
Midterm outcomes of isolated tricuspid valve surgery with a mini-thoracotomy and beating heart strategy
Highlight box
Key findings
• Mini-thoracotomy beating heart strategy for isolated tricuspid valve (TV) surgery demonstrated favorable early and midterm outcomes.
What is known and what is new?
• Conventional isolated tricuspid valve interventions carry high morbidity and mortality risks.
• This study demonstrates superior early and midterm outcomes with a minimally invasive beating heart approach.
What is the implication, and what should change now?
• Our findings demonstrate that this approach offers a superior option in properly selected patients and should be considered.
Introduction
Isolated tricuspid valve (TV) surgery is known to have high mortality and morbidity rates, reaching up to 10% and 31%, respectively, and thus it is not frequently performed (1,2). As with other valvular heart diseases, a timely surgical referral is paramount in TV disease. However, as stated above, early surgical treatment is rarely carried out, resulting in delays and an even further elevated preoperative risk (2,3).
Conventional TV surgery with median sternotomy and arrested heart strategy may predispose major postoperative complications such as myocardial ischemia and bleeding (4). In this regard, minimally invasive (mini-thoracotomy) beating heart surgery is expected to have several advantages over conventional TV surgery: (I) excellent myocardial protection with no myocardial ischemic time and (II) less bleeding due to no sternotomy with minimal tissue dissection. Our study aims to evaluate the outcomes of the isolated TV operation with a mini-thoracotomy beating heart strategy. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1868/rc).
Methods
Study population
A total of 484 patients underwent TV operations in our institution from January 2017 to May 2021. Among them, 31 patients underwent a beating heart TV operation; 2 patients with median sternotomy and 4 with concomitant surgeries were excluded. Finally, the remaining 25 patients operated by 2 surgeons were enrolled for evaluation (Figure S1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Bucheon Sejong Hospital Institutional Review Board, which waived the requirement for patient consent (IRB No. 2022-03-007, approval date April 11, 2022).
Operative techniques
After general anesthesia, a double-lumen endotracheal tube was intubated for single-lung ventilation. A 5-Fr introducer sheath (Radifocus Introducer II, Terumo Medical Corp., Tokyo, Japan) was inserted into the right internal jugular vein for the following venous drain catheter insertion. A transesophageal echocardiography (TEE) probe was inserted, and the patient was positioned supine with the right chest elevated approximately 30°. After sterile draping, a mini-thoracotomy incision was made in the right 4th intercostal space (ICS) at the anterior- to mid-axillary line along the submammary crease. A soft tissue retractor (Alexis Wound Protector/Retractor, Applied Medical Resources Group, CA, USA) was placed. If needed, a 10-mm trocar was inserted in the 5th ICS on the midaxillary line for the thoracoscope. The pleural cavity was flooded with carbon dioxide gas. Normothermic cardiopulmonary bypass (CPB) with vacuum-assisted venous drainage was established by cannulating the right internal jugular vein and the femoral vessels with a 2-cm-long oblique groin incision. Right internal jugular vein cannulation was performed with Seldinger’s technique by inserting a guidewire through the pre-inserted introducer sheath. The pericardium was opened longitudinally, 2–3 cm anterior to the phrenic nerve. After pericardial tenting, the vena cavae were snared or clamped with bulldog clamps. The vena cavae were packed with gauze sponges instead of snaring when appropriate. The right atrium (RA) was opened with standard oblique atriotomy, and TV was exposed using the Adams-Yozu atrial retractor (Geister, Tuttlingen, Germany) inserted through the mini-thoracotomy. TV procedures were performed on the beating heart with standard techniques (Video 1). After weaning from the CPB, the TV repair or replacement result was evaluated with intraoperative TEE.
Perioperative echocardiographic assessment and follow-up
Per the current guidelines, the degree of tricuspid regurgitation (TR) was assessed using qualitative and semiquantitative methods. The severity of TR was graded on a scale from 0 to 3 (0, no or minimal; 1, mild; 2, moderate; 3, severe) (5). The tricuspid annulus diameter was measured in the transthoracic apical 4-chamber view in late diastole at the time of maximal TV opening (6). The modified Bernoulli equation was used to calculate the trans-tricuspid pressure gradient (TTPG) from the maximal TR velocity measured by continuous-wave Doppler (7). Tricuspid annular plane systolic excursion (TAPSE) was estimated by measuring the distance of systolic excursion of the right ventricle (RV) annular segment along its longitudinal plane from a standard apical 4-chamber window (8). The RV systolic excursion velocity (S') was obtained by Doppler tissue imaging at an apical 4-chamber view highlighting the RV free wall (8). Estimated pulmonary artery systolic pressure (ePASP) was determined from peak TR jet velocity using the simplified Bernoulli equation combining the estimated RA pressure (8). All patients underwent transthoracic echocardiography at discharge. Follow-up echocardiography was performed as appropriate.
Statistical analysis
Descriptive statistics for the total study population were obtained. Categorical variables are presented as numbers and percentages. Continuous variables are presented as median and Q1–Q3. The distribution of a time-to-event outcome was estimated using Kaplan-Meier curves. Rates of missingness for data in our models were <1%, and no imputation was performed for missing data. All statistical analysis was carried out using R 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics and echocardiographic assessments
The median age was 65.0 years (Q1–Q3, 59.0–72.0); 18 patients (72.0%) had previous conventional cardiac surgery, including previous TV repair (n=4, 16.0%) and TV replacement (n=4, 16.0%). The median EuroSCORE II value was 3.4% (Q1–Q3, 2.1–6.8%). TV pathologies were functional (n=8), rheumatic (n=6), degenerative (n=5), prosthetic valve failure (n=4), pacemaker-related (n=2), and traumatic (n=1). Among the 25 enrolled patients, TV repair was performed in 16 patients (64.0%) and TV replacement in 9 (36.0%). There was no sternotomy conversion. Baseline patient characteristics are described in Table 1.
Table 1
| Characteristics | Values |
|---|---|
| Age, years | 65.0 [59.0–72.0] |
| Female | 13 (52.0) |
| NYHA class 3–4 | 12 (48.0) |
| Hypertension | 6 (24.0) |
| Diabetes mellitus | 4 (16.0) |
| Atrial fibrillation | 12 (48.0) |
| COPD | 1 (4.0) |
| Chronic kidney disease | 3 (12.0) |
| EuroSCORE, % | 3.4 [2.1–6.8] |
| Previous cardiac surgery | 18 (72.0) |
| Previous TV repair | 4 (16.0) |
| Previous TV replacement | 4 (16.0) |
| Type of operation | |
| TV repair | 16 (64.0) |
| TV replacement | 9 (36.0) |
| CPB time, min | 75.0 [61.0–98.0] |
Data are presented as median [Q1–Q3] or n (%). NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; TV, tricuspid valve; CPB, cardiopulmonary bypass.
Table 2 demonstrates the preoperative echocardiographic parameters. The median left ventricular ejection fraction was 55.0% (Q1–Q3, 52.0–58.0%), and 22 patients (88.0%) had severe TR. The median RA and RV diameters were 74.0 mm (Q1–Q3, 64.0–87.0) and 50.0 mm (Q1–Q3, 41.0–58.0), respectively. The median TAPSE was 15.2 mm (Q1–Q3, 12.0–17.9), and RV S' was 9.9 cm/s (Q1–Q3, 8.5–11.3).
Table 2
| Parameters | Values |
|---|---|
| LVEF, % | 55.0 [52.0–58.0] |
| Severe TR | 22 (88.0) |
| RA diameter, mm | 74.0 [64.0–87.0] |
| RV diameter, mm | 50.0 [41.0–58.0] |
| TAPSE, mm | 15.2 [12.0–17.9] |
| RV S', cm/s | 9.9 [8.5–11.3] |
| ePASP, mmHg | 33.0 [29.5–38.4] |
Data are presented as median [Q1–Q3] or n (%). LVEF, left ventricular ejection fraction; TR, tricuspid regurgitation; RA, right atrium; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; S', systolic excursion velocity; ePASP, estimated pulmonary artery systolic pressure.
Clinical and echocardiographic outcomes
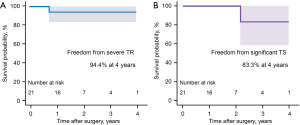
The median lengths of stay in the intensive care unit and hospital were 1.0 day (Q1–Q3, 1.0–2.0) and 9.0 days (Q1–Q3, 6.0–18.0), respectively. Early mortality (<30 days) occurred in 1 patient (4.0%) due to low cardiac output syndrome requiring mechanical circulatory support. There was no reoperation for bleeding. Permanent pacemaker insertion was required in 1 TV replacement patient (4.0%) who previously underwent (I) coronary bypass, maze procedure, mitral and TV repair, and (II) redo mitral replacement. Other early morbidities are described in Table 3. Follow-up echocardiography was available in 20 patients (median follow-up time, 31.3 months (Q1–Q3, 19.4–43.8); median last echocardiography follow-up time, 22.1 months (Q1–Q3, 12.6–27.3)]. Kaplan-Meier freedoms from all-cause mortality, severe TR, and significant tricuspid stenosis (i.e., TTPG ≥5 mmHg) were, respectively, 89.1%, 94.4%, and 83.3% at 4 years (Figures 1,2). There was no TV reoperation.
Table 3
| Variables | Values |
|---|---|
| ICU stay, days | 1.0 [1.0–2.0] |
| Hospital stay, days | 9.0 [6.0–18.0] |
| Complications | |
| Reoperation for bleeding | 0 (0.0) |
| Acute kidney injury requiring dialysis | 3 (12.0) |
| LCOS | 1 (4.0) |
| Pneumonia | 4 (16.0) |
| PPM insertion | 1 (4.0) |
| Early death (<30 days) | 1 (4.0) |
Data are presented as median [Q1–Q3] or n (%). ICU, intensive care unit; LCOS, low cardiac output syndrome; PPM, permanent pacemaker.
Discussion
The present study demonstrated favorable early and midterm outcomes of the isolated TV surgery with a mini-thoracotomy beating heart strategy.
Patients with isolated TV disease have previously been considered to have poor outcomes after TV surgery, and there are several relevant studies: Axtell et al. (9) evaluated 3,276 patients with isolated TR comparing the TV surgery group (n=171) and the medical therapy group (n=3,105). According to the study, there was no difference in overall survival between the groups in the matched analysis. In a study conducted by Zack et al. (10) analyzing the National Inpatient Sample of the United States from 2004 to 2013, the in-hospital mortality of 5,005 patients undergoing isolated TV operation was 8.8% and did not vary across the study period. Another investigation by Dreyfus et al. (1) demonstrated the result of isolated TV surgeries of 5,661 patients. In the cohort, in-hospital mortality and major complication rates were 10% and 31%, respectively. These relatively higher mortality and morbidity rates prohibit physicians from referring patients to surgery on time (2,11). As a result, delays in referral for surgery worsen the postoperative outcomes (12).
Possible conventional TV surgery-related factors responsible for poor postoperative outcomes are myocardial ischemia associated with cardioplegic arrest and mediastinal/sternal bleeding (13-16). A benefit of our strategy is that myocardial ischemia can be minimized by performing the surgery without cross-clamping the aorta. In the absence of patent foramen ovale or atrial/ventricular septal defects, TV surgery is performed safely without the risk of systemic air embolism. This strategy also enables an easier second pump run when an additional procedure is required; the only process needed for reoperation is opening the RA again with the caval occlusions without the risk of aortic clamp injury (Video 2). Furthermore, the right mini-thoracotomy provides excellent TV exposure (“en face view”) without distorting the axis (Video 1).
Meanwhile, the bleeding issue is highlighted in cardiac cirrhosis, which is not uncommon among TR patients. Compared with median sternotomy, the right mini-thoracotomy approach can minimize bleeding for the following reasons: (I) no bone cutting and (II) no mediastinal and RV dissection, especially in redo cases. Even with severe pericardial adhesions, the adhered pericardium and RA can be opened en bloc without adhesiolysis using our approach.
In addition, during the TV procedure, beating heart surgery allows real-time monitoring of conduction disturbances, such as atrioventricular block. A surgeon can modify the procedure immediately when the rhythm issue occurs, and this can minimize the postoperative permanent pacemaker insertion. It is supported by the fact that, in the present study, we had only 1 (4.0%) case of permanent pacemaker insertion in a TV replacement patient with a previous history of multiple valvular and coronary surgeries.
For these reasons, we can expect a better postoperative result by combining the right mini-thoracotomy and beating heart strategy. This assumption is advocated by several investigations reporting the favorable outcomes of isolated TV surgery with minimally invasive or beating heart strategy. Ricci et al. (17) analyzed 64 patients at high risk (EuroSCORE II, 7.3%±2.9%) who underwent minimally invasive TV surgery. In the study, 33 patients (51.5%) underwent beating heart surgery. The early mortality and pacemaker implantation rates were 7.9% and 1.6%, respectively, with a 5-year survival rate of 81.3%. Pfannmüller et al. (18) demonstrated 48 patients who underwent isolated TV operations (beating heart surgery, 87.5%) after previous cardiac surgery. The 30-day mortality rate was 0% for elective patients and 4.2% (n=2) for urgent and emergent cases. Postoperative pacemaker insertion was needed in 5 patients (10.4%). The 5-year survival rate was 72.2%±10.0% in patients who underwent elective reoperative TV surgery through minimally invasive access. In a multicenter study about isolated TV surgery by Russo et al. (19), there were fewer acute renal failures and strokes in the beating heart strategy compared with the arrested heart strategy. In the beating heart group, 30-day mortality was 5%; the 6-year survival and freedom from cardiac death were 78%±5% and 84%±4%, respectively. The 6-year composite cardiac endpoint of cardiac death and reoperation rate was worse in the arrested heart TV surgery group than the beating heart TV surgery group (P=0.024). Our study included patients who underwent TV operations using both minimally invasive and beating heart strategies. It demonstrated favorable early outcomes [early mortality, 4% (n=1); permanent pacemaker implantation, 4% (n=1)], and mid-term survival was 89.1% at 4 years [median follow-up, 30.3 months (Q1–Q3, 19.2–43.8)].
Lastly, timely surgery is also essential to improve surgical outcomes. The study by Kawsara et al. (12) analyzing 1,513 patients from the Nationwide Readmissions Database of the United States who underwent isolated TV surgery demonstrated that late referral (defined by acute heart failure decompensation, nonelective surgery status, or advanced liver disease) was the strongest predictor of in-hospital mortality (odds ratio 4.75, 95% CI: 2.74–8.25, P<0.001). Oh et al. (20) demonstrated the analysis of 131 patients with isolated TR (38 early surgery vs. 93 conservative treatments) in their prospective registry. They showed that adverse event-free survival was better in the early surgery group than in the conservative group. In another relevant study, Dreyfus et al. (21) analyzed 241 patients who underwent isolated TV surgery from a French Nationwide Database. The analysis revealed that congestive heart failure was the determinant of in-hospital mortality and major complication rates (both P=0.01). Due to high mortality and morbidity rates observed in the late surgical referral population, they concluded that early surgery might improve outcomes. Patients in our study showed relatively preserved RV function [median TAPSE, 15.2 mm (Q1–Q3, 12.0–17.9); median RV S', 9.9 cm/s (Q1–Q3, 8.5–11.3)], which reflects the early surgical referral. This factor might impact the favorable outcomes of the present study, as with the investigations above.
Our study has several limitations. First, it is a retrospective, non-randomized study in a single institution. Even though isolated TV surgery was the primary indication for the right mini-thoracotomy beating heart surgery, as surgeons decide the operative strategy, individual experience, anatomical factors, and surgical risk may have influenced the patient selection. Therefore, selection bias or unidentified confounding bias may have influenced the results. Second, our study is a single-arm study with no comparison groups, and it warrants further investigations comprising conventional TV operations performed with sternotomy or arrested heart.
Conclusions
In conclusion, the mini-thoracotomy beating heart strategy for isolated TV surgery showed favorable early and mid-term outcomes, even in high-risk patients requiring reoperations. This strategy may be a valuable option for isolated TV operations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1868/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1868/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1868/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1868/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Bucheon Sejong Hospital Institutional Review Board, which waived the requirement for patient consent (IRB No. 2022-03-007, approval date April 11, 2022).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dreyfus J, Flagiello M, Bazire B, et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J 2020;41:4304-17. [Crossref] [PubMed]
- Delgado V, Ajmone Marsan N, Bax JJ. The difficult decision of when and in whom to perform isolated tricuspid valve surgery. Eur Heart J 2020;41:4318-20. [Crossref] [PubMed]
- Lee JW, Song JM, Park JP, et al. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J 2010;74:375-80. [Crossref] [PubMed]
- Jeganathan R, Armstrong S, Al-Alao B, et al. The risk and outcomes of reoperative tricuspid valve surgery. Ann Thorac Surg 2013;95:119-24. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Foale R, Nihoyannopoulos P, McKenna W, et al. Echocardiographic measurement of the normal adult right ventricle. Br Heart J 1986;56:33-44. [Crossref] [PubMed]
- Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1-23; quiz 101-2. [Crossref] [PubMed]
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-788. [Crossref] [PubMed]
- Axtell AL, Bhambhani V, Moonsamy P, et al. Surgery Does Not Improve Survival in Patients With Isolated Severe Tricuspid Regurgitation. J Am Coll Cardiol 2019;74:715-25. [Crossref] [PubMed]
- Zack CJ, Fender EA, Chandrashekar P, et al. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J Am Coll Cardiol 2017;70:2953-60. [Crossref] [PubMed]
- Cook CC, Salman M, Wei LM, et al. Balancing risk versus reward in isolated repair of severe tricuspid regurgitation. J Thorac Cardiovasc Surg 2018;156:658-9. [Crossref] [PubMed]
- Kawsara A, Alqahtani F, Nkomo VT, et al. Determinants of Morbidity and Mortality Associated With Isolated Tricuspid Valve Surgery. J Am Heart Assoc 2021;10:e018417. [Crossref] [PubMed]
- Jacob KA, Hjortnaes J, Kranenburg G, et al. Mortality after cardiac surgery in patients with liver cirrhosis classified by the Child-Pugh score. Interact Cardiovasc Thorac Surg 2015;20:520-30. [Crossref] [PubMed]
- Hanedan MO, Çiçekçioğlu F, Aksöyek A, et al. Tricuspid Valve Replacement Through Right Thoracotomy has Better Outcomes in Redo Cases. Heart Lung Circ 2017;26:88-93. [Crossref] [PubMed]
- Kim DC, Chee HK, Song MG, et al. Comparative analysis of thoracotomy and sternotomy approaches in cardiac reoperation. Korean J Thorac Cardiovasc Surg 2012;45:225-9. [Crossref] [PubMed]
- Wahba A, Milojevic M, Boer C, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg 2020;57:210-51. [PubMed]
- Ricci D, Boffini M, Barbero C, et al. Minimally invasive tricuspid valve surgery in patients at high risk. J Thorac Cardiovasc Surg 2014;147:996-1001. [Crossref] [PubMed]
- Pfannmüller B, Misfeld M, Borger MA, et al. Isolated reoperative minimally invasive tricuspid valve operations. Ann Thorac Surg 2012;94:2005-10. [Crossref] [PubMed]
- Russo M, Di Mauro M, Saitto G, et al. Beating Versus Arrested Heart Isolated Tricuspid Valve Surgery: Long-term Outcomes. Ann Thorac Surg 2022;113:585-92. [Crossref] [PubMed]
- Oh JK, Lee S, Ji H, et al. Outcomes of Early Surgery in Patients With Isolated Severe Tricuspid Regurgitation: A Prospective Registry Data. JACC Cardiovasc Interv 2020;13:2086-7. [Crossref] [PubMed]
- Dreyfus J, Ghalem N, Garbarz E, et al. Timing of Referral of Patients With Severe Isolated Tricuspid Valve Regurgitation to Surgeons (from a French Nationwide Database). Am J Cardiol 2018;122:323-6. [Crossref] [PubMed]



