
Clinical value of peripheral blood circulating tumor DNA in predicting the efficacy of immunotherapy for non-small cell lung cancer
Highlight box
Key findings
• Circulating tumor DNA (ctDNA) levels are valuable in predicting the efficacy of immunotherapy in non-small cell lung cancer (NSCLC) patients.
What is known and what is new?
• A single biological indicator, such as programmed cell death 1–ligand 1 (PD-L1), can no longer be used to screen the patients that would benefit from immunotherapy, as it cannot meet the urgent needs of precision immunotherapy in tumor patients.
• CtDNA <3.72 ng/µL can be used to predict which NSCLC patients will achieve objective remission after immunotherapy.
What is the implication, and what should change now?
• CtDNA levels are valuable in predicting the efficacy of immunotherapy in NSCLC patients, and the use of ctDNA to guide immunotherapy may help to improve the outcomes of NSCLC patients.
Introduction
Lung cancer is one of the most common malignant tumors worldwide, second only to breast cancer (1). Nearly 85% of lung cancer patients have non-small cell lung cancer (NSCLC), among which adenocarcinoma and squamous cell carcinoma are the most common subtypes (2-4). The most common epidemiologically pathogenic factor of lung cancer is smoking (5,6). In the past 20 years, the emergence of multiple targeted therapies and the effective application of immunotherapy in some patients with advanced NSCLC have brought hope to the field of lung cancer treatment (7-9).
Immune checkpoint inhibitors (ICIs) produce a lasting anti-tumor immune response by inhibiting the programmed death cell protein-1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 on the surface of the T cells. The Food and Drug Administration (FDA) has approved 4 ICIs for the treatment of NSCLC (i.e., nivolumab, pembrolizumab, atezolizumab, and durvalumab). In addition, a variety of immune combination regimens have been approved by the FDA for the treatment of patients with NSCLC. However, NSCLC patients treated with anti-PD-1/programmed cell death 1-ligand 1 (PD-L1) have a response rate of only 30% (10-12).
Because immunotherapy does not benefit most lung cancer patients and immunotherapy drugs are also very expensive, the identification of reliable markers that can accurately predict the efficacy of immunotherapy has important clinical value and significance (10-12). The detection of PD-L1 expression in tumor tissue samples by immunohistochemistry has proven to be a relatively reliable biological indicator. However, in NSCLC patients with positive PD-L1 expression, the response rate to anti-PD-1/PD-L1 therapy is still only 15.6% to 48% (10-12). Additionally, some patients with negative PD-L1 expression can also benefit from anti-PD-1/PD-L1 immunotherapy. Thus, a single biological indicator, such as PD-L1, can no longer be used to predict treatment efficacy, as it cannot meet the urgent needs of precision immunotherapy in tumor patients.
Circulating tumor DNA (ctDNA) is a small piece of DNA derived from necrotic tumor cells in the peripheral blood, which is tumor-specific. The detection of ctDNA by second-generation gene sequencing provides doctors with biological information about tumors that can be used for early diagnosis and individualized treatment (13-16). Previous studies also showed that ctDNA may be helpful in predicting the immunotherapy efficacy in NSCLC patients (17-20). However, previous studies have not constructed predictive models based on the results. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-379/rc).
Methods
General information
A retrospective study was conducted at the Fujian Medical University Xiamen Humanity Hospital from January 2019 to January 2021. A total of 143 patients with advanced NSCLC were treated with ICIs, and the efficacy of treatment [complete remission (CR), partial remission (PR), stable disease (SD), or progression disease (PD)] was evaluated according to the efficacy evaluation criteria of solid tumors every two cycles. Patients with CR and PR were defined as an objective response (OR) group (n=67), and the other patients were defined as a control group (n=76) at the end of 4 cycles.
To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have a pathological diagnosis of stage III B or IV NSCLC, PD-L1 high expression; (II) be aged ≥18 years; and (III) have completed at least 4 cycles of anti-PD-1/PD-L1 treatment. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a co-infection; (II) had NSCLC combined with other malignant tumors; (III) had an autoimmune system disease; (IV) had missing clinical data; and/or (V) were lost to follow-up.
This retrospective clinical study was conducted in accordance with the 2013 edition of the Declaration of Helsinki and was approved by the Ethics Committee of Fujian Medical University Xiamen Humanity Hospital (No. c20220048). The requirement for informed consent was waived. Figure 1 provides a flow chart showing the process used to include the 143 patients with advanced lung cancer in this study.
Treatment methods
After the patients were admitted to the hospital, the relevant tests and examinations were performed. All the patients received Tislelizumab (200 mg/3 weeks, intravenously) for at least 4 cycles.
Efficacy evaluation
Before treatment, chest and abdomen electronic computed tomography, head magnetic resonance imaging, bone scanning, and other examinations were performed for the baseline evaluations. The efficacy of the immunotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST1.1). Efficacy was divided into CR, PR, SD, and PD. Patients with an efficacy evaluation of CR, PR, and SD continued to be treated according to the original plan, and patients with an efficacy evaluation of PD no longer received treatment. The OR rate was calculated as follows: OR = (CR + PR)/(CR + PR + SD + PD) × 100%.
Detection of ctDNA
Before treatment, 5 mL of fasting elbow vein blood was extracted and plasma ctDNA levels were measured using a magnetic bead DNA extraction kit and fluorescence quantitative polymerase chain reaction. The kit was purchased from Beijing Qunxiao Keyuan Biotechnology Co., Ltd.
Statistical analysis
SPSS26.0 (IBM, Chicago, USA) was used to complete the data analysis of this study, and a P value <0.05 indicated that the difference was statistically significant. The measurement data of the 2 groups are expressed as the mean ± standard deviation, and the differences between the 2 groups were analyzed by an independent samples t-test. The patient count data of the 2 groups are expressed as the number (percentage), and the chi-square test was used to analyze the differences between the 2 groups. The receiver operating characteristic (ROC) curve was used to analyze the value of the ctDNA in predicting the failure to achieve an OR after immunotherapy, and the optimal diagnostic threshold was calculated based on the weighted Youden index. A multivariate regression analysis was conducted to analyze the factors influencing the OR after immunotherapy in NSCLC patients. The R4.0.3 statistical software (Ross Ihaka Robert Gentleman, New Zealand) was used to establish the prediction model. Kaplan-Meier was used to analyzed the survival between the two groups.
Results
Comparison of the clinical features of the 2 groups
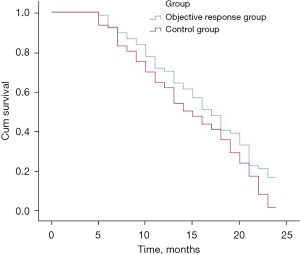
Compared to the control group, the proportion of pleural effusion in the OR group was significantly decreased (7.46% vs. 31.58%, P<0.001). Additionally, the proportion of patients treated with immunotherapy plus chemotherapy was significantly increased in the OR group compared to the control group (64.18% vs. 35.53%, P=0.001). CtDNA decreased significantly in the OR group compared to the control group (2.96±1.13 vs. 4.16±1.24 ng/µL, P<0.001). The 2-year mortality rate was significantly reduced in the OR group compared to the control group (83.58% vs. 98.68%, P=0.001; Table 1, Figure 2).
Table 1
| Category | Objective response group (n=67) | Control group (n=76) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years), mean ± SD | 58.82±11.76 | 59.38±12.61 | 0.274 | 0.785 |
| Gender, n (%) | 2.538 | 0.111 | ||
| Male | 49 (73.13) | 46 (60.53) | ||
| Female | 18 (26.87) | 30 (39.47) | ||
| Body mass index (kg/m2), mean ± SD | 25.45±2.43 | 25.39±2.65 | 0.118 | 0.906 |
| History of smoking, n (%) | 26 (38.81) | 38 (50.00) | 1.805 | 0.179 |
| History of alcoholism, n (%) | 21 (31.34) | 36 (47.37) | 3.815 | 0.051 |
| Hypertension, n (%) | 16 (23.88) | 13 (17.11) | 1.011 | 0.315 |
| Diabetes, n (%) | 7 (10.45) | 11 (14.47) | 0.525 | 0.469 |
| Tumor site, n (%) | 0.096 | 0.756 | ||
| Left | 37 (55.22) | 40 (52.63) | ||
| Right | 30 (44.78) | 36 (47.37) | ||
| Type of pathology, n (%) | 1.997 | 0.158 | ||
| Adenocarcinoma | 42 (62.69) | 56 (73.68) | ||
| Squamous cell carcinoma | 25 (37.31) | 20 (26.32) | ||
| Tumor stage, n (%) | 0.345 | 0.557 | ||
| III stage | 40 (59.70) | 49 (64.47) | ||
| IV stage | 27 (40.30) | 27 (35.53) | ||
| Maximum diameter of primary tumor (cm), mean ± SD | 6.95±1.56 | 6.78±1.60 | 0.640 | 0.523 |
| Pleural aggression, n (%) | 14 (20.90) | 18 (23.68) | 0.159 | 0.690 |
| Pleural effusion, n (%) | 5 (7.46) | 24 (31.58) | 12.810 | <0.001 |
| Treatment, n (%) | 11.699 | 0.001 | ||
| Immunotherapy in combination with chemotherapy | 43 (64.18) | 27 (35.53) | ||
| Immunotherapy | 24 (35.82) | 49 (64.47) | ||
| CtDNA (ng/µL), mean ± SD | 2.96±1.13 | 4.16±1.24 | 6.010 | <0.001 |
| 2-year mortality rate, n (%) | 56 (83.58) | 75 (98.68) | 10.564 | 0.001 |
SD, standard deviation; ctDNA, circulating tumor DNA.
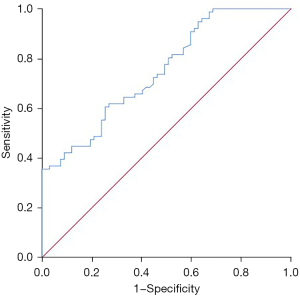
Value of ctDNA in predicting failure to achieve an OR after immunotherapy in NSCLC patients
ctDNA was valuable in predicting that an OR would not be achieved after immunotherapy in the NSCLC patients. The area under the curve (AUC) of ctDNA in predicting that an OR would not be achieved after immunotherapy in the NSCLC patients was 0.750 (95% CI: 0.673–0.828, P<0.001), the optimal diagnostic cut-off was 3.72 ng/µL, and the sensitivity and specificity were 0.618 and 0.731, respectively (Figure 3).
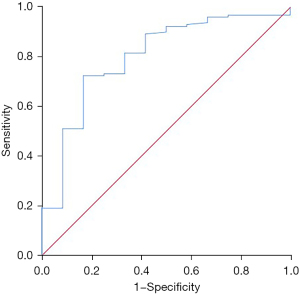
Value of ctDNA in predicting death within 2 years of immunotherapy in NSCLC patients
ctDNA was valuable in predicting death within 2 years of immunotherapy in NSCLC patients, and had an AUC of 0.800 (95% CI: 0.666–0.935, P=0.001). The best diagnostic cut-off was 2.87 ng/µL with sensitivity and specificity value of 0.725 and 0.833, respectively (Figure 4).
Analysis of influencing factors of OR in NSCLC patients after immunotherapy
The absence of pleural effusion, immunotherapy in combination with chemotherapy, and ctDNA <3.72 ng/mL were influencing factors of OR after immunotherapy in NSCLC patients (b value =1.152, 1.124 and 1.482, P=0.045, 0.004 and <0.001; Table 2).
Table 2
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Smoking history | −0.541 | 0.401 | 1.821 | 0.177 | 0.582 (0.266–1.277) |
| Pathology type | −0.845 | 0.449 | 3.537 | 0.060 | 0.430 (0.178–1.036) |
| Absence of pleural effusion | 1.152 | 0.575 | 4.013 | 0.045 | 3.164 (1.025–9.765) |
| Immunotherapy in combination with chemotherapy | 1.124 | 0.395 | 8.107 | 0.004 | 3.076 (1.419–6.666) |
| CtDNA <3.72 ng/µL | 1.482 | 0.409 | 13.132 | <0.001 | 4.403 (1.975–9.817) |
| Constant | −5.163 | 1.063 | 23.567 | <0.001 | 0.006 |
NSCLC, non-small cell lung cancer; ctDNA, circulating tumor DNA.
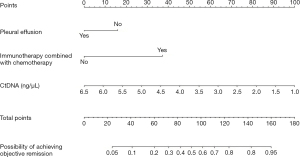
Establishment and verification of a predictive model of OR in patients with NSCLC after immunotherapy
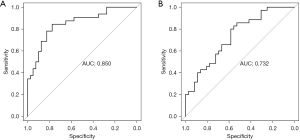
The R4.0.3 statistical software (Ross Ihaka Robert Gentleman) was used to randomly divide the data set into the training set and validation set. The sample size of the training set was 72 and that of the validation set was 71. Pleural effusion, immunotherapy in combination with chemotherapy, and ctDNA were the factors included in the predictive model. We made a nomogram and drew a ROC curve. The area under the ROC curve of the training set was 0.850 (95% CI: 0.760–0.940) and that of the verification set was 0.732 (95% CI: 0.616–0.847). The model was tested by the Hosmer-Lemeshow Goodness-of-Fit test in the validation set, and had a chi-square value of 14.227, and a P value of 0.076, which indicated that the model was valuable and credible (Figures 5,6).
Discussion
With a 5-year survival rate of <5%, the mortality rate of patients with advanced NSCLC is extremely high (1). The emergence of immunotherapy in recent years has brought hope to patients with advanced NSCLC, but immunotherapy also has a number of issues, such as its high costs and ineffectiveness in some patients (7-9). To better predict the efficacy of immunotherapy in patients with advanced NSCLC, we designed this study and found that the absence of pleural effusion, immunotherapy in combination with chemotherapy, and ctDNA <3.72 ng/µL were valuable factors of objective remission after immunotherapy in NSCLC patients (P=0.045, 0.004 and <0.001). CtDNA was valuable in predicting the efficacy of immunotherapy in NSCLC patients, and the prediction model constructed according to the relevant risk factors was also valuable in predicting objective remission after immunotherapy in NSCLC patients.
ctDNA, also known as circulating tumor cell DNA, refers to the presence of a small number of tumor cell necrosis secretion of DNA fragments in the blood. CtDNA can be used for the early diagnosis of some cancers and to guide the treatment and evaluate the prognosis of malignant tumor patients (21-24). At present, ctDNA is also used in the diagnosis and treatment of lung cancer patients. A study confirmed that ctDNA can be used for the early diagnosis of lung cancer patients (25). ctDNA is an also emerging biomarker in NSCLC. A previous study showed that ctDNA was a good predictor of chemotherapy efficacy in patients with advanced NSCLC, and decreased ctDNA was associated with longer progression-free survival (26). Moreover, another study suggested that failure to detect ctDNA after treatment may be associated with a better prognosis (27). A study of NSCLC patients receiving neoadjuvant chemotherapy and immunotherapy also confirmed that preoperative ctDNA was associated with the treatment response in lung cancer patients (17).
ctDNA is a cell-free extracellular DNA found in body fluids such as blood, synovial fluid, and cerebrospinal fluid. The ctDNA has a short half-life, and thus can accurately reflect the current situation of tumors, and represents a promising new biological index (27). Thus, ctDNA could be used to guide immunotherapy in NSCLC patients.
Limitations
This was a retrospective clinical study, and it did not dynamically monitor ctDNA levels. Moreover, we failed to study the mutant molecules of ctDNA in this retrospective study.
Conclusions
More and more scholars have begun to study the role of various biological indicators in different diseases (28-33). This study showed that ctDNA level was valuable in predicting the efficacy of immunotherapy in NSCLC patients, and ctDNA may be used to guide immunotherapy in NSCLC patients and thus improve patient outcomes. The dynamic monitoring of ctDNA levels may also be helpful, which needs to be explored in future research.
Acknowledgments
Funding: This study was supported by the Scientific and Technological Projects of Xiamen (No. 3502Z20199113).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-379/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-379/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-379/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-379/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective clinical study was conducted in accordance with the 2013 edition of the Declaration of Helsinki and was approved by the Ethics Committee of Fujian Medical University Xiamen Humanity Hospital (No. c20220048). The requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Chen K, Ye C, Gao Z, et al. Immune infiltration patterns and identification of new diagnostic biomarkers GDF10, NCKAP5, and RTKN2 in non-small cell lung cancer. Transl Oncol 2023;29:101618. [Crossref] [PubMed]
- Choi S, Yoon DW, Shin S, et al. Importance of lymph node evaluation in 2-centimeter or less pure solid non-small cell lung cancer. Ann Thorac Surg 2023; [Epub ahead of print]. [Crossref]
- Gosney JR, Peake MD, Kerr KM. Improving practice in PD-L1 testing of non-small cell lung cancer in the UK: current problems and potential solutions. J Clin Pathol 2023;jcp-2022-208643. [Crossref] [PubMed]
- Ko HW, Shie SS, Wang CW, et al. Association of smoking status with non-small cell lung cancer patients harboring uncommon epidermal growth factor receptor mutation. Front Immunol 2022;13:1011092. [Crossref] [PubMed]
- Lee TH, Chen HL, Chang HM, et al. Impact of Smoking Status in Combination Treatment with EGFR Tyrosine Kinase Inhibitors and Anti-Angiogenic Agents in Advanced Non-Small Cell Lung Cancer Harboring Susceptible EGFR Mutations: Systematic Review and Meta-Analysis. J Clin Med 2022;11:3366. [Crossref] [PubMed]
- Zeng Z, Qu J, Yao Y, et al. Clinical outcomes and risk factor of immune checkpoint inhibitors-related pneumonitis in non-small cell lung cancer patients with chronic obstructive pulmonary disease. BMC Pulm Med 2022;22:458. [Crossref] [PubMed]
- Li F, Zhai S, Lv Z, et al. Effect of histology on the efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer: A systematic review and meta-analysis. Front Oncol 2022;12:968517. [Crossref] [PubMed]
- Barsheshet Y, Voloshin T, Brant B, et al. Tumor Treating Fields (TTFields) Concomitant with Immune Checkpoint Inhibitors Are Therapeutically Effective in Non-Small Cell Lung Cancer (NSCLC) In Vivo Model. Int J Mol Sci 2022;23:14073. [Crossref] [PubMed]
- Ganguly S, Gogia A. Pembrolizumab as adjuvant therapy in non-small-cell lung cancer. Lancet Oncol 2022;23:e528. [Crossref] [PubMed]
- Chu RW, Vegas García A, Hickey C, et al. Cost-Effectiveness of First-Line Pembrolizumab Monotherapy Versus Chemotherapy in High Programmed Death-Ligand 1 Advanced Non-Small Cell Lung Cancer in the Irish Healthcare Setting. Value Health 2023;26:402-10. [Crossref] [PubMed]
- Qiao T, Zhao J, Xin X, et al. Combined pembrolizumab and bevacizumab therapy effectively inhibits non-small-cell lung cancer growth and prevents postoperative recurrence and metastasis in humanized mouse model. Cancer Immunol Immunother 2023;72:1169-81. [Crossref] [PubMed]
- Li M, Chen J, Zhang B, et al. Correction: Dynamic monitoring of cerebrospinal fluid circulating tumor DNA to identify unique genetic profiles of brain metastatic tumors and better predict intracranial tumor responses in non-small cell lung cancer patients with brain metastases: a prospective cohort study (GASTO 1028). BMC Med 2023;21:15. [Crossref] [PubMed]
- Fang X, Yu S, Jiang Y, et al. Circulating tumor DNA detection in MRD assessment and diagnosis and treatment of non-small cell lung cancer. Front Oncol 2022;12:1027664. [Crossref] [PubMed]
- Li M, Chen J, Zhang B, et al. Dynamic monitoring of cerebrospinal fluid circulating tumor DNA to identify unique genetic profiles of brain metastatic tumors and better predict intracranial tumor responses in non-small cell lung cancer patients with brain metastases: a prospective cohort study (GASTO 1028). BMC Med 2022;20:398. [Crossref] [PubMed]
- Moding EJ, Diehn M, Wakelee HA. Circulating tumor DNA testing in advanced non-small cell lung cancer. Lung Cancer 2018;119:42-7. [Crossref] [PubMed]
- Yue D, Liu W, Chen C, et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl Lung Cancer Res 2022;11:263-76. [Crossref] [PubMed]
- Jia Q, Chiu L, Wu S, et al. Tracking Neoantigens by Personalized Circulating Tumor DNA Sequencing during Checkpoint Blockade Immunotherapy in Non-Small Cell Lung Cancer. Adv Sci (Weinh) 2020;7:1903410. [Crossref] [PubMed]
- Moding EJ, Liu Y, Nabet BY, et al. Circulating Tumor DNA Dynamics Predict Benefit from Consolidation Immunotherapy in Locally Advanced Non-Small Cell Lung Cancer. Nat Cancer 2020;1:176-83. [Crossref] [PubMed]
- Li L, Wang Y, Shi W, et al. Serial ultra-deep sequencing of circulating tumor DNA reveals the clonal evolution in non-small cell lung cancer patients treated with anti-PD1 immunotherapy. Cancer Med 2019;8:7669-78. [Crossref] [PubMed]
- Jiang XE, Xu T, Wei Q, et al. DNA repair capacity correlates with standardized uptake values from (18)F-fluorodeoxyglucose positron emission tomography/CT in patients with advanced non-small-cell lung cancer. Chronic Dis Transl Med 2018;4:109-16. [PubMed]
- Pickhardt PJ, Graffy PM, Weigman B, et al. Diagnostic Performance of Multitarget Stool DNA and CT Colonography for Noninvasive Colorectal Cancer Screening. Radiology 2020;297:120-9. [Crossref] [PubMed]
- Schumann S, Scherthan H, Frank T, et al. DNA Damage in Blood Leukocytes of Prostate Cancer Patients Undergoing PET/CT Examinations with [68Ga]Ga-PSMA I&T. Cancers (Basel) 2020;12:388. [Crossref] [PubMed]
- Saini KS. New orally active DNA minor groove binding small molecule CT-1 acts against breast cancer by targeting tumor DNA damage leading to p53-dependent apoptosis. Mol Carcinog 2017;56:1266-80. [Crossref] [PubMed]
- Tailor TD, Rao X, Campa MJ, et al. Whole Exome Sequencing of Cell-Free DNA for Early Lung Cancer: A Pilot Study to Differentiate Benign From Malignant CT-Detected Pulmonary Lesions. Front Oncol 2019;9:317. [Crossref] [PubMed]
- Fiala O, Baxa J, Svaton M, et al. Combination of Circulating Tumour DNA and (18)F-FDG PET/CT for Precision Monitoring of Therapy Response in Patients With Advanced Non-small Cell Lung Cancer: A Prospective Study. Cancer Genomics Proteomics 2022;19:270-81. [Crossref] [PubMed]
- Lafata KJ, Corradetti MN, Gao J, et al. Radiogenomic Analysis of Locally Advanced Lung Cancer Based on CT Imaging and Intratreatment Changes in Cell-Free DNA. Radiol Imaging Cancer 2021;3:e200157. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- He XC, Chen HY, Qiu Y, et al. Associations of iron status with breast cancer risk factors in adult women: Findings from National Health and Nutrition Examination Survey 2017-2018. J Trace Elem Med Biol 2021;68:126867. [Crossref] [PubMed]
- Chen H, Meng X, Hao X, et al. Correlation Analysis of Pathological Features and Axillary Lymph Node Metastasis in Patients with Invasive Breast Cancer. J Immunol Res 2022;2022:7150304. [Crossref] [PubMed]
- Qiu Y, Chen H, Dai Y, et al. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients' Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J 2022;2022:6705052. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



