
Anatomical variation of sympathetic ganglia in R4+R5 sympathicotomy for primary palmar axillary hyperhidrosis
Highlight box
Key findings
• Anatomical variations of sympathetic ganglia could be visualized clearly under the near-infrared fluorescent thoracoscopy during endoscopic thoracic sympathicotomy for palmar axillary hyperhidrosis.
What is known and what is new?
• Endoscopic thoracic sympathicotomy is an effective treatment method for palmar axillary hyperhidrosis, but the surgical outcomes were reported unstable.
• The surgical outcomes may be affected by the anatomical variations of sympathetic ganglia.
What is the implication, and what should change now?
• Endoscopic thoracic sympathicotomy guided by near-infrared fluorescent thoracoscopy may be a more precise procedure for palmar axillary hyperhidrosis.
Introduction
Primary palmar axillary hyperhidrosis (PAH) is an idiopathic benign condition that seriously affects the quality of life of young people. To date, endoscopic thoracic sympathicotomy (ETS) has been widely accepted as a safe and effective treatment for PAH with durable surgical effects. The interruption of the sympathetic trunk on the fourth and fifth rib (R4+R5 sympathicotomy) is recommended as one of the standard methods for PAH in an expert consensus released by the Society of Thoracic Surgeons (STS) (1).
However, the surgical effects on sweating improvement of traditional ETS reported by previous studies were with great discrepancy, which some patients have overly dry hands, some being comfortably dry, and some have little relief (2,3). One possible explanation for this phenomenon is the anatomical variation of sympathetic ganglia, which is commonly invisible to naked eye due to the obscuring fat and pleura under normal thoracoscopy. There were 0–4.0% up-shift and 28.0–40.9% down-shift variations according to previous results (4,5). Based on these findings, an R4+R5 sympathicotomy may be possibly not a true T4+T5 sympathicotomy. Thus, we hypothesized that the anatomical variation of the thoracic sympathetic ganglia (TSG) results in unstable surgical effects.
We previously demonstrated that the TSG could be visualized under the near-infrared (NIR) fluorescent thoracoscopy combined with indocyanine green (ICG) (6,7), and this novel technique was proved safe and feasible in ETS for primary palmar hyperhidrosis (PPH) recently (8). To our knowledge, there is no literature that reported the anatomical variation of TSG in patients with PAH. Therefore, we aimed to identify anatomical variation of the T3 sympathetic ganglion (G3) and T4 sympathetic ganglion (G4) in R4+R5 sympathicotomy for PAH by utilizing the NIR fluorescent thoracoscopy in the current study. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1782/rc).
Methods
Study design and settings
This was a prospective multi-center observational cohort study carried out in Peking University People’s Hospital and Beijing Haidian Hospital from August 2016 to August 2020. All enrolled participants had completed a self-designed questionnaire (including patient demographics, basic information on sweating, impact of sweating, and quality of life) preoperatively. The data of anatomical variations were recorded when participants underwent ETS using NIR fluorescent thoracoscopy, and each participant was followed up within 3 months (short-term) and over 6 months (long-term) after surgery. Data of surgical outcomes were recorded by a self-designed questionnaire (including improvement in sweating, impact of sweating, compensatory sweating and satisfaction). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of Peking University People’s Hospital and Beijing Haidian Hospital (No. 2017PHB101-01 and No. 2017028). Individual informed consent was obtained from every patient.
Procedures
Participants recruitment
Inclusion criteria were patients with PAH who were referred to receive ETS, who should conform to the surgical indications of severe hyperhidrosis or moderate hyperhidrosis with a strong desire to surgical treatment. The severity and the therapeutic effects were assessed by self-designed questionnaires based on the questionnaire endorsed by STS expert consensus (1). Exclusion criteria included liver dysfunction, ICG allergy, intolerance to surgery and being unwilling to participate in the study. Patients whose TSG cannot be clearly identified by NIR fluorescent thoracoscopy were also excluded in the data analysis.
Preoperative procedure
All patients received intravenous injection of ICG (Tian Yi Pharmaceutical Co., Ltd. Liaoning, China) at doses of 5 mg/kg 24-hours preoperatively. FloNavi fluorescent thoracoscopy (OptoMedic Technologies Inc., Guangdong, China) was used in this study. This device combines a fluorescence image with an ordinary camera image through a patented technology called multi-channel collecting and real-time fluorescence fusion.
Operative technique
Patients were placed in a semi-Fowler’s position with both axillae exposed. The procedure was performed under general anesthesia with laryngeal mask assisted ventilation. We used a uni-portal approach of 10 mm in the third intercostal space of the midaxillary line. The sympathetic chain was observed under white light mode and fluorescent mode consecutively. A conventional R4+R5 sympathicotomy (the sympathetic trunk was transected on the surface of the fourth and fifth ribs using an electric coagulating hook under white-light mode) was performed as previously reported (2) and were not affected by any anatomical variation of TSG. After that, the thoracoscope was switched to fluorescent mode to record the anatomical variation of the G3, G4 and actual transecting level relating to the sympathetic ganglia. Operation of the right side was performed first, followed by the same procedure on the left side.
Outcome measurement
The primary outcome was the variation rates of G3 and G4. The normal position of G3 and G4 are at the 3rd and 4th intercostal spaces, respectively. Either the G3 was shifting to the 3rd rib or above, or the G4 was shifting to the 4th rib or above, we defined these situations as up-shift variation. On the contrary, we defined down-shift variation as either the G3 shifted to the 4th rib or lower, or the G4 shifted to the 5th rib or lower.
The secondary outcomes were surgical outcomes including the improvement of palmar and axillary sweating, and the degree of compensatory hyperhidrosis (CH) after surgery. Degree of patients’ sweating after operation was divided into four grades: ‘completely improved’, ‘obviously improved’, ‘partially improved’, and ‘no improvement’. Degree of CH was divided into four levels: none, mild, moderate and severe. ‘Mild CH’ means small amounts of sweating, which does not flow and does not cause embarrassment or the need to change clothes. ‘Moderate CH’ means the sweat coalesces into droplets that flow, but the patients do not have to change clothes. ‘Severe CH’ means large amount of sweat, requiring a change of clothes one or more times a day (1,2).
Sample size estimation
According to 32% variation rate of G3 from previous study (4), an estimated sample size of 171 cases were needed which produced a two-sided 95% confidence interval (CI) with a width equal to 0.140. Considering the expected drop-out rate and failure of fluorescent imaging, we increased the sample size by 20% and a total of 206 nerves and 103 patients were required.
Statistical analysis
SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA) was used for data analysis. Continuous variables were presented as means and standard deviations and normality was assessed using Shapiro-Wilk test and Kolmogorov-Smirnov test. Categorical variables were presented as counts and percentages. Proportion and 95% CI were used to describe variation rate. Chi-squared test was used to compare the difference between categorical variables. Fisher’s exact test was used when sample sizes were small. For non-parametric data, Wilcoxon test was used to compare the difference between paired variables and Mann-Whitney U test was applied in non-paired data. For parametric data, matched samples t-test and student’s t-test were conducted in paired and non-paired data, respectively. Wilcoxon rank-sum test or Kruskal-Wallis test was used to compare the difference between rank variables; McNemar’s test was used to compare the difference between paired nominal data. Statistical significance was based on two-tailed tests evaluated at a 0.05 level of significance and Bonferroni correction was used in subgroups comparisons.
Results
One hundred and sixty-two patients in total were enrolled in this study and 134 patients with bilateral clearly visualized TSG were included in data analysis, including 68 males and 66 females. The mean age was 26.63±5.95 years (range, 18–46). The success rate of fluorescent imaging of TSG was 82.7%.
All patients underwent R4+R5 sympathicotomy (the sympathetic trunk was transected on the 4th and 5th rib) under white-light mode and the anatomy of sympathatic chains were sequentially observed under fluorescent mode. No perioperative death or severe complication such as arrhythmia, pneumothorax, hemothorax, Horner syndrome and ICG-related allergy occurred. All patients completed the follow-up within 3 months (short-term) and over six months (long-term) postoperatively.
Anatomical variation of G3 and G4
There were 268 sides of sympathetic chain in total of 134 patients. There were 236 sides without anatomical variation (Figure 1, a1&a2) and 32 sides with G3 shifted downward (Figure 1, b1&b2). No upward shift variation was identified. Among patients with G3 variation, 20 patients had unilateral variation and 6 patients had bilateral variation. The variation rate of G3 was 11.9% (32/268).
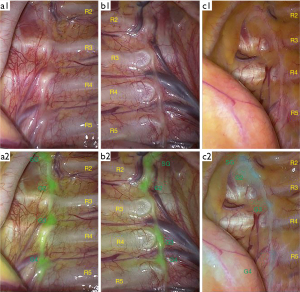
There were 216 sides without anatomical variation and 52 sides with G4 shifted downward (Figure 1, c1&c2). No upward shift variation was identified. Among patients with G4 variation, 26 patients had unilateral variation and 13 patients had bilateral variation. The variation rate of G4 was 19.4% (52/268).
The relationship between anatomical variation and clinical characteristic were analyzed. The anatomical variation is not significantly associated with age, sex, height, weight and BMI (Table 1).
Table 1
| Clinical characteristics | Normal (N=87) | Variation (N=47) | P |
|---|---|---|---|
| Gender, n (%) | 0.957 | ||
| Male | 44 (50.6) | 24 (51.1) | |
| Female | 43 (49.4) | 23 (48.9) | |
| Age (year), mean ± SD | 26.63±5.95 | 25.64±5.10 | 0.413 |
| Height (cm), mean ± SD | 168.36±7.52 | 168.72±7.63 | 0.795 |
| Weight (kg), mean ± SD | 61.55±12.30 | 63.14±11.59 | 0.469 |
| BMI (kg/m2), mean ± SD | 21.57±3.07 | 22.07±2.82 | 0.385 |
TSG, thoracic sympathetic ganglia; SD, standard deviation; BMI, body mass index.
Surgical outcomes and their correlation with the anatomical variation of G3 and G4
At short-term follow-up, the improvements of palmar sweating were ‘completely improved’ in 138 sides (51.5%), ‘obviously improved’ in 109 sides (40.7%), ‘partially improved’ in 16 sides (6.0%) and ‘no improvement’ in 5 sides (1.9%). The total improvement rate on palmar sweating was 98.1%. As to the axillary sweating, the surgical results of four grades were 59.3%, 34.0%, 3.7% and 3.0%, respectively. The total improvement rate on axillary sweating was 97%.
At long-term follow-up, the improvements of palmar sweating were ‘completely improved’ in 86 sides (32.1%), ‘obviously improved’ in 127 sides (47.4%), ‘partially improved’ in 42 sides (15.7%) and ‘no improvement’ in 13 sides (4.9%). The total improvement rate on palmar sweating was 95.1% in long-term results. As to the axillary sweating, the surgical results of four grades were 47.8%, 28.7%, 13.1% and 10.4%, respectively. The total improvement rate on axillary sweating was 89.6% in long-term results.
As to the correlation between the improvement of palmar sweating and the anatomical variation of G3, there were significant differences between normal and variation subgroups both in short-term (P=0.049) and long-term (P=0.032) follow-ups. The surgical outcomes indicated that hands were drier after surgery in the variation subgroup than in the normal subgroup (Table 2).
Table 2
| Improvement | Short-term, n (%) | Long-term, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Normal (N=236) | G3 variation (N=32) | P | Normal (N=236) | G3 variation (N=32) | P | ||
| Complete | 117 (49.6) | 21 (65.6) | 0.049 | 70 (29.7) | 16 (50.0) | 0.032 | |
| Obvious | 98 (41.5) | 11 (34.4) | 115 (48.7) | 12 (37.5) | |||
| Partial | 16 (6.8) | 0 (0.0) | 40 (16.9) | 2 (6.3) | |||
| None | 5 (2.1) | 0 (0.0) | 11 (4.7) | 2 (6.3) | |||
As to the correlation between the improvement of axillary sweating and the anatomical variation of G4, no significant difference was found between normal and variation subgroups both in short-term (P=0.635) and long-term (P=0.776) follow-ups (Table 3).
Table 3
| Improvement | Short-term, n (%) | Long-term, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Normal (N=216) | G4 variation (N=52) | P | Normal (N=216) | G4 variation (N=52) | P | ||
| Complete | 126 (58.3) | 33 (63.5) | 0.635 | 104 (48.1) | 24 (46.2) | 0.776 | |
| Obvious | 77 (35.6) | 14 (26.9) | 64 (29.6) | 13 (25.0) | |||
| Partial | 5 (2.3) | 5 (9.6) | 22 (10.2) | 13 (25.0) | |||
| None | 8 (3.7) | 0 (0.0) | 26 (12.0) | 2 (3.8) | |||
The incidence rate of CH in short-term was 84.3% (113/134), including 75 (56.0%) mild type, 29 (21.6%) moderate type and 9 (6.7%) severe type. The results were 73 (54.5%) mild type, 38 (28.4%) moderate type and 8 (6.0%) severe type with a total incidence rate of 88.8% in long-term follow-up. No significant difference was found between normal and variation subgroups on the degree of CH.
Discussion
PAH commences around adolescence and is believed to be secondary to over activity of the sympathetic nervous system (9). The activity of the eccrine sweat glands would probably decrease by the denervation of the sympathetic nerve to the hands and armpits. The sympathetic nerves that dominate the hands and armpits originate from the upper thoracic spinal cord. Based on the current findings, it is considered that the sweat glands of hand were most influenced by sympathetic ganglia T2–T4, and sympathetic ganglia T4–T5 were responsible for the armpit (10). The higher transection level of the nerve, the greater degree of denervation. It has been confirmed that T2 sympathicotomy is strongly related to severe CH (11), thus this procedure has been abandoned in treatment of PPH. According to these conclusions, T3 or T4 sympathicotomy was recommended for PPH and an additional T5 sympathicotomy was suggested for PAH (1).
In the past several years, a number of studies have compared the therapeutic effects of T3 sympathicotomy and T4 sympathicotomy (2,3,12-16). The results indicated that T3 sympathicotomy has the characteristics of drier hand, or overly dry hand, and more severe CH postoperatively. While R4 sympathicotomy has the characteristics of mild moist hand and less CH postoperatively. The conclusion of these studies is consistent that R4 sympathicotomy is superior for the treatment of PPH because of its mild side effects. However, when we interpreted the results of these studies cautiously, we would notice that there was a significant discrepancy among these reported surgical outcomes no matter of T3 sympathicotomy or T4 sympathicotomy. After the ‘same’ operation, some patients resulted in dry hand and some resulted in moist hand. In previous studies mentioned above, the localization of TSG was based on the ribs without exception. The highest posterior rib that can be seen under thoracoscopy is the second rib. The procedure to cut off the nerve on the surface of the fourth rib is called T4 sympathicotomy. In fact, according to STS expert consensus, strictly speaking, this should be called R4 sympathicotomy (1). Because of the variation of ganglion position, R4 sympathicotomy is not necessarily T4 sympathicotomy. The variation of ganglion position may be the reason of unstable curative effect.
Traditionally, given the fact that TSG are not directly visible to naked eye, surgeons can only use ribs as references to define the level of transection of sympathetic chain. However, anatomical variation of location happens to ganglia. Several studies have consistently showed 10–50% variation rate of ganglia positions (4,5,17,18), which implies a high rate of miscounted level of transection during sympathicotomy. Thus, to clarify the anatomical variation of TSG has a critical role in deepening human understanding of autonomic nerve system and improving the therapeutic effect of primary hyperhidrosis.
To the best of our knowledge, this is the first study to investigate the value of NIR fluorescent thoracoscopy to identify anatomical variation of TSG and its correlation with the surgical outcomes for PAH. Our research team reported NIR fluorescent imaging of TSG (6), and verified the fluorescence signal comes from neurocytes in the ganglia under fluorescent microscopy (7). In the meantime, this novel technique was proved safe and effective in ETS (8). Visualization and counting TSG with NIR fluorescent thoracoscopy may just provide the way of accurate sympathetic chain identification and transection for the treatment of PAH.
We successfully used NIR fluorescence thoracoscopy to display the TSG clearly with a success rate of 82.7%, and our results supplemented a set of data on the anatomical variation of TSG. Liu et al. (19) reported that the variation rate of G3 shifting downward to the upper edge of the 4th rib was 11.7%, and 1.7% of shifting downward to the surface of the 4th rib in a cadaver research. There was 10% upward shift to the lower edge of the 3rd rib according to their results. As to the G4, 25% was shifted downward to the 5th rib and 6.7% was shifted upward to the 4th rib. Street et al. (20) reported 6.25% of variation rate of TSG, and 32% of G3 variation and 50% of G4 variation was reported in the research of Zhang et al. (4). All these results were from study of cadaver. The reasons for difference between our findings and the previous results may be as follows. (I) The cases in present study were young people with PAH, while the study populations of previous cadaver researches were mostly old people. Since the bone construction of thoracic cavity may be different between these two populations, the relative position of TSG and ribs may be different accordingly. (II) The specimen in cadaver studies were fixed in formalin, which may affect the relative position of TSG to the ribs. (III) The observation of TSG in cadaver studies was by naked eyes, while NIR fluorescent thoracoscopy was applied in present study. Kim et al. (5) also reported that the variation rate of G3 and G4 was 40.9% and 81.8%, respectively. However, they indirectly identified the locations of TSG in ordinary thoracoscopy according to the inlet and outlet location of grey and white rami branches which is not exactly equivalent to the locations of TSG.
As a preliminary study, we mainly focus on the anatomical variation rate of G3 and G4. Our results showed that the fluorescent thoracoscopy can clearly show the upper TSG. Most of the G3 and G4 were normal, which is in the 3rd and 4th intercostal space. Over 10% of G3 was varied and shifted downward to the surface of the fourth rib or in the fourth intercostal space, and near one fifth of G4 was varied and shifted downward to the surface of the fifth rib or in the fifth intercostal space. This portion is consistent with data from cadaver studies (4,17-20). This can answer why the ‘same’ procedure like conventional R4 or R5 sympathicotomy resulted in quite variable outcomes, some dry, some overly dry, and some wet.
Both the short-term and long-term therapeutic effects of hands after operation were significantly correlated to the variation of ganglion position based on our consequence. In those whose G3 shifted downward, that is, the surgical resection method is T3 ganglionectomy or T3 sympathicotomy, the degree of palmar dryness is higher than those with normal position of G3, that is, the surgical resection method is T4 sympathicotomy. This is consistent with our hypothesis. But the therapeutic outcome of armpits does not support this conclusion: there was no significant difference between variation sides and normal sides. The possible reason is that the sweat glands of armpit are mainly affected by T5 but not T4. Other anatomical variation of sympathetic trunk may also affect the surgical outcomes (21). Moreover, the sample size may not be adequate to reach the statistical significance to draw this conclusion. Since the sample size estimation is based on the anatomical variation of G3, the study might be unpowered to detect differences between groups. Type II errors may occur when the surgical outcomes and their relationship with the anatomical variation of G4 were analyzed. A further redesigned study with increased sample size may come to a more reliable conclusion. The ultimate goal is to propose a more precise and real T4 or T5 sympathicotomy based on the actual location of the ganglia seen under NIR fluorescent thoracoscopy.
Conclusions
NIR fluorescent thoracoscopy provides clear identification of anatomical variations of TSG during ETS. Anatomical variation of TSG may be a possible reason for the unstable therapeutic effects of ETS for PAH. It provides a theoretical basis to develop a more accurate ETS based on the new technology of fluorescence thoracoscopy in future.
Acknowledgments
Funding: This study was funded by Capital Special Project for Featured Clinical Application of the Beijing Municipal Science and Technology Commission (No. Z181100001718201).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1782/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1782/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1782/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of Peking University People’s Hospital and Beijing Haidian Hospital (No. 2017PHB101-01 and No. 2017028). Individual informed consent was obtained from every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. [Crossref] [PubMed]
- Liu Y, Yang J, Liu J, et al. Surgical treatment of primary palmar hyperhidrosis: a prospective randomized study comparing T3 and T4 sympathicotomy. Eur J Cardiothorac Surg 2009;35:398-402. [Crossref] [PubMed]
- Chang YT, Li HP, Lee JY, et al. Treatment of palmar hyperhidrosis: T(4) level compared with T(3) and T(2). Ann Surg 2007;246:330-6. [Crossref] [PubMed]
- Zhang B, Li Z, Yang X, et al. Anatomical variations of the upper thoracic sympathetic chain. Clin Anat 2009;22:595-600. [Crossref] [PubMed]
- Kim DH, Hong YJ, Hwang JJ, et al. Topographical considerations under video-scope guidance in the T3,4 levels sympathetic surgery. Eur J Cardiothorac Surg 2008;33:786-9. [Crossref] [PubMed]
- Weng W, Liu Y, Zhou J, et al. Thoracoscopic Indocyanine Green Near-Infrared Fluorescence for Thoracic Sympathetic Ganglions. Ann Thorac Surg 2016;101:2394. [Crossref] [PubMed]
- He K, Zhou J, Yang F, et al. Near-infrared Intraoperative Imaging of Thoracic Sympathetic Nerves: From Preclinical Study to Clinical Trial. Theranostics 2018;8:304-13. [Crossref] [PubMed]
- Pei G, Liu Y, Liu Q, et al. The safety and feasibility of intraoperative near-infrared fluorescence imaging with indocyanine green in thoracoscopic sympathectomy for primary palmar hyperhidrosis. Thorac Cancer 2020;11:943-9. [Crossref] [PubMed]
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Etiology and clinical work-up. J Am Acad Dermatol 2019;81:657-66. [Crossref] [PubMed]
- Lin CC, Telaranta T. Lin-Telaranta classification: the importance of different procedures for different indications in sympathetic surgery. Ann Chir Gynaecol 2001;90:161-6. [PubMed]
- Turhan K, Cakan A, Cagirici U. Preserving T2 in thoracic sympathicotomy for palmar hyperhidrosis: less tissue trauma, same effectiveness. Thorac Cardiovasc Surg 2011;59:353-6. [Crossref] [PubMed]
- Mahdy T, Youssef T, Elmonem HA, et al. T4 sympathectomy for palmar hyperhidrosis: looking for the right operation. Surgery 2008;143:784-9. [Crossref] [PubMed]
- Wolosker N, Yazbek G, Ishy A, et al. Is sympathectomy at T4 level better than at T3 level for treating palmar hyperhidrosis? J Laparoendosc Adv Surg Tech A 2008;18:102-6. [Crossref] [PubMed]
- Kim WO, Kil HK, Yoon KB, et al. Influence of T3 or T4 sympathicotomy for palmar hyperhidrosis. Am J Surg 2010;199:166-9. [Crossref] [PubMed]
- Ishy A, de Campos JR, Wolosker N, et al. Objective evaluation of patients with palmar hyperhidrosis submitted to two levels of sympathectomy: T3 and T4. Interact Cardiovasc Thorac Surg 2011;12:545-8. [Crossref] [PubMed]
- Abd Ellatif ME, Hadidi AE, Musa AM, et al. Optimal level of sympathectomy for primary palmar hyperhidrosis: T3 versus T4 in a retrospective cohort study. Int J Surg 2014;12:778-82. [Crossref] [PubMed]
- Singh B, Ramsaroop L, Partab P, et al. Anatomical variations of the second thoracic ganglion. Surg Radiol Anat 2005;27:119-22. [Crossref] [PubMed]
- Chung IH, Oh CS, Koh KS, et al. Anatomic variations of the T2 nerve root (including the nerve of Kuntz) and their implications for sympathectomy. J Thorac Cardiovasc Surg 2002;123:498-501. [Crossref] [PubMed]
- Liu Y, Shi X, Yu E, et al. Applicative anatomy study of upper thoracic sympathetic trunk for clinical sympathictomy. Chin J Thorac Cardiovasc Surg 2005;21:75-7.
- Street E, Ashrafi M, Greaves N, et al. Anatomic Variation of Rami Communicantes in the Upper Thoracic Sympathetic Chain: A Human Cadaveric Study. Ann Vasc Surg 2016;34:243-9. [Crossref] [PubMed]
- Filion W, Lamb C. Anatomical variation of the sympathetic trunk and aberrant rami communicantes and their clinical implications. Ann Anat 2023;245:151999. [Crossref] [PubMed]


