
Association between length of stay and postoperative survival in patients with lung cancer: a propensity score matching analysis based on National Cancer Database
Highlight box
Key findings
• Patients with PLOS exhibited worse short-term and long-term survival.
What is known and what is new?
• In the past few years, LOS following thoracic surgery has been used as a quality metric for assessing the efficiency of a healthcare unit.
• LOS was first proposed as an alternative quantitative indicator of postoperative complications in NCDB lung cancer patients.
What is the implication, and what should change now?
• Avoiding PLOS could be considered to benefit patient survival after lung cancer surgery.
Introduction
Presently, lung cancer remains the leading cause of cancer-related death worldwide (1). Surgical treatment is currently recommended as the preferred therapeutic approach for resectable lung cancer (2). In recent years, the concept of enhanced recovery after surgery (ERAS) was proposed in surgical management of lung cancer, namely, to optimize perioperative treatment, reduce perioperative stress response and postoperative complications, shorten hospitalization time, and promote recovery (3,4). In the past few years, the length of stay (LOS) following thoracic surgery has been used as a quality metric for assessing the efficiency of a healthcare unit (5). However, there are few studies on the effect of postoperative LOS on survival of patients with lung cancer.
The National Cancer Database (NCDB) is a clinical oncology database co-sponsored by American College of Surgeons Commission on Cancer and the American Cancer Society, which is originated from the hospital registration data collected in more than 1,500 institutions recognized by the Cancer Committee, which can be applied to analyze and track patients with malignant tumors, as well as their treatments and outcomes (6). However, there is no records about complications in NCDB, therefore here we aimed to determine whether prolonged length of stay (PLOS) can serve as an independent prognostic factor for postoperative survival of patients with lung cancer. In the present study, LOS was first proposed as an alternative quantitative indicator of postoperative complications in NCDB lung cancer patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-407/rc).
Methods
Patient enrollment
Patients with lung cancer who underwent surgical treatment in the NCDB database from 2004 to 2015 were enrolled in this study, and their clinical data were retrospectively analyzed. The inclusion criteria included a history of surgery, and lung cancer in clinical stage I–III (6th and 7th editions). The exclusion criteria were as follows: absence of information regarding LOS, follow-up, and other clinical data; death within 30 days after surgery; follow-up period less than 3 months (Figure 1). After screening, the target population for our subsequent analysis was finally obtained. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
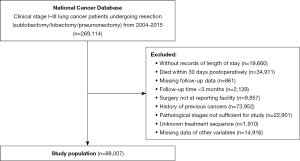
Study design
In this study, the LOS range of the included cases was 1–167 days. According to the quartile method, patients was divided into quartiles. Patients in the highest quartile were listed as the PLOS group, and the value was calculated to be more than 8 days. The remaining patients were classified as the Non-PLOS group. Deviation between the PLOS group and the Non-PLOS group was controlled by 1:1 propensity score matching (PSM) based on age, gender, race, facility type, income, insurance, Charlson/Deyo Score, year of diagnosis, laterality, clinical stage, histology type, tumor grade, surgery type, surgical margins, pathological stage, neoadjuvant therapy, and adjuvant therapy. Standardized mean difference (SMD) was calculated to evaluate the balance of baseline data between the 2 groups before and after PSM. A SMD <0.1 was considered the balanced distribution of baseline data. We selected 30-day rehospitalization rate and 90-day mortality in the PLOS group as the short-term survival indicators. The overall survival (OS) was considered the long-term survival index.
Statistical analysis
The software SPSS 25.0 (IBM Corp., Armonk, NY, USA) was used for all data analysis. Chi-square test and multivariable logistic regression analysis were used to evaluate the long-term and short-term prognostic survival before and after PSM. Kaplan-Meier analysis was used to calculate the survival rate and draw the survival curve. Log-rank test was used to compare the survival curve. Cox proportional-hazards model was used for multivariable analysis to adjust the long-term survival prognosis of patients. All statistical tests were 2-sided, and P<0.05 was considered statistically significant.
Results
Clinical characteristics
After screening, a total of 88,007 patients were enrolled in the study (Figure 1). According to the quartile of the LOS, patients hospitalized for more than 8 days were defined as the PLOS group (n=18,611, 21.1%), and the remaining patients as the Non-PLOS group (n=69,396, 78.9%). We compared the clinical characteristics between the PLOS and Non-PLOS groups (Table 1). Before PSM, there was an imbalance in baseline distribution between the PLOS group and the Non-PLOS group (SMD ≥0.1). After PSM, there were 18,585 cases in each of the PLOS group and Non-PLOS groups, with balanced variables (SMD <0.1).
Table 1
| Clinical characteristics | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Non-PLOS | PLOS | SMD | Non-PLOS | PLOS | SMD | ||
| N | 69,396 | 18,611 | 18,585 | 18,585 | |||
| Age, years, mean ± SD | 66.52±9.82 | 67.76±9.43 | 0.128 | 67.66±9.61 | 67.75±9.43 | 0.009 | |
| Gender, n (%) | 0.167 | 0.013 | |||||
| Male | 31,797 (45.8) | 10,075 (54.1) | 9,928 (53.4) | 10,051 (54.1) | |||
| Female | 37,599 (54.2) | 8,536 (45.9) | 8,657 (46.6) | 8,534 (45.9) | |||
| Race, n (%) | 0.051 | 0.019 | |||||
| White | 61,123 (88.1) | 16,542 (88.9) | 16,548 (89.0) | 16,517 (88.9) | |||
| Black | 5,907 (8.5) | 1,595 (8.6) | 1,521 (8.2) | 1,594 (8.6) | |||
| Other | 2,366 (3.4) | 474 (2.5) | 516 (2.8) | 474 (2.6) | |||
| Income, n (%) | 0.134 | 0.015 | |||||
| <$48,000 | 28,644 (41.3) | 8,923 (47.9) | 8,763 (47.2) | 8,898 (47.9) | |||
| ≥$48,000 | 40,752 (58.7) | 9,688 (52.1) | 9,822 (52.8) | 9,687 (52.1) | |||
| Insurance, n (%) | 0.012 | 0.001 | |||||
| No insured | 1,396 (2.0) | 406 (2.2) | 406 (2.2) | 406 (2.2) | |||
| Insured | 68,000 (98.0) | 18,205 (97.8) | 18,179 (97.8) | 18,179 (97.8) | |||
| Urban/rural location, n (%) | 0.033 | 0.001 | |||||
| Metro/urban counties | 67,881 (97.8) | 18,109 (97.3) | 18,081 (97.3) | 18,085 (97.3) | |||
| Rural counties | 1,515 (2.2) | 502 (2.7) | 504 (2.7) | 500 (2.7) | |||
| Facility type, n (%) | 0.142 | 0.009 | |||||
| Non-academic | 42,317 (61.0) | 12,607 (67.7) | 12,660 (68.1) | 12,582 (67.7) | |||
| Academic | 27,079 (39.0) | 6,004 (32.3) | 5,925 (31.9) | 6,003 (32.3) | |||
| Charlson Comorbidity Index score, n (%) | 0.217 | 0.055 | |||||
| 0 | 35,202 (50.7) | 7,508 (40.3) | 7,913 (42.6) | 7,508 (40.4) | |||
| 1 | 24,744 (35.7) | 7,661 (41.2) | 7,167 (38.6) | 7,656 (41.2) | |||
| ≥2 | 9,450 (13.6) | 3,442 (18.5) | 3,505 (18.9) | 3,421 (18.4) | |||
| Year of diagnosis, n (%) | 0.150 | 0.076 | |||||
| 2004–2009 | 21,759 (31.4) | 7,161 (38.5) | 7,828 (42.1) | 7,139 (38.4) | |||
| 2010–2015 | 47,637 (68.6) | 11,450 (61.5) | 10,757 (57.9) | 11,446 (61.6) | |||
| Laterality | 0.063 | ||||||
| Right | 40,272 (58.0) | 11,379 (61.1) | 11,285 (60.7) | 11,360 (61.1) | 0.008 | ||
| Left | 29,124 (42.0) | 7,232 (38.9) | 7,300 (39.3) | 7,225 (38.9) | |||
| Clinical stage | 0.070 | 0.049 | |||||
| I | 51,124 (73.7) | 13,157 (70.7) | 13,241 (71.2) | 13,147 (70.7) | |||
| II | 10,860 (15.6) | 3,353 (18.0) | 3,053 (16.4) | 3,344 (18.0) | |||
| III | 7,412 (10.7) | 2,101 (11.3) | 2,291 (12.3) | 2,094 (11.3) | |||
| Histology type, n (%) | 0.202 | 0.091 | |||||
| Adenocarcinoma | 41,110 (59.2) | 9,355 (50.3) | 9,690 (52.1) | 9,350 (50.3) | |||
| Squamous cell carcinoma | 18,601 (26.8) | 6,653 (35.7) | 5,909 (31.8) | 6,642 (35.7) | |||
| Other | 9,685 (14.0) | 2,603 (14.0) | 2,986 (16.1) | 2,593 (14.0) | |||
| Grade, n (%) | 0.180 | 0.055 | |||||
| Well differentiated | 11,803 (17.0) | 2,075 (11.1) | 2,278 (12.3) | 2,075 (11.2) | |||
| Moderately differentiated | 31,252 (45.0) | 8,434 (45.3) | 8,009 (43.1) | 8,431 (45.4) | |||
| Poorly differentiated | 25,156 (36.2) | 7,705 (41.4) | 7,831 (42.1) | 7,687 (41.4) | |||
| Undifferentiated | 1,185 (1.7) | 397 (2.1) | 467 (2.5) | 392 (2.1) | |||
| Surgery type, n (%) | 0.034 | 0.036 | |||||
| Sublobe/lobectomy | 65,913 (95.0) | 17,803 (95.7) | 17,636 (94.9) | 17,777 (95.7) | |||
| Pneumonectomy | 3,483 (5.0) | 808 (4.3) | 949 (5.1) | 808 (4.3) | |||
| Surgical margin, n (%) | 0.058 | 0.008 | |||||
| No residual tumor | 66,014 (95.1) | 17,457 (93.8) | 17,402 (93.6) | 17,437 (93.8) | |||
| Residual tumor present | 3,382 (4.9) | 1,154 (6.2) | 1,183 (6.4) | 1,148 (6.2) | |||
| Pathological stage, n (%) | 0.076 | 0.066 | |||||
| I | 45,407 (65.4) | 11,550 (62.1) | 11,977 (64.4) | 11,542 (62.1) | |||
| II | 14,187 (20.4) | 4,346 (23.4) | 3,838 (20.7) | 4,332 (23.3) | |||
| III | 9,334 (13.5) | 2,586 (13.9) | 2,615 (14.1) | 2,582 (13.9) | |||
| IV | 468 (0.7) | 129 (0.7) | 155 (0.8) | 129 (0.7) | |||
| Neoadjuvant therapy, n (%) | 0.009 | 0.016 | |||||
| No | 66,546 (95.9) | 17,812 (95.7) | 17,727 (95.4) | 17,787 (95.7) | |||
| Yes | 2,850 (4.1) | 799 (4.3) | 858 (4.6) | 798 (4.3) | |||
| Adjuvant therapy, n (%) | 0.079 | 0.025 | |||||
| No | 52,142 (75.1) | 14,605 (78.5) | 14,390 (77.4) | 14,579 (78.4) | |||
| Yes | 17,254 (24.9) | 4,006 (21.5) | 4,195 (22.6) | 4,006 (21.6) | |||
PSM, propensity score matching; PLOS, prolonged length of stay; SMD, standardized mean difference, values <0.1 indicates acceptable balance; SD, standard deviation.
The effect of the PLOS on the short-term survival
Focusing on the short-term survival, we found that 30-day re-hospitalization rate and 90-day mortality in the PLOS group were significantly higher than those in the Non-PLOS group before and after PSM (P<0.001), indicating a less favorable short-term prognosis in the PLOS group (Table 2). Further, multivariable logistic regression analysis also revealed that PLOS was an independent negative predictor of short-term postoperative survival of lung cancer patients (P<0.001).
Table 2
| Variates | Unplanned readmission within 30 days | 90-day mortality |
|---|---|---|
| Before matching, n (%) | ||
| Non-PLOS | 2,703 (3.9) | 492 (0.7) |
| PLOS | 1,147 (6.2) | 544 (2.9) |
| P value | <0.001 | <0.001 |
| After matching, n (%) | ||
| Non-PLOS | 781 (4.2) | 195 (1.0) |
| PLOS | 1,147 (6.2) | 541 (2.9) |
| P value | <0.001 | <0.001 |
| Multivariate (LOS): Non-PLOS vs. PLOS | ||
| Adjusted OR | 1.365 | 2.087 |
| 95% CI | 1.247–1.495 | 1.799–2.421 |
| P value | <0.001 | <0.001 |
PLOS, prolonged length of stay; LOS, length of stay; OR, odds ratio; CI, confidence interval.
The effect of PLOS on the long-term survival
For further study, we compared the Kaplan-Meier OS curve in the PLOS group and the Non-PLOS group before and after PSM (Figure 2). Before PSM, the median survival of the PLOS group and the Non-PLOS group was 82.1 months [95% confidence interval (CI): 80.9 to 83.3 months] and 53.2 months (95% CI: 51.6 to 54.3 months), respectively (P<0.0001). After PSM, the median survival of the PLOS group and the Non-PLOS group was 63.5 months (95% CI: 62.0 to 65.0 months) and 53.2 months (95% CI: 51.8 to 54.5 months), respectively (P<0.0001). Both results indicated a less favorable long-term prognosis in the PLOS group compared with the Non-PLOS group. Multivariable Cox regression analysis (Table 3) revealed that PLOS was an independent negative predictor for long-term survival of patients with lung cancer [hazard ratio (HR) =1.263, 95% CI: 1.227 to 1.301, P<0.001]. In addition, age (<70/≥70 years), gender, race, income, year of diagnosis, surgery type, pathological stage, and neoadjuvant therapy also were independent prognostic factors of postoperative survival for patients with lung cancer (P<0.001, respectively).
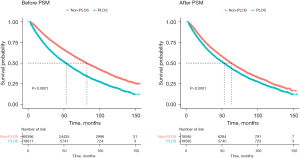
Table 3
| Covariates | Overall survival | ||
|---|---|---|---|
| Adjusted HR | 95% CI | P value | |
| Length of stay | <0.001 | ||
| Non-PLOS vs. PLOS | 1.263 | 1.227–1.301 | |
| Age | <0.001 | ||
| <70 vs. ≥70 years | 1.486 | 1.442–1.532 | |
| Sex | <0.001 | ||
| Male vs. female | 0.782 | 0.759–0.806 | |
| Race | <0.001 | ||
| White | – | – | |
| Black | 0.948 | 0.897–1.002 | |
| Other | 0.719 | 0.650–0.796 | |
| Income | <0.001 | ||
| <$48,000 vs. ≥$48,000 | 0.920 | 0.893–0.948 | |
| Urban/rural location | 0.055 | ||
| Urban vs. rural | 1.089 | 0.998–1.189 | |
| Year of diagnosis | <0.001 | ||
| 2004–2009 vs. 2010–2015 | 0.685 | 0.664–0.706 | |
| Laterality | 0.681 | ||
| Right vs. left | 1.006 | 0.977–1.037 | |
| Surgery type | <0.001 | ||
| Sub/lobectomy vs. pneumonectomy | 1.241 | 1.166–1.320 | |
| Pathological stage | <0.001 | ||
| I | – | – | |
| II | 1.707 | 1.642–1.774 | |
| III | 2.411 | 2.306–2.519 | |
| IV | 2.998 | 2.620–3.343 | |
| Neoadjuvant therapy | <0.001 | ||
| Yes vs. no | 1.283 | 1.203–1.369 | |
| Adjuvant therapy | 0.169 | ||
| Yes vs. no | 1.028 | 0.998–1.069 | |
PLOS, prolonged length of stay; HR, hazard ratio; CI, confidence interval.
Discussion
The current study mainly focused on the prognosis of lung cancer and its clinicopathological features including tumor size, lymph node metastasis, and distant metastasis, as well as patient’s self-factors, such as nutritional status, autoimmune status, and psychological status (7-10). Based on the NCDB database, PSM was performed according to age, gender, race, facility type, income, insurance, Charlson Comorbidity Index score, year of diagnosis, laterality, clinical stage, histology type, tumor grade, surgery type, surgical margins, pathological stage, neoadjuvant therapy, and adjuvant therapy in this study. After PSM, the distribution of variables in PLOS group and Non-PLOS group showed a balance (SMD <0.1).
PLOS can be attributed to various factors. A large cohort study of non-cardiothoracic surgery showed that age and anesthesia duration were risk factors for prolonged postoperative hospital stay in patients undergoing thoracoscopic single lobectomy (11). In a retrospective study of 729 TV-assisted or robot-assisted thoracoscopic lobe or segment resection for lung cancer, single segment resection, two lobes or combined lobe and segment resection, and right lobectomy were independent risk factors for postoperative pulmonary complications and were associated with increased postoperative hospitalization and total hospital cost (12). In addition, prior preoperative patient medical history may also affect the length of hospital stay, which is expressed with Charlson Comorbidity Index score in the NCDB database. Here we considered above all factors which may affect length of stay during PSM. After excluding the above factors, PLOS tends to be caused by postoperative complications. Due to the lack of relevant data of postoperative complications in the NCDB database, PLOS was therefore considered an alternative factor of complications after lung cancer surgery. To our knowledge, this study is the first to propose length of hospital stay as a quantitative indicator of postoperative complications. Our results showed less favorable long- and short-term survival in the PLOS group compared with the Non-PLOS group, which was consistent with the effect of postoperative complications of lung cancer in the previously reported studies (13,14).
As a quantitative indicator of postoperative complications, the poor prognosis of lung cancer caused by PLOS may be attributed to other multiple reasons: Infection is the most common postoperative complication of lung cancer, with the highest morbidity of 25% (15-17). The levels of inflammatory cytokines and C-reactive proteins will be increased by surgery in lung cancer patients. Colacchio et al. (18) showed that surgical stress response will increase the tumor load by inhibiting the activity of natural killer (NK) cells and downregulate the effector lymphocyte and their corresponding Th1 regulatory pathways via inhibiting endogenous mediators. This results in a promotive state of tumor growth and thereby the proliferation of residual tumor cells, which may reduce the long-term survival. Therefore, perioperative measures should be implemented systematically and normatively, including routine smoking cessation before operation, aerosol inhalation for airway preparation, expectoration training, administration of antibiotics before surgery, and getting out of bed early for expectoration after surgery (19).
Another dangerous postoperative complication of lung cancer is venous thromboembolism (VTE), including deep venous thromboembolism (DVT) and pulmonary embolism (PE) with a reported incidence rate of 15.1–16.4% (20,21). Studies have shown that the incidence of VTE within 7 days after lung cancer surgery is about 7.4% and the incidence of VTE within 30 days after surgery is about 23.1% (22,23). The incidence of VTE will be higher in advanced non-small cell lung cancer (NSCLC) and remains a higher tendency within half a year after the diagnosis of lung cancer (24). Among VTE, PE has a high mortality rate. Li et al. (25) revealed that hospital LOS was significantly prolonged in patients with PE compared with patients without PE. Based on risk evaluation of VET, active preventive measures such as moving limbs early, wearing elastic socks, and early administration of low molecular weight heparin (LMWH) should be performed after lung cancer surgery.
In addition, previous study asserted that the negative psychological state of patients should also be included in the postoperative complications of lung cancer, such as cancer-related depression and anxiety, which can reduce the treatment compliance and prolong the hospital stay (26). Therefore, we also need to pay attention to the psychological management of lung cancer patients and carry out psychological intervention when necessary to minimize the psychological burden after surgery.
Limitations
This study may have some limitations. Firstly, tumor-specific survival indicators such as progression-free survival (PFS) and disease-free survival (DFS) are not available in the NCDB database, so we could only choose OS as a long-term prognosis indicator in our study. In addition, although we tried to exclude the influence of factors on LOS through PSM, there may have been other factors that contributed to prolonged hospitalization that were not included. However, based on the results of the study, lung cancer patients with prolonged postoperative hospital stay do have poor postoperative long- and short-term outcomes. More data and mechanisms are needed to confirm our results in the future.
Conclusions
In summary, our study showed that LOS in NCDB can predict survival after lung cancer surgery, and we found that PLOS was an independent prognostic factor for poor survival after lung cancer surgery. Appropriate measures to prevent complications and thus reduce the PLOS in lung cancer patients are likely to be beneficial for survival. The results of our study are exactly consistent with the concept of ERAS. Our study undoubtedly provides an important hint for perioperative management of lung cancer patients.
Acknowledgments
Funding: This work was supported by Science and Technology Commission of Shanghai Municipality (No. 21Y31900104 to J Zhang); Industry-University-Research Projects of Shanghai University Teachers (No. RC.202111.001 to J Zhang); Scientific Research Project of Traditional Chinese Medicine of Shanghai Municipal Health Commission (No. 2020LZ004 to J Zhang); Collaborative Innovation Center for Clinical and Translational Science by Chinese Ministry of Education & Shanghai (No. CCTS-2022209 to J Zhang); Technology Transfer Promotion Program of Shanghai Jiao Tong University (No. ZT202113 to J Zhang).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-407/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-407/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-407/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw 2021;19:254-66. [Crossref] [PubMed]
- Petersen RH, Huang L, Kehlet H. Guidelines for enhanced recovery after lung surgery: need for re-analysis. Eur J Cardiothorac Surg 2021;59:291-2. [Crossref] [PubMed]
- Li R, Wang K, Qu C, et al. The effect of the enhanced recovery after surgery program on lung cancer surgery: a systematic review and meta-analysis. J Thorac Dis 2021;13:3566-86. [Crossref] [PubMed]
- McDevitt J, Kelly M, Comber H, et al. A population-based study of hospital length of stay and emergency readmission following surgery for non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:e253-9. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Cheng M, Zhang S, Ning C, et al. Omega-3 Fatty Acids Supplementation Improve Nutritional Status and Inflammatory Response in Patients With Lung Cancer: A Randomized Clinical Trial. Front Nutr 2021;8:686752. [Crossref] [PubMed]
- Bagan P, Berna P, De Dominicis F, et al. Nutritional status and postoperative outcome after pneumonectomy for lung cancer. Ann Thorac Surg 2013;95:392-6. [Crossref] [PubMed]
- Linares-Moya M, Rodriguez-Torres J, Heredia-Ciuro A, et al. Psychological distress prior to surgery is related to symptom burden and health status in lung cancer survivors. Support Care Cancer 2022;30:1579-86. [Crossref] [PubMed]
- Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338-50. [Crossref] [PubMed]
- Mazo V, Sabate S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014;121:219-31. [Crossref] [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. The long-term impact of surgical complications after resection of stage I nonsmall cell lung cancer: a population-based survival analysis. Ann Surg 2011;254:368-74. [Crossref] [PubMed]
- Nojiri T, Hamasaki T, Inoue M, et al. Long-Term Impact of Postoperative Complications on Cancer Recurrence Following Lung Cancer Surgery. Ann Surg Oncol 2017;24:1135-42. [Crossref] [PubMed]
- Radu DM, Jaureguy F, Seguin A, et al. Postoperative pneumonia after major pulmonary resections: an unsolved problem in thoracic surgery. Ann Thorac Surg 2007;84:1669-73. [Crossref] [PubMed]
- Yao L, Luo J, Liu L, et al. Risk factors for postoperative pneumonia and prognosis in lung cancer patients after surgery: A retrospective study. Medicine (Baltimore) 2021;100:e25295. [Crossref] [PubMed]
- Lee JY, Jin SM, Lee CH, et al. Risk factors of postoperative pneumonia after lung cancer surgery. J Korean Med Sci 2011;26:979-84. [Crossref] [PubMed]
- Colacchio TA, Yeager MP, Hildebrandt LW. Perioperative immunomodulation in cancer surgery. Am J Surg 1994;167:174-9. [Crossref] [PubMed]
- Heiden BT, Eaton DB Jr, Chang SH, et al. Assessment of Duration of Smoking Cessation Prior to Surgical Treatment of Non-small Cell Lung Cancer. Ann Surg 2023;277:e933-40. [Crossref] [PubMed]
- Walker AJ, Baldwin DR, Card TR, et al. Risk of venous thromboembolism in people with lung cancer: a cohort study using linked UK healthcare data. Br J Cancer 2017;116:e1. [Crossref] [PubMed]
- Akhtar-Danesh GG, Akhtar-Danesh N, Shargall Y. Venous Thromboembolism in Surgical Lung Cancer Patients: A Provincial Population-Based Study. Ann Thorac Surg 2022;114:890-7. [Crossref] [PubMed]
- Mason DP, Quader MA, Blackstone EH, et al. Thromboembolism after pneumonectomy for malignancy: an independent marker of poor outcome. J Thorac Cardiovasc Surg 2006;131:711-8. [Crossref] [PubMed]
- Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg 2011;254:131-7. [Crossref] [PubMed]
- Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost 2008;6:601-8. [Crossref] [PubMed]
- Li YP, Shen L, Huang W, et al. Prevalence and Risk Factors of Acute Pulmonary Embolism in Patients with Lung Cancer Surgery. Semin Thromb Hemost 2018;44:334-40. [Crossref] [PubMed]
- Fitch MI, Steele R. Supportive care needs of individuals with lung cancer. Can Oncol Nurs J 2010;20:15-22. [Crossref] [PubMed]
(English Language Editor: J. Jones)


