
A new risk score model based on lactate dehydrogenase for predicting prognosis in esophageal squamous cell carcinoma treated with chemoradiotherapy
Highlight box
Key findings
• Pretreatment serum LDH levels may be a reliable factor in predicting the therapeutic effect of chemoradiotherapy in ESCC.
What is known and what is new?
• TNM staging system has been correlated with survival in predicting prognosis for ESCC, and the prognostic value of LDH has been confirmed in many malignant tumors.
• LDH may be a powerful independent predictor for OS in patients with ESCC treated with chemoradiotherapy.
What is the implication, and what should change now?
• An accurate and dependable prediction model that incorporated LDH level was established for evaluating the prognosis of patients with ESCC. Studies with greater homogeneity are needed to confirm the results.
Introduction
Esophageal cancer (EC) is a common malignancy with an increasing incidence, ranking sixth as a leading cause of cancer-related death worldwide (1,2). More than 85% of all EC cases are diagnosed with esophageal squamous cell carcinoma (ESCC) (3). Despite advancement in therapeutic strategies having improved the prognosis of patients with ESCC, the long-term survival of these patients remains dismal (4). It is widely acknowledged that TNM staging system is correlated with survival in predicting prognosis for ESCC (5). However, clinical outcomes can vary greatly among patients, even at those at the same stage of disease. Therefore, identifying potential indicators and establishing an accurate and dependable prediction model for evaluating the prognosis of patients with ESCC before treatment is critically important to clinical practice.
Lactate dehydrogenase (LDH) is a key enzyme involved in anaerobic glycolysis, mainly catalyzing the conversion between pyruvate and lactate, and the expression of serum LDH levels in tumor tissue is higher than that in normal tissue due to the fact that tumor cells are mainly powered by anaerobic fermentation (6) Patients may experience elevated serum LDH levels due to active cell proliferation, enhanced metabolism, and increased normal tissue infiltration prior to progression, leading to low survival rates in patients with tumor metastasis in various cancer types (7). A growing number of studies are providing insight into the relationship between LDH and overall survival (OS) in different malignancies, such melanoma (8), lymphoma (9), nasopharyngeal carcinoma (10), lung cancer (11,12), and pancreatic carcinoma (13). Several investigators also scrutinized the prognostic value of LDH in ESCC, such as ESCC patients receiving surgical treatment (14-16) or immunotherapy (17,18), or chemoradiotherapy but reached inconsistent conclusions (19,20), serum LDH thereby remains a controversial prognostic biomarker concerning its value in ESCC prognosis and needs to be further investigated. Furthermore, studies regarding the influence of LDH on the prognosis have rarely examined those patients with ESCC who have undergone chemoradiotherapy. A prognostic classification model for predicting the outcome of ESCC patients based on genetic information obtained from ESCC samples has also been reported by Lian et al. (21). Considering the significant differences in prognosis among ESCC patients, it is crucial to develop a reliable and convenient prognostic tool to guide prognosis. Besides, serum LDH levels, which is easily available in routine clinical practice. Thus, we conducted a sing-center retrospective analysis aimed at investigating the prognostic value of LDH level in patients with ESCC who received chemoradiotherapy. We further performed univariate and multivariate analyses to identify the prognostic factors in patients with ESCC. Based on the results of the multivariate analysis, we designed a risk score model for determining the prognosis of patients with ESCC who have undergone chemoradiotherapy in order to guide personalized management. And then a nomogram is established to stratify patients at different risk of clinical outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-388/rc).
Methods
Patient characteristics and study design
The data of 614 patients were retrieved from the database of Shandong Cancer Hospital from January 1, 2012, to December 31, 2016. All patients had either rejected surgery or were unable to undergo surgery. The criteria for study inclusion were the following: (I) pathologically or cytologically proved ESCC; (II) undergone chemoradiotherapy before recurrence or progression; (III) no acute or chronic inflammatory diseases or infections, such as acute myocardial infarction, acute hepatitis B virus infection, acute cholecystitis, and bone diseases; and (IV) no evidence of prior malignant carcinoma with the previous 5 years. The following clinical data were collected from the medical records: gender, age, performance status (PS), clinical T stage (cT), clinical N stage (cN), clinical M stage (cM), tumor length, baseline LDH levels, date of diagnosis, and recurrence date. All the pathological diagnoses were confirmed by pathologists in our department. The TNM stage in this study was determined according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging system (seventh edition) (22). The PS was defined according to the criteria of Eastern Clinical Oncology Group (ECOG) (23). Our study also included several previously identified prognostic factors to adjust the prognostic effect of LDH, such as cytokeratin 19 fragment antigen 21-1 (Cyfra21-1) and carcinoembryonic antigen (CEA). The LDH, Cyfra21-1 and CEA levels were tested by the reagents used to in our hospital. Those patients with incomplete medical records were further excluded. All registered patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shandong Cancer Hospital (No. SDTHEC2023004014).
Treatment protocol and follow-up
The therapeutic strategies were based on the National Comprehensive Cancer Network (NCCN) clinical practice guidelines. All participants underwent 3-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). They underwent radiotherapy for 4–7 weeks, receiving a total dose of 45–70 Gy. Each patient with ESCC received concurrent chemoradiotherapy (CCRT) or sequential chemoradiotherapy (SCRT) based on the individualized treatment strategy. The chemotherapy regimens mainly included cisplatin plus 5-fluorouracil or cisplatin plus paclitaxel. The patients were followed up every 3–6 months, and the overall follow-up time was at least 2 years. Overall survival (OS) was defined as the date of pathological diagnosis to the date of death or last follow-up. Progression-free survival (PFS) interval was defined as the date of pathological diagnosis to the date of disease progression or the date of death or last contact.
Statistical analyses
Statistical analyses were performed using SPSS version 24.0 (IBM Corp.). The continuous variables were stratified into 2 groups by the optimal cutoff points using the X-tile program (24). Chi-squared tests were used to compare categorical data between 2 groups. The OS and PFS were analyzed with the Kaplan-Meier method using GraphPad Prism 7.0. A 1:3 optimal propensity score-matched method was used to control confounding (25). The propensity scores were estimated using a multivariable logistic regression model (25). The covariates used to calculate propensity scores included 15 variables, which were listed in Table 1. Univariable and multivariable Cox proportional hazards regression methods were used to the identify independent risk factors of ESCC. A nomogram was developed based on the results of multivariate analysis and by using the rms package in R software version 4.0.5 (http://www.r-project.cog/). The performance of the nomogram was assessed by concordance index (C-index) and calibration curve. Statistical significance was indicated by a P value ≤0.05.
Table 1
| Variable | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Low-LDH (n=68) | High-LDH (n=546) | P value | Low-LDH (n=68) | High-LDH (n=188) | P value | ||
| Gender | 0.208 | 0.653 | |||||
| Male | 57 | 421 | 57 | 153 | |||
| Female | 11 | 125 | 11 | 35 | |||
| Age (years) | 0.176 | 0.859 | |||||
| ≤69 | 58 | 427 | 58 | 162 | |||
| >69 | 10 | 119 | 10 | 26 | |||
| ECOG PS | 0.288 | 0.707 | |||||
| 0 | 34 | 236 | 34 | 99 | |||
| 1–2 | 34 | 310 | 34 | 89 | |||
| cT stage | 0.816 | 0.856 | |||||
| T1-2 | 6 | 53 | 6 | 18 | |||
| T3-4 | 62 | 493 | 62 | 170 | |||
| cN stage | 0.568 | 0.602 | |||||
| N0 | 10 | 67 | 10 | 23 | |||
| N+ | 58 | 479 | 58 | 165 | |||
| cM stage | 0.005 | 0.937 | |||||
| M0 | 62 | 417 | 62 | 172 | |||
| M1 | 6 | 129 | 6 | 16 | |||
| Differentiation | 0.077 | 0.659 | |||||
| High | 61 | 442 | 61 | 172 | |||
| Moderate or poor | 7 | 104 | 7 | 16 | |||
| Length (cm) | 0.026 | 0.655 | |||||
| ≤6.5 | 48 | 447 | 48 | 138 | |||
| >6.5 | 20 | 99 | 20 | 50 | |||
| Tumor location | 0.308 | 0.523 | |||||
| Cervical | 6 | 59 | 6 | 23 | |||
| Upper | 24 | 156 | 24 | 59 | |||
| Medium | 22 | 232 | 22 | 73 | |||
| Lower | 16 | 99 | 16 | 33 | |||
| Radiotherapy technology | 0.296 | 0.635 | |||||
| 3DCRT | 21 | 204 | 21 | 64 | |||
| IMRT | 47 | 342 | 47 | 124 | |||
| Treatment options | |||||||
| CCRT | 24 | 255 | 24 | 77 | |||
| SCRT | 44 | 291 | 0.075 | 44 | 111 | 0.413 | |
| Total dose (Gy) | 0.167 | 0.669 | |||||
| ≤58.8 | 12 | 138 | 12 | 29 | |||
| >58.8 | 56 | 408 | 56 | 159 | |||
| CEA (ng/mL) | 0.651 | 0.552 | |||||
| ≤2.4 | 25 | 190 | 25 | 62 | |||
| >2.4 | 21 | 199 | 21 | 72 | |||
| NA | 22 | 157 | 22 | 54 | |||
| Cyfra21-1 (ng/mL) | 0.150 | 0.665 | |||||
| ≤6.4 | 35 | 311 | 35 | 103 | |||
| >6.4 | 2 | 41 | 2 | 9 | |||
| NA | 31 | 194 | 31 | 76 | |||
ESCC, esophageal squamous cell carcinoma; LDH, lactate dehydrogenase; ECOG, Eastern Clinical Oncology Group; PS, performance status; Cyfra21-1, cytokeratin 19 fragment antigen 21-1; CEA, carcinoembryonic antigen; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; CCRT, concurrent chemoradiotherapy; SCRT, sequential chemoradiotherapy; cT stage, clinical T stage; cN stage, clinical N stage; cM stage, clinical M stage; NA, not applicable.
Results
Patient characteristics
The study examined the data from 614 patients with ESCC. There were 478 (77.9%) males and 136 (22.1%) females, with a median age of 63 years (range 35–85). According to the X-tile program, the optimal cutoff points for age, CEA, Cyfra21-1, tumor length, total dose, and LDH were respectively 69 years, 2.4 ng/mL, 6.4 ng/mL, 6.5 cm, 58.8 Gy and 134 U/L. The X-tile analyses for LDH are shown in Figure 1. The patients then were stratified into low and high groups based on LDH for further analyses (LDH ≤134 and LDH >134). A total of 546 (88.9%) patients were placed in the high-LDH group, whereas 68 (11.1%) patients were placed in the low-LDH group.
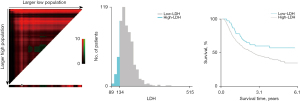
We found that patients with a high-LDH level were associated with more advanced cM stage (P=0.005) and larger tumor length (P=0.026). No statistically significant association was observed between LDH level and other clinical features. To balance differences in the clinical features among groups, all patients were randomly selected and matched in a 1:3 ratio to another group with similar characteristics. A total of 256 patients were matched successfully, with 68 patients in the low-LDH group and 188 in the high-LDH group. Patients’ clinical features were balanced between the low-LDH group and the high-LDH group after matching. The correlation between patient characteristics with LDH level is summarized in Table 1.
Prognostic value of pretreatment serum LDH levels
In the whole cohort, the median PFS was 31.5 and 17.5 months for the low-LDH group and the high-LDH group, respectively, while the median OS was 32.4 and 25.5 months for the low-LDH group and the high-LDH group, respectively. Notably, more than half of the patients in the low-LDH group survived to the last follow-up. Before matching, the patients in the high-LDH group had significantly shorter PFS and worse OS than did those in the low-LDH group according to Kaplan-Meier analysis (all log-rank P values <0.05, Figure S1).
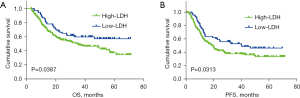
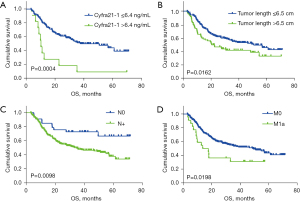
For the matched cohort, Kaplan-Meier analysis showed that the PFS and OS in the high-LDH group were significantly shorter than those in the low-LDH group (Figure 2). The survival curves on OS for Cyfra21-1 level (P=0.0004), tumor length (P=0.0162), cN stage (P=0.0098), and cM stage (P=0.0198) are shown in Figure 3A-3D, respectively.
Survival risk according to univariate and multivariate Cox regression analysis
The results of univariate and multivariate Cox regression analyses of PFS and OS after chemoradiotherapy of patients with ESCC before matching are presented in Tables S1,S2. After matching, according to the univariate Cox regression analysis of PFS, Cyfra21-1 level, LDH level, and tumor length were significantly associated with tumor recurrence (all P values <0.05). We additionally found there to be a significant correlation between the following characteristics and OS in the univariate analysis: Cyfra21-1 level, tumor length, cN stage, cM stage, and LDH level (all P values <0.05). The significant factors were then subjected to the multivariate analysis to identify the independent prognostic factors. Multivariate analysis revealed that Cyfra21-1 level [hazard ratio (HR) =2.37, 95% CI: 1.22–4.60; P=0.011], LDH level (HR =1.50; 95% CI: 1.03–2.19; P=0.035), and tumor length (HR =1.62; 95% CI: 1.15–2.28; P=0.005) were independent factors associated with PFS in patients with ESCC; meanwhile, Cyfra21-1 level (HR =2.81; 95% CI: 1.43–5.50; P=0.003), tumor length (HR =1.61; 95% CI: 1.11–2.34; P=0.013), cN stage (HR =1.94; 95% CI: 1.01–3.72; P=0.047), cM stage (HR =2.04; 95% CI: 1.18–3.53; P=0.011), and LDH level (HR: 1.56; 95% CI: 1.02–2.39; P=0.039) were independent factors associated with OS in patients with ESCC (Tables 2,3).
Table 2
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender (female vs. male) | 0.99 (0.66, 1.49) | 0.960 | – | – | |
| Age (>69 vs. ≤69 years) | 1.16 (0.75, 1.81) | 0.501 | – | – | |
| ECOG PS (1–2 vs. 0) | 1.17 (0.86, 1.61) | 0.324 | – | – | |
| cT stage (T3–4 vs. T1–2) | 1.06 (0.61, 1.83) | 0.843 | – | – | |
| cN stage (N+ vs. N0) | 1.54 (0.92, 2.58) | 0.104 | – | – | |
| cM stage (M1 vs. M0) | 1.40 (0.82, 2.39) | 0.213 | – | – | |
| Differentiation (moderate/poor vs. high) | 1.40 (0.83, 2.35) | 0.204 | – | – | |
| Tumor length (>6.5 vs. ≤6.5 cm) | 1.58 (1.12, 2.21) | 0.009 | 1.62 (1.15, 2.28) | 0.005 | |
| Tumor location | 0.108 | ||||
| Upper vs. cervical | 0.60 (0.36, 1.03) | 0.062 | – | – | |
| Medium vs. cervical | 0.82 (0.49, 1.36) | 0.437 | – | – | |
| Lower vs. cervical | 1.00 (0.58, 1.74) | 0.991 | – | – | |
| Radiotherapy technology (IMRT vs. 3DCRT) | 0.83 (0.60, 1.15) | 0.263 | – | – | |
| Treatment options (CCRT vs. SCRT) | 1.29 (0.93, 1.79) | 0.126 | – | – | |
| Dose (>58.8 vs. ≤58.8 Gy) | 0.78 (0.52, 1.17) | 0.234 | – | – | |
| CEA (ng/mL) | |||||
| >5.5 vs. ≤5.5 | 1.09 (0.74, 1.59) | 0.668 | – | – | |
| NA vs. ≤5.5 | 1.04 (0.70, 1.54) | 0.855 | – | – | |
| Cyfra21-1 (ng/mL) | |||||
| >6.4 vs. ≤6.4 | 2.49 (1.29, 4.83) | 0.007 | 2.37 (1.22, 4.60) | 0.011 | |
| NA vs. ≤6.4 | 0.80 (0.58, 1.12) | 0.194 | 0.80 (0.58, 1.12) | 0.196 | |
| LDH (>134 vs. ≤134 U/L) | 1.51 (1.03, 2.20) | 0.033 | 1.50 (1.03, 2.19) | 0.035 | |
HR, hazard ratio; CI, confidence interval; LDH, lactate dehydrogenase; ECOG, Eastern Clinical Oncology Group; PS, performance status; Cyfra21-1, cytokeratin 19 fragment antigen 21-1; CEA, carcinoembryonic antigen; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; CCRT, concurrent chemoradiotherapy; SCRT, sequential chemoradiotherapy; cT stage, clinical T stage; cN stage, clinical N stage; cM stage, clinical M stage; ESCC, esophageal squamous cell carcinoma; PFS, progression-free survival; NA, not applicable.
Table 3
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender (female vs. male) | 0.89 (0.56, 1.41) | 0.625 | – | – | |
| Age (>69 vs. ≤69 years) | 1.04 (0.63, 1.70) | 0.891 | – | – | |
| ECOG PS (1–2 vs. 0) | 1.17 (0.83, 1.65) | 0.368 | – | – | |
| cT stage (T3–4 vs. T1–2) | 0.87 (0.49, 1.54) | 0.628 | – | – | |
| cN stage (N+ vs. N0) | 2.29 (1.20, 4.38) | 0.012 | 1.94 (1.01, 3.72) | 0.047 | |
| cM stage (M1 vs. M0) | 1.88 (1.10, 3.22) | 0.022 | 2.04 (1.18, 3.53) | 0.011 | |
| Differentiation (moderate/poor vs. high) | 1.40 (0.80, 2.44) | 0.236 | – | – | |
| Tumor length (>6.5 vs. ≤6.5 cm) | 1.56 (1.08, 2.26) | 0.017 | 1.61 (1.11, 2.34) | 0.013 | |
| Tumor location | |||||
| Upper vs. cervical | 0.68 (0.39, 1.20) | 0.134 | – | – | |
| Medium vs. cervical | 0.68 (0.39, 1.20) | 0.134 | – | – | |
| Lower vs. cervical | 1.04 (0.57, 1.88) | 0.904 | – | – | |
| Radiotherapy technology (IMRT vs. 3DCRT) | 0.80 (0.56, 1.14) | 0.220 | – | – | |
| Treatment options (CCRT vs. SCRT) | 1.43 (0.99, 1.79) | 0.054 | – | – | |
| Dose (>58.8 vs. ≤58.8 Gy) | 0.75 (0.48, 1.17) | 0.206 | – | – | |
| CEA (ng/mL) | |||||
| >5.5 vs. ≤5.5 | 1.08 (0.71, 1.63) | 0.733 | – | – | |
| NA vs. ≤5.5 | 1.13 (0.74, 1.73) | 0.584 | – | – | |
| Cyfra21-1 (ng/mL) | |||||
| >6.4 vs. ≤6.4 | 3.17 (1.63, 6.18) | 0.001 | 2.81 (1.43, 5.50) | 0.003 | |
| NA vs. ≤6.4 | 0.98 (0.68, 1.40) | 0.895 | 1.00 (0.70, 1.44) | 0.983 | |
| LDH (>134 vs. ≤134 U/L) | 1.55 (1.02, 2.35) | 0.040 | 1.56 (1.02, 2.39) | 0.039 | |
HR, hazard ratio; CI, confidence interval; LDH, lactate dehydrogenase; ECOG, Eastern Clinical Oncology Group; PS, performance status; Cyfra21-1, cytokeratin 19 fragment antigen 21-1; CEA, carcinoembryonic antigen; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; CCRT, concurrent chemoradiotherapy; SCRT, sequential chemoradiotherapy (SCRT); cT stage, clinical T stage; cN stage, clinical N stage; cM stage, clinical M stage; ESCC, esophageal squamous cell carcinoma; NA, not applicable.
A new risk score model and a prediction nomogram for OS based on LDH level
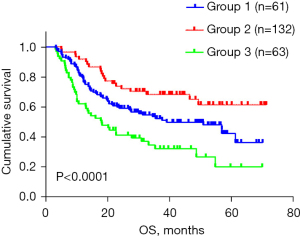
A new risk score model for OS among ESCC patients underwent chemoradiotherapy was constructed incorporating the 5 adverse factors (LDH level, tumor length, cN stage, cM stage, and Cyfra21-1 level) identified in the multivariate analysis, According to this prediction model, patients were stratified into 3 risk groups with distinct prognoses: low-risk group (0–1 adverse factors), intermediate-risk group (2 adverse factors) and high-risk group (3–5 adverse factors), as shown in Figure 4 (P<0.0001).
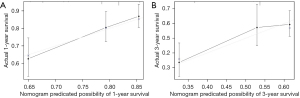
The prediction nomogram that integrated the significant independent factors for OS is shown in Figure 5. The C-index for OS prediction was 0.599 (95% CI: 0.569–0.629). It could be observed that lower total points correspond to worse prognosis. The calibration curve for the probability of survival demonstrated good agreement between the prediction and actual observation in the probability of 1-year survival, but a relatively poor agreement for 3-year survival probability (see Figure 6A,6B).

Discussion
This study demonstrated that lower LDH levels were associated with a better prognosis compared with higher LDH levels, as shown in both multivariable analysis based on the whole cohort of 614 patients and the propensity score-matched cohort of 256 patients. More importantly, it is among the few to establish a new risk prognostic scoring model based on the baseline LDH levels, and it stratified patients into 3 groups with different prognoses. Moreover, our study found that elevated LDH was linked to distant metastasis and larger tumor length, suggesting that a high level LDH is likely to reflect a heavier tumor burden and may represent a more aggressive disease in ESCC.
Studies on whether increased LDH is related to tumor survival have been reported for several solid tumors (9-13). The underlying mechanism between LDH and poor survival remains unknown. It has been hypothesized that elevated serum LDH levels were considered as a marker of tumor hypoxia or immunosuppression in cancer patients (26,27). Alderuccio et al. confirmed that the prognosis of patients with lymphoma with high LDH level was poor (9) whereas Ali et al. reported pretreatment LDH level to be an independent predictor of OS in nasopharyngeal carcinoma (10). Additionally, de Jong et al. reviewed 593 patients with advanced non-small cell lung cancer (NSCLC) and found that a high pretreatment LDH level was related to lower OS (11). Wang et al. also reported that high LDH levels indicated poor prognosis for patients with NSCLC and brain metastases (12). Additionally, in a study by Xiao et al., baseline LDH levels were proven to have significant prognostic value in patients with pancreatic cancer (13). Several other studies have evaluated the prognostic value of LDH in ESCC (14-20). The study on the prognostic value of LDH in ESCC published by Wei et al. is the largest study of its kind, including 906 patients with ESCC, which showed that the survival time of patients with a high level of LDH is shorter than those with a lower level (14). Li et al. identified LDH to be a powerful independent factor for OS in patients with advanced ESCC treated with anti-programmed cell death protein 1 (PD-1) therapy, which is in line with the findings of Wang et al.’s research, but both these studies included fewer than 50 patients (17,18). Additionally, an investigation that recruited 567 patients with ESCC conducted by Luo et al. also demonstrated an elevated LDH level to be an independent indicator for poor prognosis (19). Similarly, our results in patients with ESCC who had undergone chemoradiotherapy showed that those with high LDH at baseline had shorter OS than did those with low LDH at baseline. However, this conflicted with the results of another retrospective study on 212 patients with ESCC undergoing chemoradiotherapy by Zhang et al., which indicated LDH to not be associated with OS or PFS (20). Another 2 studies also reported that LDH was not a prognostic factor regarding the OS of patients with ESCC (15,16). It is worth mentioning that the cutoff LDH values in these articles were inconsistent, and the cutoff value of LDH in our study was significantly lower than that of the others. Another possible explanation for the discrepancy is that the patients included in our study might have been in the earlier stages, implying a relatively lower tumor burden than that in previous studies. Furthermore, there may be a difference in the method for determining the optimal cutoff value of LDH. Moreover, only 68 patients were enrolled in our study according to the calculation of X-tile software in the low-LDH group. In summary, there was no standard point for optimal cutoff value of LDH, which might be affected by various conditions in clinical practice. Thus, more prospective studies are urgently need to solve the problem of inconsistent optimal LDH cutoff values. Overall, although definitive conclusions exist regarding pretreatment serum LDH levels as a predictor for prognosis in ESCC, our results suggest it may be a reliable factor in predicting the therapeutic effect of chemoradiotherapy in patients with ESCC. However, given the small sample sizes and the different disease stage distributions within each sample, additional research is warranted to further validate the prognostic value of LDH before it can be widely used in clinical settings.
This is the first report identifying LDH, ECOG-PS, Cyfra-21, tumor-length, cN stage and cM stage as the dependent parameters to construct a new risk score model with P<0.0001 and a nomogram for predicting the survival rate with a C-index of 0.599. To note, the significance of the risk score model is guiding the individual treatment. But the nomogram were not performed very well in predicting survival. However, we still recommend a comprehensive treatment for ESCC patients with high serum LDH levels. If no distant metastasis was found during pre-treatment examinations but with high serum LDH value, attention should be paid to the presence of small distant metastases that were not clinically detected, or the tendency for distant metastasis. It may provide a practical, economical, and reliable detection indicator for the selection of treatment plans for ESCC patients. Further large-scaled prospective trials are warranted to verify our results.
Moreover, according to the result of Cox multivariate analysis, we found that, in addition to LDH, tumor length was another independent prognostic factor for OS, while advanced cN stage and advanced cM stage were shown to be associated with worse OS. This was consistent with the findings of a previous study by Yu et al., with the difference being that our findings indicated OS to be related to cM stage, while Yu et al.’s results indicated OS was related to T stage (28). This can be explained by the fact that the patient selection in our study included patients with ESCC who underwent chemoradiotherapy, while in the retrospective study mentioned above, more than 80% of the patients underwent curative esophagectomy. Although the cT stage and cN stage in our study were determined using endoscopic ultrasound (EUS), enhanced-scanning computed tomography (CT), positron emission tomography (PET)-CT, or pathological biopsy, it was not completely equivalent to postoperative pathological staging. With regard to the Cyfra21-1 level in our study, multivariate analysis indicated that a high level of Cyfra21-1 was an adverse prognostic factor and was better than CEA level as a predictor for prognosis in ESCC. Our study results support the prognostic value of Cyfra21-1 in predicting OS and PFS. The finding was in accordance with 2 earlier studies (29,30). However, Yang et al. reported a conflicting result, with CEA being superior to other tumor biomarkers as prognostic indicators in ESCC (31). The prognostic value of tumor marker index (TMI) based on Cyfra21-1 and squamous cell carcinoma antigen (SCC-Ag) has been reported in recent years (32,33). Unfortunately, SCC-Ag was not assessed in our study. Overall, studies with greater homogeneity are needed to identify the prognostic factors for ESCC patients in the context of inconsistent results.
Recently, Alderuccio et al. proposed a new prognostic index based on LDH to better predict the survival of recognize patients (9). Moreover, Luo et al. developed a prognostic risk scoring model that included the levels and neutrophil count to help verify the prognosis of patients with ESCC (19). However, no studies thus far have examined the combination of LDH levels with tumor markers for the prognosis of patients with ESCC treated with chemoradiotherapy. Using the findings derived in our analysis, we established a model based on serum LDH levels, tumor biomarkers, and the TNM staging system to identify those patients with ESCC who were most likely to benefit from chemoradiotherapy. No widely used predictive model for prognosis has been constructed for patients with ESCC receiving chemoradiotherapy until now, and developing a more effective and reliable prediction model for estimating the prognosis will be our main focus in the subsequent studies.
Our study also has some limitations that should be noted. First, we used a single-center retrospective design, which likely introduced some degree of selection bias. Second, it was difficult to obtain the complete pathological data for the patients in this study who underwent chemoradiotherapy but not surgery. Third, the role of LDH in predicting prognosis was limited due to there being some other factors influencing the LDH levels but not tumors. Third, the most sensitive cutoff points of serum LDH need to be determined through large-scale clinical trials.
Conclusions
In summary, serum LDH level was found to be a predictive factor for poor survival in ESCC patients undergone chemoradiotherapy. LDH should be considered a relevant clinical variable and included in the prognostic classification of patients with ESCC, with the aim to better determine the most appropriate treatment strategies and to better stratify patients included in clinical trials. Therefore, a multicenter, large-sample prospective study is needed to further verify the conclusions before this method can be applied to routine clinical studies.
Acknowledgments
We would like to thank our colleagues who provided extensive support in the protocol design, data collection and analysis, and the manuscript writing of this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-388/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-388/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-388/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-388/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Shandong Cancer Hospital (No. SDTHEC2023004014). All registered patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cai Y, Lin J, Wei W, et al. Burden of esophageal cancer and its attributable risk factors in 204 countries and territories from 1990 to 2019. Front Public Health 2022;10:952087. [Crossref] [PubMed]
- The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:582-97. [Crossref] [PubMed]
- Uhlenhopp DJ, Then EO, Sunkara T, et al. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol 2020;13:1010-21. [Crossref] [PubMed]
- Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer 2010;116:3763-73.
- Claps G, Faouzi S, Quidville V, et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol 2022;19:749-62. [Crossref] [PubMed]
- Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 2015;54:961-70. [Crossref] [PubMed]
- Petrelli F, Ardito R, Merelli B, et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res 2019;29:1-12. [Crossref] [PubMed]
- Alderuccio JP, Reis IM, Habermann TM, et al. Revised MALT-IPI: A new predictive model that identifies high-risk patients with extranodal marginal zone lymphoma. Am J Hematol 2022;97:1529-37. [Crossref] [PubMed]
- Ali WAS, Huang X, Wu Y, et al. Pretreatment Serum Lactate Dehydrogenase and Metastases Numbers as Potential Determinants of Anti-PD-1 Therapy Outcome in Nasopharyngeal Carcinoma. Cancer Control 2023;30:10732748221148912. [Crossref] [PubMed]
- de Jong C, Deneer VHM, Kelder JC, et al. Association between serum biomarkers CEA and LDH and response in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Thorac Cancer 2020;11:1790-800. [Crossref] [PubMed]
- Wang S, Lv J, Lv J, et al. Prognostic value of lactate dehydrogenase in non-small cell lung cancer patients with brain metastases: a retrospective cohort study. J Thorac Dis 2022;14:4468-81. [Crossref] [PubMed]
- Xiao Y, Chen W, Xie Z, et al. Prognostic relevance of lactate dehydrogenase in advanced pancreatic ductal adenocarcinoma patients. BMC Cancer 2017;17:25. [Crossref] [PubMed]
- Wei XL, Zhang DS, He MM, et al. The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour Biol 2016;37:1879-87. [Crossref] [PubMed]
- Huang H, Wang XP, Li XH, et al. Prognostic value of pretreatment serum alanine aminotransferase/aspartate aminotransferase (ALT/AST) ratio and gamma glutamyltransferase (GGT) in patients with esophageal squamous cell carcinoma. BMC Cancer 2017;17:544. [Crossref] [PubMed]
- Feng JF, Wang L, Yang X, et al. Gustave Roussy Immune Score (GRIm-Score) is a prognostic marker in patients with resectable esophageal squamous cell carcinoma. J Cancer 2020;11:1334-40. [Crossref] [PubMed]
- Li Y, Wang K, Zhao E, et al. Prognostic Value of Lactate Dehydrogenase in Second-Line Immunotherapy for Advanced Esophageal Squamous Cell Carcinoma. Pathol Oncol Res 2022;28:1610245. [Crossref] [PubMed]
- Wang X, Zhang B, Chen X, et al. Lactate dehydrogenase and baseline markers associated with clinical outcomes of advanced esophageal squamous cell carcinoma patients treated with camrelizumab (SHR-1210), a novel anti-PD-1 antibody. Thorac Cancer 2019;10:1395-401. [Crossref] [PubMed]
- Luo HS, Xu HY, Du ZS, et al. Prognostic Significance of Baseline Neutrophil Count and Lactate Dehydrogenase Level in Patients With Esophageal Squamous Cell Cancer Treated With Radiotherapy. Front Oncol 2020;10:430. [Crossref] [PubMed]
- Zhang P, Xi M, Li QQ, et al. The modified glasgow prognostic score is an independent prognostic factor in patients with inoperable thoracic esophageal squamous cell carcinoma undergoing chemoradiotherapy. J Cancer 2014;5:689-95. [Crossref] [PubMed]
- Lian L, Teng SB, Xia YY, et al. Development and verification of a hypoxia- and immune-associated prognosis signature for esophageal squamous cell carcinoma. J Gastrointest Oncol 2022;13:462-77. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest 2013;123:3685-92. [Crossref] [PubMed]
- Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark 2017;19:353-63. [Crossref] [PubMed]
- Yu L, Zhang XT, Guan SH, et al. The Number of Negative Lymph Nodes is Positively Associated with Survival in Esophageal Squamous Cell Carcinoma Patients in China. Open Med (Wars) 2020;15:152-9. [Crossref] [PubMed]
- Zhang HQ, Wang RB, Yan HJ, et al. Prognostic significance of CYFRA21-1, CEA and hemoglobin in patients with esophageal squamous cancer undergoing concurrent chemoradiotherapy. Asian Pac J Cancer Prev 2012;13:199-203. [Crossref] [PubMed]
- Yan HJ, Wang RB, Zhu KL, et al. Cytokeratin 19 fragment antigen 21-1 as an independent predictor for definitive chemoradiotherapy sensitivity in esophageal squamous cell carcinoma. Chin Med J (Engl) 2012;125:1410-5. [PubMed]
- Yang Y, Huang X, Zhou L, et al. Clinical use of tumor biomarkers in prediction for prognosis and chemotherapeutic effect in esophageal squamous cell carcinoma. BMC Cancer 2019;19:526. [Crossref] [PubMed]
- Qiao Y, Chen C, Yue J, et al. Tumor marker index based on preoperative SCC and CYFRA 21-1 is a significant prognostic factor for patients with resectable esophageal squamous cell carcinoma. Cancer Biomark 2019;25:243-50. [Crossref] [PubMed]
- Yin N, Liu W. Clinical Value of Tumor Marker Index Based on Preoperative CYFRA 21-1 and SCC-Ag in the Evaluation of Prognosis and Treatment Effectiveness in Patients with Esophageal Squamous Cell Carcinoma. Onco Targets Ther 2020;13:4135-43. [Crossref] [PubMed]
(English Language Editor: J. Gray)


