
Upgrading extra corporeal life support to ECMELLA using Impella 5.0 in rescued INTERMACS 1 patients, lactate level matters!
Highlight box
Key findings
• Lactate levels above 7.9 mmol/L should postpone the implantation of an Impella 5.0 in a rescued patient on ECLS.
What is known and what is new?
• Optimal timing for left ventricle unloading under ECLS still controversial. The benefit of bridging patients presenting cardiogenic shock under ECLS to ECMELLA still to be proven.
• In this single center analysis, patients under ECLS upgraded to ECMELLA with an elevated serum lactate level above 7.9 mmol/L did not benefit from this strategy.
What is the implication, and what should change now?
• Dynamic course of lactate during ECLS therapy and an elevated lactate level above 7.9 mmol/L should be considered as a reason to defer the LV discharge.
Introduction
To improve the characterization of patients with advanced heart failure (HF) who were previously classified as having New York Heart Association (NYHA) functional class 3 or 4, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) classification proposes an appropriate matching of patients’ profile with intervention. There are seven INTERMACS profiles (1), with INTERMACS 1 describing “crash and burn” patients following acute myocardial infarction, acute decompensated HF, biventricular failure, and myocarditis who requires immediate restoration of circulatory hemodynamics using venoarterial extracorporeal life support (ECLS).
In rescued INTERMACS 1 patients, ECLS is the treatment of choice as a bridge to long-term device support, transplantation or recovery (2). One of the major complications of ECLS is that it increases left ventricular overload, acting as a vicious cycle intensifying myocyte loss, delaying myocardial recovery, prolonging the length of stay in the critical care unit and sometimes worsening the prognosis (3).
The Impella 5.0 pump is a microaxial mechanical circulatory support device capable of generating a flow of up to 5 L/minute. Its implementation in addition to ECLS, i.e., Impella used in combination with venoarterial extracorporeal membrane oxygenation (ECMELLA), has been described to counteract ECLS related complications, mainly the left ventricle (LV) overload that strongly affect the outcome (4,5). Percutaneous ventricle assist device (PVAD) is an alternative but we have no good data to support this at this stage. With a mortality rate varying from 40% to 60% in INTERMACS 1 patients receiving ECLS, the question of bridging INTERMACS 1 patients from ECLS to the costly and invasive Impella 5.0 pump has to be addressed. Indeed, there are conflicting or even negative results regarding the use of the Impella (6,7). If both the learning curve and the route of implantation are crucial to obtain good results (8,9), careful patient selection seems to be the most important part of clinical success (10). Therefore, we aimed to identify parameters that could identify INTERMACS 1 patients already under ECLS who are good candidates for ECMELLA upgrading. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1297/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). French law does not require ethics committee or institutional review board approval or informed consent relative to retrospective data collection. All data were anonymized and compiled according to the requirements of the Commission Nationale Informatique et Liberté, the organization dedicated to privacy, information technology and civil rights in France.
Population
The population consisted of INTERMACS 1 patients with acute myocardial infarction, acute decompensated HF, biventricular failure such as found in postcardiotomy failure, and myocarditis, preceded or not by cardiac arrest. INTERMACS 1 patients were defined as having “crash and burn” critical cardiogenic shock requiring intensive care management both with respiratory mechanical and/or inotropic support. All patients were sequentially managed with initial ECLS implantation since their baseline clinical condition indicated refractory and durable cardiogenic shock, followed by Impella 5.0 pump implantation as a bridge to recovery, transplantation, or long-term left ventricular assistance device (LVAD). Combining ECLS to Impella 5.0 pump was decided by a heart team composed of cardiac surgeons, anesthesiologists, and cardiologists. The clinical decision for ECMELLA support was based on clinical evidence of left ventricular overload, including the occurrence of early pulmonary edema, invasive or noninvasive elevated pulmonary capillary pressure, blood sludge formation due to reduced LV emptying velocities assessed by echocardiography severely dilated LV above 70 mm and constant aortic valve closure.
Surgical technique for Impella 5.0 pump implantation
The catheter-based Impella 5.0 (ABIOMED, Danvers, USA) left ventricular temporary assist device is a safe, reliable pump that can provide hemodynamic support in low cardiac output syndrome (11). The concept of ECMELLA, combining the utilization of Impella on top of ECLS, was described in several studies (5,12,13). Technically, in our institution, both femoral and axillary sites were considered for Impella 5.0 pump implantation if arteries had a minimum open caliber of 6.2 mm after computed tomography (CT) scan or ultrasound imaging. The procedure took place in a hybrid operating room. The anesthetized patient was placed in the supine position and monitored with fluoroscopy and transesophageal echocardiography (TEE). Once the axillary or femoral site was properly prepared, both arteries were exposed through a 4-cm incision, followed by a 10-mm Dacron graft anastomosis using a running 5-0 Proline in an end-to-side fashion between two vessel loops. Care was taken to achieve an activated clotting time (ACT) >200–250 seconds with heparin. The distal orifice of the Dacron graft was used to insert a dedicated introducer (ABIOMED) in which a left Amplatz catheter (TERUMO, Somerset, USA) was inserted and directed toward the left ventricular apex in a 2-step manner successively using a 0.035 normal and stiff guidewire to cross the aortic valve. Fluoroscopic and TEE guidance were used to prevent papillary muscle injury or aortic valve injury during this step. Before removing the left catheter, the 0.035 stiff guidewire was replaced by a 0.018 stiff guidewire to backload the pump. Under fluoroscopic and TEE guidance, the pump was subsequently positioned 4 cm below the aortic valve. While the 0.018 stiff guidewire was removed, the pump was progressively turned on from its controller. The surgical field was closed after the Dacron graft was clamped and shortened into the groin access.
Data collection and endpoint
Forty-one consecutive INTERMACS 1 patients (12 women, mean age 49±13 years) were retrospectively included between 2011 and 2020. Demographic, clinical, imaging, and biological parameters, including available hemodynamics from echocardiography or right-side heart catheterization data, were collected at the implementation of ECLS and at the upgrade to ECPELLA. Metabolic data (serum lactate, creatinine, bilirubin, troponin, and liver enzymes) were collected daily until the patient was considered for pump implantation. In addition, data on the preoperative etiology of INTERMACS 1 presentation, occurrence of cardiac arrest, preoperative coronary angiography and subsequent revascularization were also recorded. Safety data of the Impella 5.0 pump were collected.
The combined endpoint included the following: 30-day hospital mortality, recovery, transplantation, or long-term left ventricular assistance device.
Statistical analysis
SPSS 13.0 was employed for statistics. Continuous variables are expressed as the mean ± standard deviation (SD). Student’s t-test was used to assess differences between mean values, and categorical variables were compared with the χ2 test and Fisher’s exact test when appropriate. Significant continuous variables with P<0.05 were dichotomized into categorical variables with the use of receiver-operator characteristic curve analysis to define cutoff values that best distinguished the issue. Thus for lactate level and troponin concentration, the best cutoffs were points on the curve with minimum distance from the left-upper corner of the unit square; and the point where the Youden’s index is maximum. All significant continuous and categorical variables entered a univariate Cox regression model and then a multivariate Cox regression procedure to detect independent predictors of endpoints. Cumulative event-free survival analysis was performed with the log-rank test, while curves were drawn with the Kaplan-Meier method. P<0.05 was considered significant.
Results
Patient characteristics at baseline
Baseline characteristics are summarized in Table 1. Smoking habits, hypercholesterolemia, obesity, and type 2 diabetes were the four most common cardiovascular risk factors. Etiologies of cardiogenic shock leading to emergency ECLS implantation were mostly related to acute coronary syndrome (n=18, 44%) and decompensated chronic heart disease (n=12, 29%, Table 1). Together, they accounted for 73% of all ECLS implantations. Cardiac arrest was reported in 63% of the patients whether in asystole or shockable rhythm (ventricular tachycardia/fibrillation). For both, the no flow duration was of 0±1 min and the low flow duration was 42±44.8 min (Table 1). The echocardiographic LV ejection fraction average at Impella 5.0 implantation was 18%±12%.
Table 1
| Parameters | Total (n=41) | Survivors (n=16) | Nonsurvivors (n=25) | P value |
|---|---|---|---|---|
| Age (years) | 49±12.6 | 43.2±11.9 | 53.9±1.7 | 0.014 |
| Male sex | 29 [71] | 8 [50] | 21 [84] | 0.019 |
| BMI (kg/m2) | 28.0±4.9 | 26.2±4.4 | 29.4±4.9 | NS |
| Cardiovascular risk factors | ||||
| Arterial hypertension | 7 [17] | 2 [13] | 5 [20] | NS |
| Type 2 diabetes | 8 [20] | 1 [6] | 7 [28] | NS |
| Smoking habits | 17 [41] | 6 [38] | 11 [44] | NS |
| Hypercholesterolemia | 10 [24] | 1 [6] | 9 [36] | 0.031 |
| Obesity (BMI >30 kg/m2) | 9 [22] | 3 [19] | 6 [24] | NS |
| Comorbidities | ||||
| Atrial fibrillation | 3 [7] | 0 [0] | 3 [12] | NS |
| Previous cardiomyopathy | 22 [54] | 8 [50] | 14 [56] | NS |
| Ischemic heart disease | 7 [17] | 2 [13] | 5 [20] | NS |
| Cardiac surgery | 6 [15] | 2 [13] | 4 [16] | NS |
| Periph. vascular disease | 1 [2] | 1 [6] | 0 [0] | NS |
| Stroke | 0 [0] | 0 [0] | 0 [0] | NS |
| Chronic kidney disease | 0 [0] | 0 [0] | 0 [0] | NS |
| Previous PTCI | 29 [71] | 13 [81] | 16 [64] | NS |
| Cancer | 0 [0] | 0 [0] | 0 [0] | NS |
| Etiology of cardiogenic shock | ||||
| Acute coronary syndrome | 18 [44] | 2 [13] | 16 [64] | 0.0007 |
| Acute myocarditis | 2 [5] | 2 [13] | 0 [0] | NS |
| Postcardiotomy | 1 [2] | 0 [0] | 1 [4] | NS |
| Chronic heart disease | 12 [29] | 7 [44] | 5 [20] | NS |
| Other | 8 [20] | 5 [31] | 3 [12] | NS |
| Status at ECLS implantation | ||||
| LV ejection fraction (%) | 17.9±12.3 | 20.8±14.4 | 20.7±10.3 | NS |
| Cardiac arrest | 26 [63] | 7 [44] | 19 [76] | 0.037 |
| Shockable (VT/VF) | 4 [10] | 2 [13] | 2 [8] | NS |
| Asystole | 2 [5] | 1 [6] | 1 [4] | NS |
| Low flow (min) | 42.0±44.8 | 15.9±26.1 | 52.2±42.9 | 0.048 |
Data are shown as n [%] or mean ± SD. BMI, body mass index; NS, non specific; PTCI, percutaneous coronary intervention; ECLS, extracorporeal life support; LV, left ventricle; VT/VF, ventricular tachycardia/ventricular fibrillation; SD, standard deviation.
Patient characteristics at Impella 5.0 pump implantation and follow-up
The Impella 5.0 pump was set up in average 9 [0–30] hours after ECLS implantation (Table 2). Left ventricular unloading was the main reason for pump implantation. Other hemodynamic support included inotropic drugs (85%), intra-aortic balloon counterpulsation (10%), and preoperative invasive ventilation (90%). No patient had a primary Impella 5.0 pump implantation.
Table 2
| Parameters | Total (n=41) | Survivors (n=16) | Nonsurvivors (n=25) | P value |
|---|---|---|---|---|
| Time between ECLS and Impella (h) | 9 [0–30] | 3 [0–14] | 13 [2–48] | NS |
| Hemodynamics at Impella implantation | ||||
| Median SAP (mmHg) | 83 [72–90] | 88 [82–90] | 75 [63–90] | 0.017 |
| Mean PAP (mmHg) | 29.2±9.0 | 31±5 | 28±10.5 | NS |
| Heart rate (bpm) | 89 [71–120] | 84 [71–116] | 96 [70–126] | NS |
| Inotropic support | 35 [85] | 13 [81] | 22 [88] | NS |
| Intra-aortic balloon counterpulsation | 5 [12] | 2 [12] | 3 [12] | NS |
| Biology at Impella implantation | ||||
| Creatinine (µmol/L) | 138 [105–199] | 118 [79–175] | 172 [118–211] | NS |
| Hemoglobin (mmol/L) | 11 [9–12] | 10 [9–11] | 11 [9–12] | NS |
| Arterial pH | 7.4 [7.3–7.5] | 7.4 [7.4–7.5] | 7.4 [7.2–7.5] | NS |
| Lactate (mmol/L) | 4 [2–11] | 3 [2–7] | 6 [3–12] | 0.049 |
| Troponin I (ng/L) | 3,930 [539–13,800] | 1,103 [176–2,666] | 6,797 [2,338–26,454] | 0.048 |
| AST (IU/L) | 252 [100–791] | 169 [90–282] | 362 [101–1,096] | NS |
| GGT (IU/L) | 339±490 | 284±311 | 378±591 | NS |
| Total bilirubin (mg/dL) | 12 [10–23] | 11 [10–23] | 16 [11–23] | NS |
| Prothrombin ratio | 48 [36–64] | 50 [39–68] | 46 [30–64] | NS |
| NT pro BNP (pg/mL) | 7,988 [1,241–33,168] | 7,999 [656–29,336] | 5,100 [3,446–34,114] | NS |
Data are shown as n [%] or median [min–max] or mean ± SD. ECLS, extracorporeal life support; NS, non specific; SAP, systolic arterial pressure; PAP, pulmonary arterial pressure; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; NT pro BNP, N-terminal pro brain natriuretic peptide; SD, standard deviation.
At Impella 5.0 implantation, the mean arterial pressure and mean pulmonary arterial pressure were 79±16 and 29±10 mmHg, respectively. The lactate level ranged from 0.9 to 19.8 mmol/L (mean ± SD, 6.7±6.5 mmol/L), troponin level ranged from 10 to 158,299 mg/dL (mean ± SD, 15,964±31,578 mg/dL) and NT pro-BNP ranged from 260 to 63,127 pg/mL (mean ± SD, 19,546±24,491 pg/mL). Both renal and liver functions were deteriorated.
Major bleeding at the device exit site was the most frequent complication, including 4 cases of pectoral hematoma all necessitating surgical revision with a reversible brachial plexus injury in 1 case. Nine patients developed sustained ventricular arrhythmia, which was treated by speed flow reduction. One patient with refractory ventricular fibrillation underwent multiple defibrillations. Pump dislodgement was repositioned under echocardiographic guidance in 10 cases. A new pump was implanted through an alternative arterial site after an unsuccessful subclavian artery crossing attempt. Eight patients developed acquired von Willebrand syndrome leading to major gastrointestinal bleeding, 3 cases of severe hemolysis, explantation or device exchange were performed, and two of them died (Table 3). Coronary angiogram was performed in 66% (Table 3) and percutaneous coronary intervention was performed in 29 patients.
Table 3
| Parameters | Total (n=41) | Survivors (n=16) | Nonsurvivors (n=25) |
|---|---|---|---|
| Transplantation | 2 [5] | 2 [13] | – |
| LVAD | 6 [15] | 2 [13] | 4 [16] |
| TAH | 1 [2] | 1 [6] | – |
| Recovery | 11 [27] | 11 [69] | – |
| Brain death | 8 [20] | – | 8 [32] |
| ECLS related | 6 [15] | – | 6 [24] |
| Non-ECLS related | 2 [5] | – | 2 [8] |
| Major bleeding | 4 [10] | – | 4 [16] |
| Multi organ failure | 9 [22] | – | 9 [36] |
| Renal replacement therapy | 13 [32] | 2 [13] | 11 [44] |
| Duration of mechanical ventilation (days) | 9 [3–15.8] | 14 [9.5–22] | 5.5 [2–10.3] |
| Time on ECLS (days) | 4.5 [2.3–5] | 3.5 [2–5] | 7 [5–11.3] |
| Time on Impella (days) | 6 [3.8–7] | 6 [3.8–7] | 8 [6–9.5] |
| Hospital length of stay (days) | 15 [4.8–32.8] | 44 [23–58] | 8 [2–15] |
| CAG performed | 27 [66] | 6 [38] | 21 [84] |
| Coronary intervention (PCI) | 29 [71] | 13 [81] | 16 [64] |
Data are shown as n [%] or median [min–max]. LVAD, left ventricular assist device; TAH, total artificial heart; ECLS, extracorporeal life support; CAG, coronarography angiogram; PCI, percutaneous coronary intervention.
Predictors of outcome
The length of stay in the intensive care unit was 19±24 days, while the length of stay in the hospital was 27±38 days. Outcomes were collected at 30 days. Among the 25 patients of the non-survivor group, 9 died from multiple organ failure, 8 for brain death with ECMELLA discontinuation, and 4 patients for major bleeding (Table 3). Four patients weaned from ECMELLA after long term LVAD implantation died from right ventricular failure. Among the 16 patients who survived, 2 patients were transplanted, 3 patients were weaned from ECMELLA, 1 converted to total artificial heart implantation and 2 bridged to long-term LVAD implantation, while 11 recovered. The characteristics of survivors and nonsurvivors at baseline and Impella 5.0 pump implantation are detailed in Tables 1,2, respectively. At baseline, patients were older and more frequently men. Comorbidities were similar between groups. Nonsurvivors more frequently had an acute coronary syndrome and required revascularization. Cardiac arrest was the main clinical presentation. At Impella 5.0 implantation, the mean arterial pressure was lower in nonsurvivors. Both troponin and lactate levels were higher in nonsurvivors. A serum lactate level of >7.9 mmol/L (area under the curve =0.66) and a troponin level of >2,700 mg/dL (area under the curve =0.77) were selected by receiver-operator characteristic curve analysis as the best cutoff values for predicting the patient’s outcome. When tested as categorical variables, a troponin level of >2,700 mg/dL and a lactate level of >7.9 mmol/L were more prevalent in nonsurvivors. Other parameters were unchanged between groups.
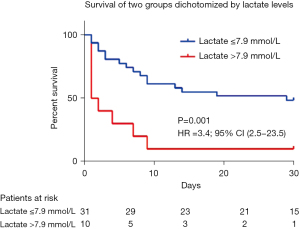
In univariate analysis (Table 4), acute coronary syndrome, cardiac arrest before ECLS implantation, serum lactate >7.9 mmol/L and troponin >2,700 mg/dL were independent predictors of death. However, in multivariate analysis (Table 4), adjusted for age and sex as well as others confounding factors, only serum lactate >7.9 mmol/L was independently associated with mortality. The Kaplan-Meier curve demonstrated a significant (log-rank =0.0001, Figure 1) survival rate between groups, with a mortality rate of 90% for patients with a serum lactate level of >7.9 mmol/L.
Table 4
| Parameters | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| χ2 | 95% CI | P value | χ2 | 95% CI | P value | ||
| Hypercholesterolemia | 4.73 | 0.17–0.93 | 0.03 | – | 0.37–13.5 | 0.37 | |
| Acute coronary syndrome | 10.5 | 1.60–8.70 | 0.001 | – | 0.40–7.97 | 0.78 | |
| Cardiac arrest | 5.67 | 1.16–7.40 | 0.017 | – | 0.036–3.89 | 0.41 | |
| Lactate >7.9 mmol/L | 10.1 | 1.56–9.09 | 0.001 | – | 1.47–12.90 | 0.02 | |
| Troponin >2,700 mg/dL | 12.0 | 0.08–0.55 | 0.001 | – | 1.12–8.48 | 0.27 | |
| Low-flow (min) | 5.18 | 1.00–1.025 | 0.023 | – | 0.98–1.02 | 0.88 | |
CI, confidence interval.
Discussion
Impella 5.0 pump following ECLS implantation, the ECMELLA upgrade
The benefit of bridging patients presenting cardiogenic shock under ECLS to ECMELLA still to be proven in patients admitted in INTERMACS profile I. In our small retrospective study, among the 41 patients upgraded to ECMELLA, in hospital mortality remains high (61%; n=25). Our major finding was that high serum lactate >7.9 mmol/L at the time of Impella 5.0 implantation was associated with significantly poorer outcomes, namely, a 30-day survival of 10% vs. 48% (P=0.001).
Despite providing an adequate supply of oxygenated blood, ECLS has numerous adverse effects related to retrograde blood flow into the aorta. From a pathophysiological perspective, left ventricular mechanical overload is the major adverse effect with an obvious mechanistic and prognostic challenge for contemporary ECLS (14). The resulting LV dilation increases cardiac metabolism, promotes myocardial ischemia, delays myocardial recovery, and leads to pulmonary edema and potential thrombus formation (15). Several LV unloading strategies during ECLS support have been described, such as inotropic support, intra-aortic balloon pumping (16), direct left atrial decompression (percutaneous transeptally placed left atrial vent (17), or atrial septostomy (15) and LV decompression with the use of vent or Impella 5.0 (5,12,18).
Percutaneous implantation of an Impella 5.0 pump is the treatment of choice in our center to unload the LV in patients receiving ECLS with evidence of refractory pulmonary edema. In our INTERMACS 1 population, the surgical route was axillary in 46% of the patients and femoral in the remaining 54%. Axillary access was preferred when extubation was expected once the patient was stabilized. Thus, 8 patients were able to fully recover after sequential ECLS followed by Impella 5.0 pump implantation with no extra support, while 25 died. There are conflicting results in the literature regarding mortality. In a retrospective cohort of 157 patients, Pappalardo et al. suggested a concomitant implantation of an Impella 5.0 pump and ECLS and found a significantly lower rate of hospital mortality (47% vs. 80%, P<0.001) and a higher rate of successful bridging to either recovery or further therapy (68% vs. 28%, P<0.001) compared with ECLS alone (19). Here, the Impella 5.0 pump was implanted 9 [0–30] hours (Table 1) after ECLS. Optimal timing for LV unloading under ECLS still controversial. However, Schrage et al. (20) demonstrated that the delay of LV unloading in ECLS patients of more than 2 hours negatively impacts the survival. and despite the relatively short time between ECLS and Impella 5.0 Implantion, the mortality rate remained elevated in our cohort. Thus, the selection of good candidates for bridging from ECLS to ECMELLA remains challenging, considering that not all patients are candidates for escalation therapy, as their prognosis is poor. It seems relevant to identify biomarkers for predicting survival/mortality when considering Impella 5.0 pump implantation.
Available scores in ECLS
Venoarterial-extracorporeal membrane oxygenation (ECMO) is the treatment of choice in “crash and burn” INTERMACS 1 patients due to its rapid setup and simplicity. However, despite better knowledge and management of cardiogenic shock, the mortality rate of INTERMACS 1 patients with ECLS is still very high, with a reported 61% (n=25) of deaths in our population. Thus, in INTERMACS 1 patients under ECLS support, the importance of appropriate patient selection for adjunctive Impella pump implantation remains an unsolved question. The SAVE score (21), the REMEMBER (22) score and the ENCOURAGE (23) score are useful tools for predicting survival. However, these scores have several limitations, including the use of multiple parameters and equations available only on the internet, an estimation with no clinical decision making, a calculation that does not consider recent biological parameters such as lactate level, an assessment not always possible, and sometimes only dedicated to selected populations.
Here, we propose a new simple parameter based on one biological marker that can be used at any time in the management of INTERMACS 1 patients for adding Impella 5.0 to ECLS. Briefly, patients with an elevated serum lactate above 7.9 mmol/L should not be considered for Impella 5.0 pump implantation, while those with non-elevated biological markers have a 47% mortality risk similar to that reported by Pappalardo et al. (19).
Lactate
Biological markers are the most powerful and independent predictors of mortality with adjustment for age and sex. Lactate is a metabolic product of anaerobic glycolysis that may result in inadequate oxygen delivery. By restoring adequate blood flow, ECLS support improves tissue perfusion. Therefore, the time-varying lactate level is expected to normalize with ECLS, and the dynamic course of lactate during ECLS therapy within the first 24 hours seems superior to a single lactate measurement as a predictive marker of 30-day mortality (24). In a cohort of 70 patients receiving only Impella 5.0 and 5.5 support for a broad spectrum of INTERMACS 1, 2 and 3 patients, Nersesian et al. identified a cut-off of 8 mmol/L for lactate level before and on ECLS support to predict a poor outcome (4). This threshold was similar in our work, suggesting that Impella 5.0 pump implantation should not be used in patients on ECLS with a serum lactate level of >7.9 mmol/L. Ott et al. describing a standard operating procedure for the management of cardiogenic shock have used the 8 mmol/L lactate level cut-off in their decision tree for upgrading from ECLS to ECMELLA (13).
Beside, lactate level has confounding factors with mortality in this manuscript like cardiac arrest, duration of low-flow, acute coronary syndrome that may have change the lactate level at baseline. However, in our manuscript we should mentioned that lactate level was that one measures just before Impella 5.0 implantation and not at baseline with a mean of 9 hours when upgrading to ECMELLA. We thought that lactate level at the time of implantation is more a marker of a persistent poor condition than an initial presentation. Thus, time varying lactate level could be more relevant than baseline or at 5.0 Impella implantation to be correlated to outcomes. Second, outcomes were corrected to all conditions that were significantly different between survivors and non-survivors, i.e., cardiac arrest, duration of low flow, acute coronary syndrome and still lactate level after multivariate analysis remain significantly linked to mortality.
Study limitations
First, we would like to mention that the collected data carry all the drawbacks of a small cohort size. Limitations also include the single-center, small and retrospective nature of the study and the lack of a prospective design. In addition, we acknowledge the complexity of accurately reporting hemodynamic conditions at the time of ECLS implantation given the difficulty of collecting accurate data during cardiopulmonary resuscitation (CPR). Furthermore, our population was heterogeneous at baseline, with a majority of acute myocardial infarction and few chronic left ventricular dysfunctions. The time to Impella 5.0 pump implantation was relatively short, within the first 9 hours in average, but varied on a broad spectrum, meaning that death may be related to other causes, such as multiorgan failure or brain death, in which lactate plays a minor role. We already mentioned that delaying LV unloading in INTERMACS 1 patients beyond 2 hours might have a detrimental effect on mortality as reported by Schrage et al. (20), meaning that outcomes are not only driven by lactate level. The value of just using lactate levels as a single decision-making parameter should be put in perspective with the clinical context which is extremely difficult to assess in case of cardiogenic shock.
Conclusions
Upgrading ECLS to ECMELLA INTERMACS 1 patients, using Impella 5.0 can be challenging. Despite receiving acute mechanical support, patients may still have a poor prognosis. In this single center analysis, patients under ECLS upgraded to ECMELLA with an elevated serum lactate level above 7.9 mmol/L did not benefit from this strategy. Ultimately, a larger multicenter cohort analysis is needed to define optimal criteria for upgrading ECLS to ECMELLA.
Acknowledgments
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1297/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1297/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1297/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1297/coif). CF reports grants from Pfizer, and Novartis, consulting fees from JANSSEN and payment for lectures from Pfizer, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). French law does not require ethics committee or institutional review board approval or informed consent relative to retrospective data collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alba AC, Rao V, Ivanov J, et al. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant 2009;28:827-33. [Crossref] [PubMed]
- Keebler ME, Haddad EV, Choi CW, et al. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail 2018;6:503-16. [Crossref] [PubMed]
- Schrage B, Ibrahim K, Loehn T, et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019;139:1249-58. [Crossref] [PubMed]
- Nersesian G, Tschöpe C, Spillmann F, et al. Prediction of survival of patients in cardiogenic shock treated by surgically implanted Impella 5+ short-term left ventricular assist device. Interact Cardiovasc Thorac Surg 2020;31:475-82. [Crossref] [PubMed]
- Bertoldi LF, Pappalardo F, Lubos E, et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J Crit Care 2020;57:259-63. [Crossref] [PubMed]
- Zhang B, Guo S, Ning J, et al. Continuous-flow left ventricular assist device versus orthotopic heart transplantation in adults with heart failure: a systematic review and meta-analysis. Ann Cardiothorac Surg 2021;10:209-20. [Crossref] [PubMed]
- Denisenko A, Ouattara A, Guinot PG, et al. Left ventricular unloading did not decrease mortality in cardiogenic shocks patients supported with VA-ECMO: A propensity score matching study. Anaesth Crit Care Pain Med 2022;41:101042. [Crossref] [PubMed]
- Scolari FL, Trott G, Schneider D, et al. Cardiogenic shock treated with temporary mechanical circulatory support in Brazil: The effect of learning curve. Int J Artif Organs 2022;45:292-300. [Crossref] [PubMed]
- Schultz J, Duval S, Shaffer A, et al. Axillary or Subclavian Impella 5.0 Support in Cardiogenic Shock: A Systematic Review and Meta-analysis. ASAIO J 2022;68:233-8. [Crossref] [PubMed]
- Panuccio G, Neri G, Macrì LM, et al. Use of Impella device in cardiogenic shock and its clinical outcomes: A systematic review and meta-analysis. Int J Cardiol Heart Vasc 2022;40:101007. [Crossref] [PubMed]
- Batsides G, Massaro J, Cheung A, et al. Outcomes of Impella 5.0 in Cardiogenic Shock: A Systematic Review and Meta-analysis. Innovations (Phila) 2018;13:254-60. [Crossref] [PubMed]
- Rao P, Mosier J, Malo J, et al. Peripheral VA-ECMO with direct biventricular decompression for refractory cardiogenic shock. Perfusion 2018;33:493-5. [Crossref] [PubMed]
- Ott S, Lewin D, Nersesian G, et al. Improving Survival in Cardiogenic Shock-A Propensity Score-Matched Analysis of the Impact of an Institutional Allocation Protocol to Short-Term Mechanical Circulatory Support. Life (Basel) 2022;12:1931. [Crossref] [PubMed]
- Truby LK, Takeda K, Mauro C, et al. Incidence and Implications of Left Ventricular Distention During Venoarterial Extracorporeal Membrane Oxygenation Support. ASAIO J 2017;63:257-65. [Crossref] [PubMed]
- Rupprecht L, Flörchinger B, Schopka S, et al. Cardiac decompression on extracorporeal life support: a review and discussion of the literature. ASAIO J 2013;59:547-53. [Crossref] [PubMed]
- Petroni T, Harrois A, Amour J, et al. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med 2014;42:2075-82. [Crossref] [PubMed]
- Kim AR, Park H, Lee SE, et al. Outcomes of left ventricular unloading with a transseptal cannula during extracorporeal membrane oxygenation in adults. Artif Organs 2021;45:390-8. [Crossref] [PubMed]
- Patel DK, Duncan MS, Shah AS, et al. Association of Cardiac Rehabilitation With Decreased Hospitalization and Mortality Risk After Cardiac Valve Surgery. JAMA Cardiol 2019;4:1250-9. [Crossref] [PubMed]
- Pappalardo F, Schulte C, Pieri M, et al. Concomitant implantation of Impella(®) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19:404-12. [Crossref] [PubMed]
- Schrage B, Becher PM, Bernhardt A, et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation 2020;142:2095-106. [Crossref] [PubMed]
- Chen WC, Huang KY, Yao CW, et al. The modified SAVE score: predicting survival using urgent veno-arterial extracorporeal membrane oxygenation within 24 hours of arrival at the emergency department. Crit Care 2016;20:336. [Crossref] [PubMed]
- Wang JI, Lu DY. Outcomes of Hospitalizations for Cardiogenic Shock at Left Ventricular Assist Device Versus Non-Left Ventricular Assist Device Centers. J Am Heart Assoc 2020;9:e017326. [Crossref] [PubMed]
- Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016;42:370-8. [Crossref] [PubMed]
- Slottosch I, Liakopoulos O, Kuhn E, et al. Lactate and lactate clearance as valuable tool to evaluate ECMO therapy in cardiogenic shock. J Crit Care 2017;42:35-41. [Crossref] [PubMed]


