
Outcomes after thymectomy in non-thymomatous myasthenia gravis
Highlight box
Key findings
• Patients who undergo thymectomy for non-thymomatous myasthenia gravis (NTMG) benefit from a decrease in rescue therapy requirements postoperatively. Thymectomy for NTMG appears to be underutilized.
What is known and what is new?
• Thymectomy leads to a clinical improvement in patients with NTMG as demonstrated in a randomized controlled trial. Here, we report an associated reduction in rescue therapy costs following surgery as well as the acceptable morbidity following thymectomy in this database.
What is the implication, and what should change now?
• In a selected population, thymectomy affords a clear benefit to patients with NTMG and should be considered more often in these patients to minimize use of rescue therapies and possibly improve long-term outcomes.
Introduction
Myasthenia gravis (MG) is a chronic neuromuscular disorder that can significantly affect a patient’s functional status and quality of life, often necessitating costly recurring treatments (1,2). Patients with symptomatic MG are initially managed with pyridostigmine. Step-up treatment strategies involve corticosteroids and nonsteroidal immunosuppressive agents (NSIS) (3) which are associated with significant side effects and adverse reactions (4). In refractory cases, rescue therapies such as intravenous immunoglobulin and plasmapheresis can lead to meaningful improvement (3).
Prior studies demonstrated that patients with thymomas have increased rates of MG, and thus benefited greatly from thymectomy (5,6). More recently, the Thymectomy Trial in Non-Thymomatous Myasthenia Gravis Patients Receiving Prednisone Therapy (MGTX) reported that patients with non-thymomatous MG (NTMG) undergoing thymectomy plus alternate-day prednisone have reduced disease severity and lower steroid requirements, as well as less need for NSIS, compared to those treated with prednisone alone (7,8). National and international guidelines have subsequently adopted recommendations for consideration of thymectomy in patients 18 to 50 years old based on the evidence from this trial (9-12).
Although the benefits of thymectomy were considerable in MGTX, little is known as to how this evidence has translated into clinical practice more broadly and within a national dataset. Moreover, studies evaluating the association between surgical resection and NTMG treatment costs are lacking but certainly merit attention given the chronic nature of this disease. Our objectives were to (I) report the utilization of thymectomy within NTMG patients and (I) investigate short-term treatment needs, surgical outcomes, and costs before and after surgery. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1589/rc).
Methods
Data source
Data were collected from the Optum de-identified Clinformatics Data Mart Claims (CDM) from January 2007 to March 2021. CDM is a commercial insurance database that covers approximately 17–19 million patients annually. International Classification of Diseases (ICD) codes and Current Procedural Terminology (CPT) codes, as well as pharmaceutical generic and brand names, were used to identify the study cohort and service utilization. NTMG-related costs were calculated from the CDM standard price, which reflects the allowed payment for all provider services. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was exempt from approval and informed consent by the institutional review board for use of de-identified data.
Patient population, and operative characteristics
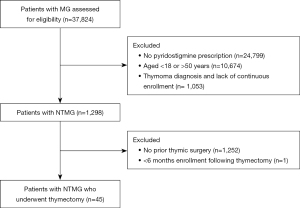
Patients aged 18–50 years with a claim for MG were identified. To avoid errant claims for MG, only those with a prescription for pyridostigmine within 3 months of the MG diagnosis were included. Additionally, a washout period of 12-month before the MG diagnosis was applied. Only patients who had no diagnosis of thymoma and underwent thymectomy (identified by CPT codes) within 12 months of MG diagnosis were included. To ensure the completeness of information from claims, continuous enrollment during the entire study period, i.e., 12 months prior to and after the MG diagnosis including 6 months post-thymectomy, was required (Figure 1). Operative approach (thoracotomy or thoracoscopy), and hospital length of stay were also recorded, as were post-operative complications (13), emergency department (ED) visits, and hospital admission in the 90 days following the operative date. Patients with unknown length of stay after thymectomy were excluded from the comparison analysis of length of stay, postoperative ED visits and hospital readmissions.
Study period and outcomes
The study period covered the 6-month duration before and after the date of thymectomy. Outcomes were use of steroids, NSIS, and rescue therapies, including plasmapheresis and immunoglobulin. Costs associated with these treatments were defined as the costs of treatment-associated claims incurred during the study period and standardized to the 2020 US dollar. Inpatient costs associated with surgery were excluded. ED visits and hospital admissions were also identified if a claim indicated NTMG as a reason for the visit (see Appendix 1 for formulations and codes).
Statistical analysis
As a paired study design, the rate of different therapies between the 6 months preceding and following thymectomy were compared using a McNemar’s test. Also, due to the skewed distribution, all the medical costs related to NTMG management between the pre- and post-period were compared using Wilcoxon Signed Rank test. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). All P values reported are two-sided with significance considered <0.05.
Results
Patient and operative characteristics
There were 1,298 patients identified from the CDM Database, aged 18–50, who received pyridostigmine for a diagnosis of NTMG. Among them, 45 patients (3.47%) underwent thymectomy and had a 6-month follow-up observation period (Figure 1). Thymectomy was performed using minimally invasive surgery (MIS) in just over half of patients (n=24, 53.33%) with the remainder undergoing an open approach (n=21, 46.67%). The hospital length of stay associated with MIS (mean =2.71 days) was significantly shorter than with open thymectomy (mean =4.17 days, P=0.027) (Table 1). Two (4.44%) patients who underwent open thymectomy had a readmission within 30 days of their surgery. The length of stay for both post-operative readmissions was 2 days. Among patients resected with a MIS approach, no readmissions were observed in the 90 days post-surgery.
Table 1
| Operative characteristics | Operative approach | |
|---|---|---|
| Open* | MIS | |
| Number of patients, n (%) | 21 (46.67) | 24 (53.33) |
| Average LOS (days) | 4.17 | 2.71 |
| Post-operative hospital readmission**, n (%) | 2 (9.52) | 0 (0.00) |
*, 3 patients were excluded from the calculations of LOS and post-operation readmission due to missing of LOS information; **, post-operative readmission observed over the 90 days following resection. MIS, minimally invasive surgery; LOS, length of stay.
Use of pharmacotherapy and rescue therapies
During the 6 months prior to thymectomy, 53.33% (n=24) of the cohort was prescribed steroids, and 4.44% (n=2) required NSIS. In the 6 months following thymectomy, steroid use increased to 66.67% (n=30, P=0.034), while the rate of NSIS remained stable at 2.22% (n=1, P=0.564). Regarding rescue therapies, in the 6 months prior to resection 44.44% (n=20) of patients received plasmapheresis or intravenous immunoglobulin. Following surgery, use of these rescue therapies declined to 24.44% (n=11, P=0.007) in the 6 months post-operatively (Table 2).
Table 2
| Management modality and cost | Study period | P value | |
|---|---|---|---|
| 6 months prior to thymectomy | 6 months following thymectomy | ||
| Steroid use, n (%) | 24 (53.33) | 30 (66.67) | 0.034 |
| NSIS use, n (%) | 2 (4.44) | 1 (2.22) | 0.564 |
| Rescue therapy use*, n (%) | 20 (44.44) | 11 (24.44) | 0.007 |
| Costs of steroids ($) | 0.317 | ||
| Mean (SD) | 16.95 (25.57) | 22.34 (33.94) | |
| Median (IQR) | 5.59 (29.42) | 7.17 (32.26) | |
| Costs of NSIS ($) | 0.500 | ||
| Mean (SD) | 78.35 (510.49) | 1.91 (12.84) | |
| Median (IQR) | 0.00 (0.00) | 0.00 (0.00) | |
| Costs of rescue therapies ($) | 0.035 | ||
| Mean (SD) | 13,243.98 (20,438.60) | 8,486.26 (21,941.92) | |
| Median (IQR) | 0.00 (24,385.00) | 0.00 (0.00) | |
| ED visits**, n (%) | 9 (21.43) | 6 (14.29) | 0.257 |
| Hospital admissions**, n (%) | 9 (21.43) | 4 (9.52) | 0.096 |
*, rescue therapies: defined as use of plasmapheresis or intravenous immunoglobulin; **, 3 patients were excluded from the analysis due to missing of the length of stay for the thymectomy. NSIS, nonsteroidal immunosuppressant; SD, standard deviation; IQR, interquartile range; ED, emergency department.
Costs of NTMG care
In an effort to evaluate the financial implications of thymectomy, NTMG-related treatment costs were evaluated before and after surgery. The average cost of steroid use prior to thymectomy was $16.95 (median =$5.59), and this was similar to the costs following resection with a mean cost of $22.34 (median =$7.17, P=0.317). The mean cost of NSIS was $78.35 (median =$0.00) preceding thymectomy, and remained stable following surgery (mean =$1.91; median =$0.00, P=0.500). The mean cost of rescue therapy was $13,243.98 (median =$0.00) prior to resection, and significantly decreased to $8,486.26 (median =$0.00, P=0.035) following surgery (Table 2).
ED visits and hospital admissions
There were 9 (21.43%) patients who had ED visits for management of their NTMG prior to thymectomy, compared to 6 (14.29%) patients requiring emergency care following resection (P=0.257). NTMG-related hospital admission occurred in 9 (21.43%) patients prior to thymectomy, and in 4 (9.52%) patients after resection (P=0.096) (Table 2).
Discussion
In this analysis of a large national claims database, we found that few patients with NTMG underwent thymectomy despite guidelines and clinical trial evidence suggesting benefit in this patient population. Patients who did undergo resection experienced a significant reduction in the need for rescue therapies in the 6 months following surgery, albeit with an increase in steroid use postoperatively. Additionally, surgery was associated with a decrease in NTMG-related treatment costs. Compared to patients who underwent an open surgery, those resected with a minimally-invasive approach had a shorter inpatient stay after thymectomy.
While thymic resection is reported to improve long-term post-operative steroid needs and remission rates (14), the possibility of myasthenic crises and symptom flare resulting from surgical stress is well documented (15). This may explain the increased use of steroids observed in our study during the short-term post-operative period. These findings are similar to those reported by Wolfe and colleagues who also found an increased need for steroids following thymectomy (7).
Overall, the rate of operative management in this cohort remains low. Our study period included years prior to the incorporation of thymectomy into evidence-based guidelines and this may be explanatory (7,8). However, additional considerations may include inability to access experienced thoracic surgeons, concerns regarding surgical morbidity, and lack of awareness of the disease-specific benefits associated with resection from both a provider and payer perspective. The increased adoption of minimally-invasive surgical techniques for thymic disease (16), and the associated reduction in pain and morbidity with these approaches (17), may mitigate previous concerns regarding the use of surgery for disease control in NTMG. Finally, as many studies within medicine have noted, randomized trial and guideline-based evidence often takes several years to affect “real world” clinical practice (18,19). In this regard, NTMG patients may not be referred in a timely manner by primary care physicians and neurologists, reflecting the lags seen more broadly when translating evidence into practice.
Though our analysis highlights several important findings, including the cost implications of thymectomy, a reduction in use of rescue therapies postoperatively, and the sporadic use of surgery overall within NTMG patients, there were several notable limitations. First, we included only patients within a specific insurance claims database, potentially limiting the generalizability of these results. Second, given the limited sample size, we restricted our analysis to the period immediately prior to and after surgery. However, a long-term analysis in a larger population would be valuable. Finally, quality of life metrics and direct assessments of MG severity are not reported in this study because such information is not captured in claims data.
Conclusions
In conclusion, in patients with NTMG between the ages of 18 and 50, thymectomy appears underutilized despite safe perioperative outcomes and a significant reduction in the need for rescue therapies postoperatively.
Acknowledgments
The authors would like to acknowledge the generous philanthropic contributions from the Mason Family Research Fund.
Funding: This work was supported by the generous philanthropic contributions from the Mason Family Research Fund. YCTS acknowledges funding from the National Cancer Institute (NCI) (No. R01CA207216).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1589/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1589/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1589/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1589/coif). YCTS received funding from National Cancer Institute (NCI) (No. R01CA207216), and consulting fees, travel, and accommodations in 2019 for serving on a grants review panel for Pfizer Inc and an advisory board for AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was exempt for approval and informed consent by the institutional review board for use of de-identified data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Omorodion JO, Pines JM, Kaminski HJ. Inpatient cost analysis for treatment of myasthenia gravis. Muscle Nerve 2017;56:1114-8. [Crossref] [PubMed]
- Alekseeva TM, Gavrilov YV, Kreis OA, et al. Fatigue in patients with myasthenia gravis. J Neurol 2018;265:2312-21. [Crossref] [PubMed]
- Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016;87:419-25. [Crossref] [PubMed]
- Bacci ED, Coyne KS, Poon JL, et al. Understanding side effects of therapy for myasthenia gravis and their impact on daily life. BMC Neurol 2019;19:335. [Crossref] [PubMed]
- Taioli E, Paschal PK, Liu B, et al. Comparison of Conservative Treatment and Thymectomy on Myasthenia Gravis Outcome. Ann Thorac Surg 2016;102:1805-13. [Crossref] [PubMed]
- Bulkley GB, Bass KN, Stephenson GR, et al. Extended cervicomediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg 1997;226:324-34; discussion 334-5. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol 2019;18:259-68. [Crossref] [PubMed]
- Narayanaswami P, Sanders DB, Wolfe G, et al. International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology 2021;96:114-22. [Crossref] [PubMed]
- Gronseth GS, Barohn R, Narayanaswami P. Practice advisory: Thymectomy for myasthenia gravis (practice parameter update): Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2020;94:705-9. [Crossref] [PubMed]
- Kim SW, Choi YC, Kim SM, et al. Effect of thymectomy in elderly patients with non-thymomatous generalized myasthenia gravis. J Neurol 2019;266:960-8. [Crossref] [PubMed]
- Zhang J, Chen Y, Zhang H, et al. Effects of thymectomy on late-onset non-thymomatous myasthenia gravis: systematic review and meta-analysis. Orphanet J Rare Dis 2021;16:232. [Crossref] [PubMed]
- The Society of Thoracic Surgeons' General Thoracic Registry Training Manual 2022 3/2/2022. Available online: https://www.sts.org/sites/default/files/Database%20Manuals/Training%20Manual%20V4_20_2%20April%202022.pdf
- Cataneo AJM, Felisberto G Jr, Cataneo DC. Thymectomy in nonthymomatous myasthenia gravis - systematic review and meta-analysis. Orphanet J Rare Dis 2018;13:99. [Crossref] [PubMed]
- Geng Y, Zhang H, Wang Y. Risk factors of myasthenia crisis after thymectomy among myasthenia gravis patients: A meta-analysis Medicine (Baltimore) 2020;99:e18622. [published correction appears in Medicine (Baltimore) 2022 Jun 03;101(22):e29480]. [Crossref] [PubMed]
- Salfity HV, Timsina L, Ceppa DP, et al. Minimally invasive surgery in the management of resectable thymoma: a retrospective analysis from the National Cancer Database. J Thorac Dis 2021;13:6353-62. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011;104:510-20. [Crossref] [PubMed]
- Rodríguez-Lopéz JL, Ling DC, Heron DE, et al. Lag Time Between Evidence and Guidelines: Can Clinical Pathways Bridge the Gap? J Oncol Pract 2019;15:e195-201. [Crossref] [PubMed]


