
Treatment of rheumatoid arthritis-associated interstitial lung disease in a multi-center registry cohort
Highlight box
Key findings
• The real-world approach to the pharmacologic treatment of RA-ILD demonstrates heterogenous treatment patterns, and many patients do not persist on immunomodulatory therapy which reflects the paucity of robust evidence and lack of guidelines for RA-ILD.
What is known and what is new?
• A lack of robust evidence in RA-ILD makes it difficult to make treatment decisions in this condition.
• Patients with RA-ILD frequently experience loss of lung function and risk of mortality, irrespective of radiologic pattern.
What is the implication and what should change now?
• These real-world findings highlight the urgent need for randomized trials of therapeutics for RA-ILD.
Introduction
Rheumatoid arthritis (RA) is the most common connective tissue disease, with an incidence of 0.5–1% (1). RA is a progressive, systemic autoimmune disease, with both articular and extra-articular manifestations including interstitial lung disease (ILD) (2). ILD is the most common manifestation of lung involvement in RA and is a leading cause of morbidity and mortality (3-5). The most common high-resolution computed tomography (HRCT) patterns in RA are usual interstitial pneumonia (UIP) and non-specific interstitial pneumonia (NSIP), although organizing pneumonia (OP), lymphocytic interstitial pneumonia (LIP), acute interstitial pneumonia, and desquamative interstitial pneumonia also occur, though less frequently (6-9). Previous studies have identified several variables associated with mortality risk in patients with RA-ILD including older age, male sex, disease activity score, radiologic UIP pattern, reduced forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), and fibrosis on histopathology (10,11).
Treatment regimens for RA-ILD include varying doses of prednisone with or without other immunomodulatory medications such as azathioprine (AZA), mycophenolate mofetil (MMF), rituximab (RTX), or cyclophosphamide (CYC) (12-19). This treatment approach is largely based on retrospective case series data or clinical experience. There are no evidence-based guidelines to inform the therapeutic approach to RA-ILD and limited randomized placebo-controlled therapeutic trials outside of antifibrotic therapy (20-23) for those with a progressive fibrotic phenotype. Retrospective data suggest efficacy of certain immunomodulatory drugs with stabilization of lung function in connective tissue disease-associated ILD on AZA or MMF and a reduction in required oral corticosteroid therapy (13,14,24,25). The paucity of robust evidence makes treatment decisions challenging for the management of patients with RA-ILD. Given this lack of guidance, it is also not known if specific clinical variables influence therapeutic decision-making by individual clinicians, and whether those decisions impact lung function and survival. The Canadian Registry for Pulmonary Fibrosis (CARE-PF) provides a unique opportunity to understand how patients with RA-ILD are being treated, and whether specific treatments are associated with improved outcomes in RA-ILD (26).
The objective of this retrospective study was to characterize the real-life pharmacologic treatment approach to patients with RA-ILD in a multi-center national registry. We further sought to determine if radiographic patterns are associated with specific treatment choices, and to compare subsequent changes in lung function and survival by HRCT pattern and treatment status in this patient population. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1820/rc).
Methods
Study population
The CARE-PF is a prospective multi-center registry of ILD patients that included six different ILD centers from across Canada at the time of this sub-study initiation (26). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study received approval from ethics boards at all participating sites [coordinating site: University of British Columbia Research Ethics Office (H18-00993)]. All patients provided written informed consent at the time of enrollment.
Patients enrolled in the registry between January 2015 and September 2018 were included, with this date range chosen to ensure adequate follow-up time for the longitudinal analyses. Registry data are collected at time of enrolment, including all relevant clinical data preceding and following the date of enrolment. For the purposes of this study, a detailed chart review was performed on every registry patient to ascertain treatment details and outcomes. Patients were included if they had a diagnosis of RA-ILD, with their RA confirmed by a rheumatologist, and a HRCT pattern of either UIP or NSIP, as determined by their treating physician. Patients were excluded if they had an overlapping connective tissue disease, including secondary Sjogren’s, or if they were missing a reported HRCT pattern. Patients were also excluded for missing lung function data or key demographic variables necessary to standardize lung function to the same reference range. Variables collected at first ILD clinic visit included baseline age, patient reported sex, smoking status (current/ex-smoker vs. never smoker), race, absolute lung function (FVC and DLCO) and HRCT pattern. All follow-up absolute lung function (FVC and DLCO) were also collected, and date of death or lung transplantation. Absolute FVC and DLCO were then standardized to Global Lung Initiative reference ranges to provide percent predicted (%) values, including correcting DLCO for ambient barometric pressure at each of the locations in Canada (27,28). Patients were censored on November 10, 2021.
Radiographic patterns
Radiographic HRCT patterns were defined at registry enrolment as per the treating clinician’s assessment, as either UIP, NSIP, LIP, or OP, in accordance with contemporaneous radiologic definitions (29-31). The radiographic pattern was designated as UIP if felt to be either probable or UIP. Probable UIP is defined as peripheral, basal predominant reticulation with traction bronchiectasis and UIP as peripheral, basal predominant reticulation/honeycombing with or without traction bronchiectasis (29). NSIP pattern is typically peripheral and basal with ground-glass opacities, reticulation, traction bronchiectasis, subpleural sparing, and little to no honeycombing (30). While not independently or blindly scored by radiologists, this approach is thought to be reflective of the clinician’s HRCT interpretation which is being used to guide therapeutic decision making in clinical practice. All sites have access to formal multidisciplinary rounds discussion, HRCT images, and the radiology report when designating the HRCT pattern on the case-report form at the time of registry enrollment. Only those patients with either UIP or NSIP patterns were included in the analyses.
Treatment determination
Patients were categorized as ‘treated’ if they received what were potentially therapeutic doses of MMF, AZA, RTX, and/or CYC. This was defined as MMF at a minimum total dose of 500 mg per day for over 120 days, AZA at a minimum total dose of 50 mg per day for over 120 days, any dose of RTX, and any dose of CYC (intravenous or oral) for over 120 days. These doses were chosen to favor inclusivity, recognizing that there are no robust studies informing the optimal dosing for this clinical indication. Patients were considered ‘untreated’ if none of these criteria were met. To gain further insight into the use of these medications, we also identified the first medication patients were prescribed, regardless of dose or duration of therapy. The first of these medications recorded in the registry, regardless of duration or dose, was considered ‘first medication received’. Prednisone was not specifically evaluated due to challenges with accurate doses and duration of use. Additionally, there is no significant evidence that prednisone is an effective treatment for patients with fibrotic RA-ILD. Treatment data were ascertained via a standardized retrospective chart review at each participating site.
Statistical analysis
Unpaired t-test, chi-square, and Kruskal-Wallis tests were used to compare baseline characteristics between patients with UIP and NSIP patterns, as well as across treatment groups and for first drug choice. Linear mixed models were used to compare changes in FVC% and DLCO% over time, unadjusted and in models adjusted for age, sex, smoking status, and baseline lung function, with these covariates chosen as potentially relevant confounders. Cox proportional hazards regression models were used to compare the risk of death or lung transplant between patients with UIP and NSIP patterns, and according to treatment status, in unadjusted models and in models adjusted for age, sex, smoking status, and baseline lung function. Kaplan-Meier curves were used to visually compare transplant-free survival for patients with UIP vs. NSIP, and according to treatment status, along with the log-rank test. All statistical analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Study population
A total of 181 patients with RA-ILD were identified from the registry, with 161 included in the analyses shown in Figure 1. Baseline characteristics are presented in Table 1. The mean age was 64 years (±10) and 94/161 (58%) were female. Mean FVC% was 78% (±19) and mean DLCO% was 63% (±22). There were 90 (56%) patients with a UIP pattern and 71 (44%) with an NSIP pattern. Median follow-up time was 4.0 years [interquartile range (IQR), 2.8–5.9] during which 39 patients died and 6 underwent lung transplantation. Patients with a UIP pattern were older, more likely male, and more likely to have smoked compared to patients with an NSIP pattern.
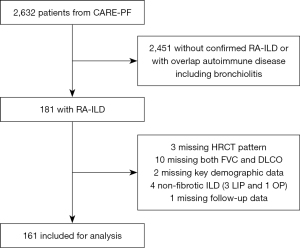
Table 1
| Variables | Total cohort (n=161) | UIP (n=90) | NSIP (n=71) |
|---|---|---|---|
| Age (years), mean ± SD | 64±10 | 66±10 | 62±11 |
| Female sex, n [%] | 94 [58] | 44 [49] | 50 [70] |
| Current or ex-smoker, n [%] | 112 [70] | 66 [73] | 45 [65] |
| Median pack years [IQR]# | 23 [10–38] | 25 [12–38] | 15 [8–33] |
| FVC (L), mean ±SD | 2.6±0.9 | 2.8±0.9 | 2.5±0.8 |
| FVC%, mean ±SD | 78±19 | 79±18 | 78±21 |
| DLCO (mL/mmHg/min), mean ± SD | 13.6±5.8 | 13.5±6.1 | 13.8±5.3 |
| DLCO%, mean ± SD | 63±22 | 60±22 | 66±23 |
| Deceased, n [%] | 39 [24] | 29 [32] | 10 [14] |
| Transplant, n [%] | 6 [4] | 5 [6] | 1 [1] |
| Median follow-up time (years), [IQR] | 4.0 [2.8–5.9] | 3.8 [2.5–5.8] | 4.1 [2.8–6.1] |
| Treated*, n [%] | 44 [27] | 28 [31] | 16 [23] |
#, based on available information from n=111; *, based on ‘treatment’ definition. UIP, usual interstitial pneumonia; NSIP, non-specific interstitial pneumonia; SD, standard deviation; IQR, interquartile range; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
Medication usage
Of these 161 patients, 44 (27%) met the specified treatment definition, with 117 (73%) not meeting this definition, indicating that only about a quarter of patients received drug therapy at what would be considered a minimally effective dose over a meaningful duration of time. The mean dose of MMF in those who met treatment criteria was 1,733 mg/day (±372) with a dose range of 1,500–2,000 mg/day, and for AZA the mean dose was 132 mg (±36) with a dose range of 125–150 mg/day. The median duration of therapy for MMF was 457 (IQR, 294–972) days, for AZA was 1,101 (IQR, 223–1,393) days, for RTX was 478 (IQR, 273–876) days and for CYC was 159 (IQR, 151–366) days. A higher proportion of patients with a UIP pattern were treated (31%) than patients with an NSIP pattern (22%) although this was not statistically different (P=0.22). There were no associations identified between treatment status with patient age, sex, baseline FVC%, or baseline DLCO% shown in Table 2, suggesting that medication prescription or usage are not necessarily determined by these clinical variables.
Table 2
| Variables | Treated (n=44) | Untreated (n=117) | P value |
|---|---|---|---|
| Age (years), mean ± SD | 63±10 | 65±11 | 0.34 |
| Female sex, n [%] | 26 [59] | 68 [58] | 0.91 |
| Baseline FVC%, mean ± SD | 76±15 | 79±20 | 0.25 |
| Baseline DLCO%, mean ± SD | 58±21 | 64±23 | 0.19 |
| Median follow-up time (years), median (IQR) | 5.1 (2.9–6.7) | 3.8 (2.6–5.4) | 0.07 |
SD, standard deviation; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; IQR, interquartile range.
There were 104/161 patients (65%) who received either AZA, MMF, RTX, or CYC at any point, but did not meet the treatment definition that required a specific minimum dose and duration of therapy. The most frequently first prescribed drug was MMF (n=39) followed by AZA (n=37) then RTX (n=24). CYC was the least frequent first attempted medication, being used first in only 4 patients. The remaining 57 patients did not receive any of these four medications at any time shown in Table 3. More patients were initially started on an ILD-specific medication than persisted to meet the definition of ‘treated’ for the purposes of this study, suggesting a high discontinuation rate after attempted initiation. Specific data on reasons for discontinuation were not available.
Table 3
| Variables | MMF (n=39) | AZA (n=37) | RTX (n=24) | CYC (n=4) | Untreated (n=57) |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 63±10 | 61±12 | 66±8 | 58±9 | 67±10 |
| Female sex, n [%] | 21 [54] | 24 [65] | 13 [54] | 3 [75] | 33 [58] |
| FVC (L), mean ± SD | 2.5±0.9 | 2.5±0.8 | 2.8±0.7 | 2.6±0.9 | 2.7±0.9 |
| FVC%, mean ± SD | 73±18 | 75±19.3 | 80±18 | 73±14 | 83±19 |
| DLCO (mL/mmHg/min) mean ± SD | 13.2±5.9 | 12.1±4.6 | 13.3±4.7 | 12.5±7.7 | 15.1±6.6 |
| DLCO%, mean ± SD | 59 ±21 | 55±19 | 61±22 | 52 ±25 | 71±23 |
| Median follow-up time (years), (IQR) | 3.5 [2.5–5.8] | 4.8 [3.0–6.4] | 3.9 [2.8–6.0] | 5.6 [4.4–7.3] | 3.8 [2.6–5.5] |
| Died, n [%] | 9 [23] | 8 [22] | 8 [33] | 1 [25] | 13 [23] |
| Transplant n [%] | 1 [3] | 3 [8] | 0 [0] | 0 [0] | 2 [4] |
| HRCT pattern, n [%] | |||||
| UIP | 15 [38] | 22 [60] | 18 [75.0] | 2 [50] | 33 [58] |
| NSIP | 24 [62] | 15 [40] | 6 [25.0] | 2 [50] | 24 [42] |
MMF, mycophenolate mofetil; AZA, azathioprine; RTX, rituximab; CYC, cyclophosphamide; SD, standard deviation; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; IQR, interquartile range; HRCT, high-resolution computed tomography; UIP, usual interstitial pneumonia; NSIP, non-specific interstitial pneumonia.
Changes in lung function
Mean annual decline in FVC% and DLCO% for the total cohort were 1.42% (±0.35) and 2.11% (±0.34), respectively. Mean annual decline in FVC% and DLCO% in those with NSIP pattern was 0.87% (±0.54) and 1.27% (±0.52), while mean annual decline in those with UIP was 1.81% (±0.46) and 2.7% (±0.43). The difference in annual decline in both FVC% and DLCO% were not statistically significant between patients with a UIP pattern compared to those with an NSIP pattern shown in Table 4. There were no differences in FVC% decline within the NSIP or UIP pattern groups, whether patients met the definition for being treated or untreated.
Table 4
| HRCT pattern and treatment status | FVC% decline | DLCO% decline | |||
|---|---|---|---|---|---|
| Mean ± SD | P value | Mean ± SD | P value | ||
| NSIP | n=69 | n=63 | |||
| Untreated | 1.0±0.6 | 0.63 | 1.2±0.6 | 0.84 | |
| Treated | 0.4±1.1 | 1.4±1.0 | |||
| UIP | n=84 | n=80 | |||
| Untreated | 1.4±0.6 | 0.30 | 2.8±0.6 | 0.77 | |
| Treated | 2.4±0.8 | 2.5±0.7 | |||
HRCT, high-resolution computed tomography; FVC, forced vital capacity; SD, standard deviation; DLCO, diffusion capacity of the lung for carbon monoxide; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia.
Transplant-free survival
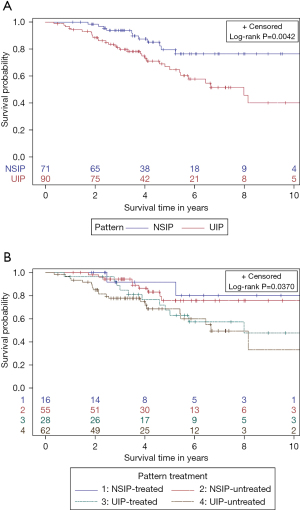
Patients with an NSIP pattern had lower risk of death or transplant, compared to patients with UIP pattern (P=0.0042) (shown in Figure 2A). There was a trend towards improved transplant-free survival among the subgroups of patients with NSIP treated or untreated compared to UIP patients who were treated or untreated (P=0.0370) (shown in Figure 2B). Median survival time for UIP was 7.98 years [95% confidence interval (CI): 5.40–11.49]. There was no difference in time to death or lung transplant in patients with NSIP who were treated compared to those untreated in unadjusted [hazard ratio (HR) =0.71; 95% CI: 0.15 to 3.30; P=0.66] or adjusted models (HR =0.73; 95% CI: 0.15 to 3.62; P=0.70). Similarly, there was no difference in time to death or lung transplant in patients with a UIP pattern who were treated compared to those untreated in unadjusted (HR =0.88; 95% CI: 0.44 to 1.8; P=0.73) or adjusted models (HR =1.06; 95% CI: 0.49 to 2.28; P=0.89).
Discussion
This multi-center registry-based retrospective cohort study found that patients with RA-ILD were often trialed on treatment, but a majority did not persist on it long enough to potentially derive benefit. A higher number of patients were initiated on an ILD-specific pharmacologic therapy (65%) compared to those who persisted on it long enough to meet the treatment definition (27%). Additionally, several different treatments were attempted with MMF and AZA being most frequently initiated, followed by RTX. This heterogenous medication approach appeared independent of HRCT patterns or patient demographics, suggesting that other factors influence medication choice in patients with RA-ILD. Our findings are consistent with, and supportive of, prior data showing that a UIP pattern is associated with worse transplant-free survival compared to an NSIP pattern (9,32-34). Among the full cohort, 24% died and only 3.7% underwent lung transplantation, highlighting potential opportunities for improved approaches to clinical management of patients with RA and ILD.
Whether patients were treated or not was not associated with important clinical outcomes including lung function decline and mortality in this cohort, which may be due to several reasons. Current treatment may be insufficient to relent disease progression, particularly with historical reliance on immunosuppression alone. We cannot exclude confounding by indication in that sicker patients were more likely to be started on therapy, and thus those receiving treatment were at higher baseline risk of disease progression. Additionally, this retrospective cohort study is likely underpowered to detect a true difference if present, again reiterating the need for prospective randomized therapeutic trials. We also found that patients with NSIP demonstrate clinically relevant lung function decline over time, an important finding when clinicians are considering timing of drug initiation as well as key target populations for future clinical trial enrollment. We were not able to evaluate response to treatment in those who experienced acute exacerbations as this outcome is difficult to accurately ascertain within the registry.
The evidence informing pharmacologic management of RA-ILD is sparse and largely based on retrospective cohort studies and case series (13-18). Treatment decisions for ILD are complex and guided by several factors, including disease severity, risk-benefit considerations for immunomodulatory medications, concomitant use of disease-modifying anti-rheumatic drugs (DMARDs) for extra-pulmonary manifestations of disease, access to and funding of medications, and critically, patient preferences and values. Based on current evidence, there is no single correct approach to treatment. While this study did not identify differences in outcomes between patients who were treated or untreated, it is likely that any efficacy signal would be masked by confounding by indication and lack of randomization. Presumably, clinicians are more likely to prescribe drug therapy to patients with more severe disease, or those who are anticipated to be at greater risk of disease progression and clinical decline. Further work should aim to understand the parameters that guide clinical decision-making regarding drug initiation and selection.
While retrospective non-randomized data cannot definitively characterize treatment response, our study notably demonstrates that most patients in this cohort were untreated. The majority were started on an initial therapy, but few persisted to meet our treatment definitions. Reasons for this could include intolerance, complications, or use of other drugs not captured in the registry, including those for the extra-pulmonary manifestations of RA. This study also pre-dates findings from relatively recent trials utilizing the antifibrotics, nintedanib and pirfenidone, in this patient population. Two recent trials using antifibrotic drugs in patients with progressive fibrosing-ILD (PF-ILD) including RA-ILD, have shown positive results in this disease phenotype. Nintedanib and pirfenidone were both shown to slow the rate of disease progression in patients with a PF-ILD phenotype (20-22). These trials do not necessarily inform first-line therapy, or a pharmacologic approach in patients without a PF-ILD phenotype. Recent results from the TRAIL1 trial demonstrated a slowed decline in FVC in RA-ILD patients treated with pirfenidone although the trial was ended prematurely and the primary endpoint was not met (23). In TRAIL1, participants were on a multitude of therapies including both traditional DMARDs and biologic DMARDs and these data do not inform the optimal first lines of pharmacologic therapy for patients with RA-ILD. A phase 2 study evaluating the effect of abatacept on lung function in RA-ILD is currently underway (NCT03084419), as is a phase 4 study comparing tofacitinib to methotrexate to slow parenchymal abnormalities on HRCT (NCT04311567). It is hoped that the results from these trials will inform therapeutic approaches to treating RA-ILD, across radiographic patterns and disease severity at the time of presentation. Another recent study reported improvement in FVC% and DLCO% in RA-ILD patients 12 months after treatment initiation with MMF, AZA or RTX, although theirs included a lower proportion of UIP HRCT pattern (38%) compared to our study (56%) and lung function changes were estimated over a shorter time period (35).
This study is unique in its multi-center well-characterized population, relatively large sample size, and application of standardized lung function values. Conversion of absolute lung function to a standardized norm set is infrequently done in multi-center observational studies of ILD, where pooling of values from differing norm sets may introduce important sources of error, when comparing across individuals. However, our study also has limitations including its retrospective non-randomized design, low numbers of patients on treatment, and potential for selection bias for those initiated on treatment. We cannot conclude efficacy or effectiveness for any of the presented drugs in the absence of randomized clinical trial data. Additionally, there was infrequent reporting of other medications including abatacept, tumor necrosis factor inhibitors and other RA biologics/DMARDs, as these are not systematically collected in this ILD-focused registry but instead are collected by patient self-reporting. We were unable to evaluate the effect of prednisone given the limitations for accuracy in dosing or duration of therapy and could not exclude important misclassification bias. Antifibrotics including nintedanib and pirfenidone were also not considered, as they were not under approved indication in Canada at the time of data collection. We do not have data on RA disease activity or timing of RA diagnosis relative to ILD, both of which should be considered in future studies. While we used a shortened treatment period as opposed to longer for inclusion in the treatment group, and relatively low medication doses, this would be expected to bias the results toward the null, and thus was considered preferable to longer duration and higher drug doses. CARE-PF sites are referral centers for ILD and may have more advanced or complicated patients than typically seen in a community setting, thereby potentially limiting the generalizability of these findings. Additionally, we are not able to accurately ascertain the proportion of patients in Canada with RA-ILD who are cared for at a CARE-PF site or subsequently in the registry. This study utilized HRCT chest imaging to evaluate treatment response, however future study adding surgical lung biopsy results to further delineate cellular and fibrotic NSIP may be of interest.
Conclusions
In summary, there is significant heterogeneity in the treatment of patients with RA-ILD and the majority of patients in this study did not receive the most frequently used medications for treatment of their lung disease. Patients with a UIP radiologic pattern have worse outcomes, compared to patients with an NSIP pattern, as shown in previous smaller studies. However, patients with NSIP pattern also demonstrate progression over time, including lung function decline, and death or lung transplant. These real-world findings highlight the urgent need for randomized trials of therapeutics for RA-ILD, with treatments for early disease as well as the progressive fibrosing phenotype.
Acknowledgments
The authors would like to thank Dr. Brian Graham for his assistance in using the Global Lung Initiative conversion equations, and notably, the patients for their valuable participation in clinical research through their contributions to CARE-PF.
Funding: CARE-PF is funded by Boehringer Ingelheim.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1820/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1820/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1820/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1820/coif). All authors report that Canadian Registry for Pulmonary Fibrosis is sponsored by Boehringer Ingelheim Canada but it has had no influence on this study design/manuscript. VM reports personal fees from Boehringer Ingelheim Canada and Hoffman-La Roche Ltd., and Astra Zeneca, grants from the University of Saskatchewan, Royal University Hospital Foundation, Boehringer Ingelheim, Astra Zeneca and Hoffman-La Roche. SL reports consulting fees and moderator honoraria from Boehringer-Ingelheim and a grant from the University of Saskatchewan. SL has served on prior advisory boards of Boehringer-Ingelheim. DA reports consulting fees from Boehringer-Ingelheim and Hoffman-La Roche, payments for a lecture from Boehringer-Ingelheim, and research grants from Boehringer Ingelheim. JHF reports grants from the Canadian Pulmonary Fibrosis Foundation and the University of Toronto, and personal fees from Boehringer-Ingelheim and AstraZeneca, outside the submitted work. JHF serves as an unpaid medical advisory board member of Canadian Pulmonary Fibrosis Foundation. SS reports advisory board participation and personal fees from Boehringer-Ingelheim, Hoffman-La Roche, and AstraZeneca. JM reports personal fees from Boehringer-Ingelheim and Hoffman-La Roche. HM reports research grants from Boehringer-Ingelheim, Galapagos, BMS and Hoffman-La Roche, personal fees from Boehringer-Ingelheim, and participates on an advisory board for Boehringer Ingelheim. CDF reports educational grants and research grants from Boehringer-Ingelheim, Roche and the Canadian Pulmonary Fibrosis Foundation; personal fees from Roche and Boehringer Ingelheim; Chair of the Board for the Canadian Pulmonary Fibrosis Foundation. NH reports research grants from Boehringer-Ingelheim and Janssen, and personal fees from Boehringer-Ingelheim, Janssen, and Hoffman-La Roche. MK serves as an unpaid editorial board member of Journal of Thoracic Disease. MK reports grants from Boehringer-Ingelheim, Hoffman-La Roche and Pieris, personal fees from Boehringer Ingelheim, Hoffman-La Roche, Horizon, Cipla, Abbvie, Bellerophon, Algernon, CSL Behring, United Therapeutics Novartis and LabCorp, participation on the advisory board of United Therapeutics Novartis and LabCorp, and allowance for serving as Chief Editor of the European Respiratory Journal. MS reports honoraria for attending symposia from Boehringer-Ingelheim. AWW reports speaker’s honoraria from Boehringer-Ingelheim and AstraZeneca. PGW reports payment for presentation from Vertex. PGW is a member of the Cystic Fibrosis Foundation Drug Safety Monitoring Board. CJR reports personal fees from Boehringer-Ingelheim, Hoffman-La Roche, Veracyte, AstraZeneca, Pliant Therapeutics, Cipla Ltd., and Ensho Health. KAJ reports grants from University Hospital Foundation, Three Lakes Foundation and University oof Calgary CSM, and personal fees from Boehringer-Ingelheim, Hoffman-La Roche, Pliant Therapeutics and Three Lakes Foundation. KAJ is a member of the board for PFOX Trial. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study received approval from ethics boards at all participating sites [coordinating site: University of British Columbia Research Ethics Office (H18-00993)]. All patients provided written informed consent at the time of enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023-38. [Crossref] [PubMed]
- Kadura S, Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev 2021;30:210011. [Crossref] [PubMed]
- Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017;76:1700-6. [Crossref] [PubMed]
- Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford) 2010;49:1483-9. [Crossref] [PubMed]
- Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183:372-8. [Crossref] [PubMed]
- Nakamura Y, Suda T, Kaida Y, et al. Rheumatoid lung disease: prognostic analysis of 54 biopsy-proven cases. Respir Med 2012;106:1164-9. [Crossref] [PubMed]
- Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010;35:1322-8. [Crossref] [PubMed]
- Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005;127:2019-27. [Crossref] [PubMed]
- Solomon JJ, Ryu JH, Tazelaar HD, et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Respir Med 2013;107:1247-52. [Crossref] [PubMed]
- Assayag D, Lubin M, Lee JS, et al. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 2014;19:493-500. [Crossref] [PubMed]
- Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016;47:588-96. [Crossref] [PubMed]
- Kawano-Dourado L, Lee JS. Management of Connective Tissue Disease-Associated Interstitial Lung Disease. Clin Chest Med 2021;42:295-310. [Crossref] [PubMed]
- Fischer A, Brown KK, Du Bois RM, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol 2013;40:640-6. [Crossref] [PubMed]
- Swigris JJ, Olson AL, Fischer A, et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest 2006;130:30-6. [Crossref] [PubMed]
- Keir GJ, Maher TM, Ming D, et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology 2014;19:353-9. [Crossref] [PubMed]
- Braun-Moscovici Y, Butbul-Aviel Y, Guralnik L, et al. Rituximab: rescue therapy in life-threatening complications or refractory autoimmune diseases: a single center experience. Rheumatol Int 2013;33:1495-504. [Crossref] [PubMed]
- Vadillo C, Nieto MA, Romero-Bueno F, et al. Efficacy of rituximab in slowing down progression of rheumatoid arthritis-related interstitial lung disease: data from the NEREA Registry. Rheumatology (Oxford) 2020;59:2099-108. [Crossref] [PubMed]
- Zhang G, Xu T, Zhang H, et al. Randomized control multi-center clinical study of mycophenolate mofetil and cyclophosphamide in the treatment of connective tissue disease related interstitial lung disease. Zhonghua Yi Xue Za Zhi 2015;95:3641-5. [PubMed]
- Barnes H, Holland AE, Westall GP, et al. Cyclophosphamide for connective tissue disease-associated interstitial lung disease. Cochrane Database Syst Rev 2018;1:CD010908. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019;381:1718-27. [Crossref] [PubMed]
- Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 2021;9:476-86. [Crossref] [PubMed]
- Behr J, Neuser P, Prasse A, et al. Exploring efficacy and safety of oral Pirfenidone for progressive, non-IPF lung fibrosis (RELIEF) - a randomized, double-blind, placebo-controlled, parallel group, multi-center, phase II trial. BMC Pulm Med 2017;17:122. [Crossref] [PubMed]
- Solomon JJ, Danoff SK, Woodhead FA, et al. Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir Med 2023;11:87-96. [Crossref] [PubMed]
- Mira-Avendano IC, Parambil JG, Yadav R, et al. A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis. Respir Med 2013;107:890-6. [Crossref] [PubMed]
- Deheinzelin D, Capelozzi VL, Kairalla RA, et al. Interstitial lung disease in primary Sjögren's syndrome. Clinical-pathological evaluation and response to treatment. Am J Respir Crit Care Med 1996;154:794-9. [Crossref] [PubMed]
- Ryerson CJ, Tan B, Fell CD, et al. The Canadian Registry for Pulmonary Fibrosis: Design and Rationale of a National Pulmonary Fibrosis Registry. Can Respir J 2016;2016:3562923. [Crossref] [PubMed]
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324-43. [Crossref] [PubMed]
- Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J 2017;50:1700010. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Yunt ZX, Chung JH, Hobbs S, et al. High resolution computed tomography pattern of usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease: Relationship to survival. Respir Med 2017;126:100-4. [Crossref] [PubMed]
- Kim EJ, Collard HR, King TE Jr. Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 2009;136:1397-405. [Crossref] [PubMed]
- Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143-8. [Crossref] [PubMed]
- Matson SM, Baqir M, Moua T, et al. Treatment Outcomes for Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Real-World, Multisite Study of the Impact of Immunosuppression on Pulmonary Function Trajectory. Chest 2023;163:861-9. [Crossref] [PubMed]


