSCLC) (1-4). Until now, the application scope of IO has covered nearly all aspects of NSCLC, from later line treatment in the early stages (5) to neoadjuvant therapy more recently (6,7). Although in the later line, single IO could bring about long-term survival benefit for part of advanced NSCLC, the objective response rate (ORR) has been reported at only 20% (5), and the efficiency seems to be inferior for cases of advanced NSCLC with driver gene mutations such as EGFR and ALK. Therefore, it is still of great significance to seek new strategies to improve the therapeutic efficiency of IO in second or above line treatment (8).
Although single PD-1 shows relatively higher efficiency (up to 50%) toward advanced NSCLC with higher PD-L1 expression (9), the incidence of such patients was reported at only 10–15% totally. Therefore, except for studies exploring the priority population, to improve the efficiency, clinical evidence has increasingly shown that the more practical treatment approach is to implement drug combination strategies. Considering the combination of PD-1/PD-L1 inhibitors and chemotherapy has become one of the main modes for the first-line treatment of NSCLC (10,11), it is also theoretically reasonable to combine PD-1/PD-L1 inhibitors and single chemotherapy in the second line or above treatment to further enhance the therapeutic efficiency. Since either single docetaxel or PD-1/PD-L1 inhibitors has become the standard regimen with lower efficiency under such conditions (12), the drug combination of PD-1/PD-L1 inhibitors and single docetaxel would be more practical (13). However, compared to nanoparticle albumin-bound paclitaxel (nab-ptx) (14), the relatively higher toxicity and the requirement for dexamethasone premedication (15) of docetaxel has displayed intrinsic disadvantages, indicating that the regimen needs to be further optimized. Correspondingly, considering the higher penetrance capacity relative to docetaxel or paclitaxel, nab-ptx would probably be a more suitable partner for the combination of PD-1/PD-L1 inhibitors (11). Accumulating evidence has shown that the drug combination of nab-ptx and PD-1/PD-L1 inhibitors in solid tumors exerts obvious synergistic effect with lower toxicities (16-19), although the mechanism remains unknown (20). With regard to PD-1/PD-L1 inhibitors, in China, camrelizumab was the first PD-1/PD-L1 antibody to be approved by the National Medical Products Administration (NMPA) in 2019 for the first-line treatment of advanced NSCLC (21,22). However, until now, no study about the drug combination of nab-ptx and camrelizumab had been reported in the second or above line treatment of advanced NSCLC. Therefore, in this study, we retrospectively analyzed the data of advanced NSCLC patients who accepted the treatment of camrelizumab and nab-ptx with the aim to uncover a new effective way to improve the efficiency of advanced NSCLC treatment with IO. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-387/rc).
Methods
Study design
During June 2019 to January 2022, we retrospectively collected the medical records of patients in Hubei Cancer Hospital who received the combination of nab-ptx and camrelizumab in the second or above line treatment of advanced NSCLC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Committee of Hubei Cancer Hospital Affiliated to Tongji Medical College (Wuhan, China) (No. HBCHEn2023125), and patients or their families signed an informed consent form. A total of 53 patients with advanced NSCLC accepted the drug combination of camrelizumab and nab-ptx in the second- or above-line treatment.
Inclusion criteria
Patients age between 18 and 70 years with histologically confirmed advanced NSCLC were eligible for enrollment. The enrollment criteria included prior lack of response or intolerance to at least one chemotherapeutic regimen (including both doublet platinum regimen), and drug resistance confirmation was necessary for patients who had previously undergone EGFR tyrosine kinase inhibitor (TKI) therapy. Before disease progression, no patient had accepted immune checkpoint therapy such as anti-PD-1 or anti-cytotoxic T lymphocyte antigen-4 (CTLA-4). The criteria for progression to second or above line chemotherapy or targeted therapy were based on computed tomography (CT) and magnetic resonance imaging (MRI) evaluation. The additional enrollment criteria were as follows: at least 1 measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors (RECIST); an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 2; acceptable hematologic, hepatic, and renal function; and a life expectancy of more than 3 months.
Exclusion criteria
The key exclusion criteria included symptomatic brain metastasis, cachexia, and a life expectancy of less than 3 months.
Gene or biomarker detection
Most of the patients had undergone CT-guided needle aspiration for diagnosis. Once the diagnosis was confirmed pathologically, part of the sample was used for multiple gene detection, such as EGFR, ALK, C-Met, and K-RAS, the detection was performed using standard assay. The detection of PD-L1 was conducted according to the standard assay (Dako 22C-3 antibody), and the cutoff value for PD-L1 was set as 1%.
Lymph cell subpopulation analysis by fluorescence-activated cell sorting (FACS)
Samples of ethylenediamine tetraacetic acid (EDTA) anticoagulated peripheral blood (2 mL) were collected from patients with advanced NSCLC baseline and subsequent treatment cycles. All samples were detected within 6 hours of being obtained. Briefly, CD3+/CD4+/CD8+ T-cell, CD19+ B-cell, and CD16+CD56+ natural killer (NK)-cell counts (cells/µL) were measured by multiple-color flow cytometry with human monoclonal anti-CD3-fluorescein isothiocyanate (FITC), anti-CD4-phycoerythrin (PE), anti-CD8-allophycocyanin (APC), anti-CD19-PE, anti-CD16-APC, and anti-CD56-PE antibodies [BD Multitest; Becton, Dickinson, and Co. (BD) Biosciences, Franklin Lakes, NJ, USA] correspondingly, according to the manufacturer’s instructions. The cells were analyzed on a BD FACS Canto II flow cytometry system (BD Biosciences).
Drug application
All participants had received 1 or more lines of treatment. Following disease progression or drug resistance by clinic confirmation, the regimen was performed by modified dosage, namely, the dosage of nab-ptx was 100–200 mg/m2 every 3 weeks. The dosage for camrelizumab was fixed at 200 mg, repeated every 3 weeks, and the treatment was continued until the occurrence of disease progression or intolerable toxicities. Upon practical application, PD-1/PD-L1 inhibitor was applied firstly, followed by nab-ptx.
Efficiency evaluation and adverse event (AE) monitoring
All patients had undergone at least 2 cycles of treatment. Patients were followed up every 6 weeks by using lung CT and MRI. The main criterion was RECIST v1.1. The primary study endpoints were ORR and progression-free survival (PFS), the secondary study endpoints were OS and disease control rate (DCR). The ORR was the ratio of complete response (CR) plus partial response (PR) patients to total patients; the DCR was the ratio of non-progressive disease (PD) patients (including CR, PR and SD) to total patients. PFS was defined as the duration of treatment from the start of treatment until disease progression or death from any cause. If the disease had not progressed or no death had occurred, the time of the last imaging study was set. OS was defined as the time from the start of treatment until death from any cause or the last follow-up if no death occurred.
Safety was assessed by recording the incidence of treatment-related adverse reactions and classifying adverse reactions according to National Cancer Institute and Terminology Criteria for Adverse Events, version 4.0. Data were recorded for all events, irrespective of whether they were immunotherapy-related or not, and were reported within the first dose to 30 days after the last dose. The safety analysis included all patients who had received at least one more treatment, and no treatment-related grade 5 adverse reactions were reported prior to data lock. AEs leading to discontinuation of treatment or requiring management with immunomodulatory drugs were also recorded.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 (IBM Corp., Chicago, IL, USA). Two-tailed tests were performed at a significance level of α=0.05, with P<0.05 indicative of statistical significance. Subgroup comparisons of count date were performed using the chi-square test or Fisher’s exact test. The relationship between the variables and survival was assessed using Kaplan-Meier curves and the log-rank test’s subgroup differences in survival were assessed. The corresponding figures were drawn using Graph Prism 5.0 (GraphPad Software, San Diego, CA, USA). A P value <0.05 was regarded as statistically significant.
Results
Patient characteristics
A total of 53 patients were enrolled in this study, including 31 males and 22 females, with a median age of 64 years. The general performance status of the patients was 0–2, including 8 cases of brain metastasis and 7 cases of liver metastasis. Most of PS status was 0–1. A minority of patients (approximately 36%) had a performance score of 2. Most of the female patients were non-smokers, and the majority of the male patients had smoking history. According to the detection results of PD-L1 expression before treatment, 13 patients with high expression of PD-L1 (≥1%) and 10 patients with low expression of PD-L1 (<1%) were classified. The main pathological types of the patients were adenocarcinoma or squamous cell carcinoma, including 34 cases of adenocarcinoma and 19 cases of squamous cell carcinoma (Table 1).
Table 1
| Characteristics | No. of patients (%) |
|---|---|
| Age (years) | |
| Median | 64 |
| Range | 47–75 |
| Gender | |
| Male | 31 (58.49) |
| Female | 22 (41.51) |
| Smoking history | |
| Never smoker | 16 (30.19) |
| Former smoker | 37 (69.81) |
| Histology | |
| Adenocarcinoma | 34 (64.15) |
| Squamous carcinoma | 19 (35.85) |
| ECOG score | |
| 0–1 | 34 (64.15) |
| ≥2 | 19 (35.85) |
| Previous radiotherapy | |
| Yes | 16 (30.19) |
| No | 37 (69.81) |
| Brain metastasis | |
| Yes | 8 (15.09) |
| No | 45 (84.91) |
| Liver metastasis | |
| Yes | 7 (13.21) |
| No | 46 (86.79) |
| Stage | |
| IIIB/IIIC | 10 (18.86) |
| IV | 43 (81.14) |
| PD-L1 expression | |
| Positive | 13 (24.53) |
| Negative | 10 (18.87) |
| Unknown | 30 (56.60) |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1.
Previous treatment
All participants had undergone at least 1 line treatment of chemotherapy. The first-line treatment regimen was gemcitabine or docetaxel plus platinum and pemetrexed for squamous cell carcinoma; for adenocarcinoma, the combination of pemetrexed and platinum (cisplatin or carboplatin) was the priority regimen. A total of 4 patients had received radiotherapy, and the treatment interval before enrollment was not less than 3 weeks.
Efficiency
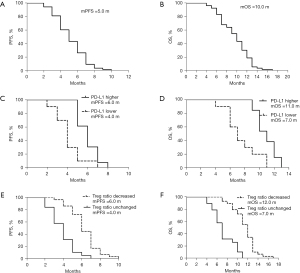
All 53 participants received the combination therapy of camrelizumab with nab-ptx. The preliminary results showed that the ORR of camrelizumab combined with nab-ptx in the treatment of advanced NSCLC patients was 35.85%, with 19 PR, 16 stable disease (SD), and 18 PD; the DCR was 66.03% (see Table 2), and the median PFS (mPFS) was 5.0 months, whereas the median OS (mOS) was 10.0 months (see Figure 1A,1B). In subgroup analysis, compared to these patients with lower PD-L1 expression (<1%), patients with higher PD-L1 expression (≥1%) exhibited higher efficiency (mPFS 6.0 vs. 4.0 months, HR =0.15, 95% CI: 0.04–0.51, P=0.0025; mOS 11.0 vs. 7.0 months, HR =0.14, 95% CI: 0.04–0.44, P=0.001) (see Figure 1C,1D).
Table 2
| Variables | Values |
|---|---|
| Complete response | 0 |
| Partial response, patient No. (%) | 19/53 (35.85) |
| Stable disease, patient No. (%) | 16/53 (30.19) |
| Progressive disease, patient No. (%) | 18/53 (33.96) |
| Objective response (%) | 35.85 |
| Median PFS (months) | 5.0 |
| Disease control rate (%) | 66.03 |
| Median OS (months) | 10.0 |
Nab-ptx, nanoparticle albumin-bound paclitaxel; NSCLC, non-small cell lung cancer; PFS, progression-free survival; OS, overall survival.
Lymph cell subpopulation changes
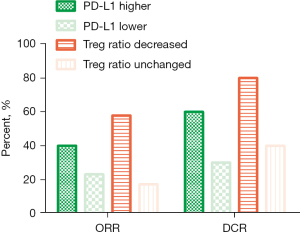
We also compared the effect of treatment on the lymph cell subpopulation before and after the treatment; no obvious difference was observed in the total amount of baseline lymph cells, such as CD4+ T cell, CD8+ T cell, B cell, and NK cell. However, the decrease of regulatory T cell (Treg) ratio seemed to be correlated with the efficiency. Compared to the patients without decreased Treg cells, the efficiency of patients with decreased Treg cells was better (ORR 57.89% vs. 17.24%, respectively; mPFS 6.0 vs. 4.0 months, HR =0.12, 95% CI: 0.05–0.28, P<0.0001, respectively; mOS 12.0 vs. 7.0 months, HR =0.08, 95% CI: 0.03–0.20, P<0.0001, respectively) (Figure 1E,1F, Figure 2 and Table 2), indicating that the synergistic mechanism of this regimen may be related to the depletion of Treg cells in the peripheral blood.
Toxicity
Besides efficiency, we also record the type and degree of AEs during the whole treatment period. The main toxicities of this regimen were leukopenia, neutropenia, anemia, thrombocytopenia peripheral neuropathy, vomiting, nausea, fatigue, decreased appetite, and so on. The incidence of severe side effects was about 53%. In regard to immune-related AEs, the commonly observed AEs were reactive cutaneous capillary endothelial proliferation (RCCEP), hypothyroidism, hyperthyroidism, fatigue, rash, itching, and so on, whereas the occurrence ratio of degree 3 or 4 was 17% (Table 3). Severe immune-related toxic side effects were interstitial pneumonia, infusion reaction, and so on. One patient had third degree interstitial pneumonia which was reversed through high-dose hormone treatment.
Table 3
| Adverse events | PD-1 plus nab-ptx, n (%) | |
|---|---|---|
| Any grade | Grade 3 or 4 | |
| Fatigue | 31 (58.49) | 4 (7.55) |
| Alopecia | 21 (39.62) | 1 (1.89) |
| Nausea | 20 (37.74) | 1 (1.89) |
| Neuropathy | 16 (30.19) | 2 (3.77) |
| Anemia | 15 (28.30) | 2 (3.77) |
| Vomiting | 15 (28.30) | 4 (7.55) |
| Neutropenia | 11 (20.75) | 2 (3.77) |
| Hyporexia | 10 (18.87) | 2 (3.77) |
| Diarrhea | 5 (9.43) | 1 (1.89) |
| Paronychia | 4 (7.55) | 0 (0.00) |
| Hypomagnesemia | 3 (5.66) | 0 (0.00) |
| Pruritus | 5 (9.43) | 0 (0.00) |
| Fluid retention | 4 (7.55) | 0 (0.00) |
| Immune-related adverse events | ||
| RCCEP | 35 (66.04) | 2 (3.77) |
| Hypothyroidism | 21 (39.62) | 2 (3.77) |
| Hyperthyroidism | 18 (33.96) | 2 (3.77) |
| Pneumonitis | 6 (11.32) | 2 (3.77) |
| Hepatitis | 5 (9.43) | 1 (1.89) |
| Xerostomia | 2 (1.89) | 0 (0.00) |
| Nephritis | 1 (1.89) | 0 (0.00) |
| Vitiligo | 1 (1.89) | 0 (0.00) |
| Sjogren’s syndrome | 1 (1.89) | 0 (0.00) |
Nab-ptx, nanoparticle albumin-bound paclitaxel; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; RCCEP, reactive cutaneous capillary endothelial proliferation.
Discussion
Numerous studies had shown that although IO could deliver persistent efficiency for some advanced patients, the efficiency still needed to be further improved, especially under the circumstances of single PD-1/PD-L1 inhibitors application (5,9,12). During the past decades, drug combination has become a main treatment mode to improve the efficiency of IO, such as dual IO (4), in combination with chemotherapy (1), combined with anti-angiogenesis therapy and so on (23). However, from the perspective of clinical application feasibility, the combination of PD-1/PD-L1 inhibitors and chemotherapy has exhibited more advantages, the potential of which has been confirmed in many solid tumors, such as lung cancer (1), head and neck squamous cell carcinoma (HNSCC) (24), triple negative breast cancer (TNBC) (19), esophageal cancer (25), and stomach cancer (26).
However, although almost all of these regimens are utilized in the first line due to the widespread recognition of IO in treatment-naïve patients with advanced cancer, limited study about the drug combination of PD-1/PD-L1 inhibitors and single CT had been reported in the second or above line treatment, even for NSCLC (27,28). Since it is widely recognized that single drug has limited efficiency in the second-line and above treatment of NSCLC, so it is of great significance to explore the combination of PD-1/PD-L1 inhibitors and nab-ptx in the second-line and above treatment of NSCLC. Although the efficiency and safety of several commonly used regimens for advanced NSCLC such as TP (docetaxel plus platinum) (3), GP (gemcitabine plus platinum) (29), and PC (pemetrexed plus carboplatin (1) have been reported in the era of IO combination, it is still necessary to seek a suitable partner for drug combination from the perspective of efficiency or safety. It is not hard to imagine that with different mechanisms, not all of the chemotherapeutic drugs could enhance the therapeutic efficiency of IO by activating the immune effect (30). Among these commonly used potential drugs in clinic, the potential of paclitaxel had been extensively reported, indicating that paclitaxel or analogs may be an ideal partner for IO combination (19,31).
Actually, several studies have exhibited that the drug combination of docetaxel and PD-1/PD-L1 inhibitors such as pembrolizumab or sintilimab could exhibit promising efficiency for advanced NSCLC in the second or above line treatment (13,32). However, either from cytotoxicity or convenience of clinical application, this regimen still needed to be further optimized. In this setting, we evaluated the combination possibility of nab-ptx and camrelizumab in the second or above line treatment of advanced NSCLC. Consistent with our expectation, the drug combination of nab-ptx and camrelizumab was shown to lead to about 36% ORR, with mean PFS and OS of 5 months and 10 months, respectively, all of which appeared to be non-inferior to those reported in previous studies (13,33). Besides promising efficiency, the toxicity of this regimen seemed to be lower, except the relatively higher incidence of neuropathy (34,35), most degree of which was 1 or 2 and could be tolerated. Further analysis indicated that compared to these patients with lower PD-L1 expression, patients with higher PD-L1 expression obtained better efficiency, indicating that expression level of PD-L1 can be regarded as a biomarker to predict the efficiency of this regimen (1,8). Importantly, we also found that compared to patients without a Treg ratio decrease, the ORR of patients with a Treg ratio decrease after treatment was obviously higher, indicating that the decrease of Treg ratio can be utilized to predict efficiency. Since the changes of immune cells imposed by regimen can produce a direct effect (36,37), from the perspective of mechanism, such a discovery would be of great significance in elaborating the mechanism, indicating that the mechanism of nab-ptx likely lies in depleting the Treg ratio to further release the immune function to synergize with PD-1/PD-L1 inhibitors (11). In order to further exclude the possible influence of confounding factors on efficiency, such as the gender (38), the severity of tumor burden, the proportion of liver and brain metastases, and the expression level of lactate dehydrogenase (LDH), no significant changes are observed among these subgroups. Therefore, lymphocyte subset changes are expected to serve as a potential predictor of efficacy of this regimen. It is not hard to image that according to the preliminary conclusions of our study and the published research data, advanced NSCLC patients with the following characteristics are expected to benefit more from this regimen, such as better physical fitness score (PS score 0–1 points), no liver and brain metastases, patients with a significantly lower proportion of Treg after treatment. However, in regard to the exact target or key signal pathway involved in regulating Treg production (39), much work needs to be conducted in the future; fortunately, such work is currently in progress.
Conclusions
To our knowledge, this is the first study to report the efficiency and safety of nab-ptx and camrelizumab in the second or above treatment line of advanced NSCLC. We further found that the synergistic mechanism of this regimen may lie in depleting Treg ratio to activate an immune response. However, due to the limitations of sample size, retrospective and single-center design of this study, many problems need to be resolved, such as the exact molecular mechanism (20), further optimization of the regimen, screening for the priority population, elucidating resistance mechanism (40), and so on. With the rapid development and accumulation of clinical experience of IO application, these puzzles will be gradually resolved and more and more patients will be able to benefit from this regimen.
Acknowledgments
Funding: The study was supported by Natural Science Foundation of Hubei Province (No. 2019CFC929).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-387/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-387/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-387/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-387/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Ethics Committee of Hubei Cancer Hospital Affiliated to Tongji Medical College (Wuhan, China) (No. HBCHEn2023125), and patients or their families signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J Thorac Oncol 2022;17:289-308. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non-Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:586-97. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol 2019;30:219-35. [Crossref] [PubMed]
- Galluzzi L, Humeau J, Buqué A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020;17:725-41. [Crossref] [PubMed]
- Borghaei H, Gettinger S, Vokes EE, et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021;39:723-33. [Crossref] [PubMed]
- Zhang Y, Song L, Zeng L, et al. Sintilimab plus docetaxel as second-line therapy of advanced non-small cell lung cancer without targetable mutations: a phase II efficacy and biomarker study. BMC Cancer 2022;22:952. [Crossref] [PubMed]
- Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release 2013;170:365-72. [Crossref] [PubMed]
- Arbour KC, Mezquita L, Long N, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2872-8. [Crossref] [PubMed]
- Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol 2020;15:1351-60. [Crossref] [PubMed]
- Li JJ, Wang JH, Dingv Y, et al. Efficacy and safety of anti-PD-1 inhibitor combined with nab-paclitaxel in Chinese patients with refractory melanoma. J Cancer Res Clin Oncol 2022;148:1159-69. [Crossref] [PubMed]
- Liu Q, Zhao G, Zhang X, et al. Nab-paclitaxel plus S-1 with or without PD-1 inhibitor in pancreatic ductal adenocarcinoma with only hepatic metastases: a retrospective cohort study. Langenbecks Arch Surg 2022;407:633-43. [Crossref] [PubMed]
- Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:44-59. [Crossref] [PubMed]
- Zhang J, Tang Z, Guo X, et al. Synergistic effects of nab-PTX and anti-PD-1 antibody combination against lung cancer by regulating the Pi3K/AKT pathway through the Serpinc1 gene. Front Oncol 2022;12:933646. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. [Crossref] [PubMed]
- Ren S, Chen J, Xu X, et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J Thorac Oncol 2022;17:544-57. [Crossref] [PubMed]
- Socinski MA, Nishio M, Jotte RM, et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol 2021;16:1909-24. [Crossref] [PubMed]
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915-28. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Chen B, Wang J, Pu X, et al. The efficacy and safety of immune checkpoint inhibitors combined with chemotherapy or anti-angiogenic therapy as a second-line or later treatment option for advanced non-small cell lung cancer: a retrospective comparative cohort study. Transl Lung Cancer Res 2022;11:2111-24. [Crossref] [PubMed]
- Gao G, Zhao J, Ren S, et al. Efficacy and safety of camrelizumab plus apatinib as second-line treatment for advanced squamous non-small cell lung cancer. Ann Transl Med 2022;10:441. [Crossref] [PubMed]
- Zhou C, Wu L, Fan Y, et al. Sintilimab Plus Platinum and Gemcitabine as First-Line Treatment for Advanced or Metastatic Squamous NSCLC: Results From a Randomized, Double-Blind, Phase 3 Trial (ORIENT-12). J Thorac Oncol 2021;16:1501-11. [Crossref] [PubMed]
- Glorieux C, Xia X, You X, et al. Cisplatin and gemcitabine exert opposite effects on immunotherapy with PD-1 antibody in K-ras-driven cancer. J Adv Res 2022;40:109-24. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Arrieta O, Barrón F, Ramírez-Tirado LA, et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:856-64. [Crossref] [PubMed]
- Zhang F, Huang D, Zhao L, et al. Efficacy and safety of PD-1/PD-L1 inhibitors plus nab-paclitaxel for patients with non-small cell lung cancer who have progressed after platinum-based chemotherapy. Ther Adv Med Oncol 2020;12:1758835920936882. [Crossref] [PubMed]
- Yoneshima Y, Morita S, Ando M, et al. Phase 3 Trial Comparing Nanoparticle Albumin-Bound Paclitaxel With Docetaxel for Previously Treated Advanced NSCLC. J Thorac Oncol 2021;16:1523-32. [Crossref] [PubMed]
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055-62. [Crossref] [PubMed]
- Diederichsen AC. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother 2003;52:423-8. [Crossref] [PubMed]
- Katz SC, Pillarisetty V, Bamboat ZM, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol 2009;16:2524-30. [Crossref] [PubMed]
- Choi MG, Choi CM, Lee DH, et al. Impact of gender on response to immune checkpoint inhibitors in patients with non-small cell lung cancer undergoing second- or later-line treatment. Transl Lung Cancer Res 2022;11:1866-76. [Crossref] [PubMed]
- Kumagai S, Koyama S, Itahashi K, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 2022;40:201-218.e9. [Crossref] [PubMed]
- Passaro A, Brahmer J, Antonia S, et al. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J Clin Oncol 2022;40:598-610. [Crossref] [PubMed]
(English Language Editor: J. Jones)


