
Efficacy and safety of immunoradiotherapy for advanced non-small cell lung cancer: a retrospective comparative cohort study
Highlight box
Key findings
• In clinical practice, combination therapy using immune checkpoint inhibitors (ICIs) and radiation may improve progression-free survival (PFS) and tumor response rates in advanced non-small cell lung cancer (NSCLC) patients and radiotherapy concurrent with ICI can boost the distant effect.
What is known and what is new?
• Ample mechanistic evidence suggests that radiotherapy can enhance the immune response.
• In clinical practice, radiotherapy concurrent with ICI can boost the distant effect; Subgroup analyses revealed that single-site, high biologically effective dose (BED) (≥72 Gy), planning target volume (PTV) size (<213.7 mL) radiotherapy groups had better PFS. In multivariate analysis, PTV size was an independent predictor of immunotherapy PFS.
What is the implication, and what should change now?
• More prospective and external cohort validations are required to confirm the results of the present study.
Introduction
The rapid advancement of immunotherapy has led to the development of immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) expression, which have demonstrated promising therapeutic effects. PD-1/PD-L1 inhibitors, including pembrolizumab and atezolizumab, have been recommended as the first-line treatment for patients with advanced non-small cell lung cancer (NSCLC) exhibiting high PD-L1 expression (1-3). KEYNOTE-024 study demonstrated that the 5-year overall survival (OS) rate of patients with advanced NSCLC (with high PD-L1 expression) who received immunotherapy was approximately doubled (31.9% vs. 16.3%) compared with that of patients who received chemotherapy (4). However, the efficacy of ICIs remains controversial because effective antitumor activity can only be observed in patients sensitive to the drugs, which have been reported in the minority (17–48%) (2,5). Therefore, exploring the means to improve the systemic efficacy of ICIs remains an important research topic (6-8). One of the interesting research areas is immunoradiotherapy (iRT), which involves a combination of ICIs and radiotherapy (RT) (9-11).
Ample mechanistic evidence suggests that RT can enhance the immune response (12-15), which can be attributed mainly to its in-situ tumor vaccination effect and distant effect. RT leads to the release of antigens to the whole body from the primary tumor tissue, which are recognized by antigen-presenting cells (e.g., dendritic cells), and these cells finally present the antigens to T lymphocytes (especially cytotoxic CD8+ T cells). Therefore, RT can turn cold tumors into hot and inflamed tumors and enhance anti-PD-1/PD-L1 immune response (e.g., increase in capillary permeability and promotion of PD-L1 expression in tumor cells). The results of several clinical trials, including PACIFIC, have demonstrated that iRT has a promising impact on patients with specific types of cancer (9-11,16-19). For patients with metastatic NSCLC, clinical supporting evidence is available for the use of RT in combination with immunotherapy (13). In the PEMBRO-RT trial, although no significant difference was observed in the remission rate between the pembrolizumab combined with RT group and the pembrolizumab alone group (36% vs. 18%, P=0.07) (20), a pooled analysis of two randomized trials by Theelen et al. showed that RT along with pembrolizumab immunotherapy could significantly improve the response and prognosis of patients with metastatic NSCLC (12). For locally advanced non-metastatic cases, the randomized PACIFIC trial demonstrated the efficacy of RT with subsequent immunotherapy (21). Two retrospective studies with a small sample size have shown that the combination of RT and nivolumab immunotherapy can improve OS and progression-free survival (PFS) without increasing acute toxicity in patients with advanced NSCLC (22,23). The data so far is compelling and supports the need for additional research into combination treatment schedules.
A review by Li et al. (24) showed that the incidence of immune-associated or radiation-induced pneumonia was higher in patients with advanced NSCLC who received combined iRT. A study by Bang et al. (25) reported high overall toxicity after RT within 14 days of ICI treatment. Although extensive attention has been paid to the strategy of RT combined with ICIs in recent years, the efficacy and safety of PD-1/PD-L1 inhibitors combined with RT in NSCLC treatment remain to be investigated.
This retrospective study was conducted to investigate the efficacy and safety of ICI monotherapy or combined iRT in patients with advanced NSCLC in the real-world clinical setting and to explore independent predictors for the efficacy of RT combined with immunotherapy and the incidence of immune-related pneumonitis associated with this treatment strategy. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1685/rc).
Methods
Participants
Patients treated with immunotherapy were retrospectively collected from Shandong Cancer Hospital between September 2018 and March 2020. The last follow-up and data collection were by June 2022. The inclusion criteria were as follows: (I) patients histologically or cytologically diagnosed with unresectable stage III/IV NSCLC; (II) patients with at least one evaluable target lesion (except those with uncontrolled brain metastases) according to Response Evaluation Criteria In Solid Tumours (RECIST, version 1.1); (III) patients who had undergone at least one efficacy evaluation during treatment. (IV) patients with wild-type epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase tumors met the inclusion criteria regardless of the type of PD-1/PD-L1 inhibitor used. The exclusion criteria were as follows: (I) patients pathologically diagnosed with small cell lung cancer (SCLC); (II) patients previously treated with a PD-1 inhibitor, PD-L1 inhibitor. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Shandong Cancer Hospital Affiliated to Shandong First Medical University (No. SDTHEC2022011004) and individual consent for this retrospective analysis was waived.
The following patient data were collected from the medical records: age; sex; number of prior systemic chemotherapies; histological type; PD-L1 expression; EGFR mutation; smoking history; presence or absence of liver disease; presence of bone and brain metastases; previous treatments; treatment response; characteristics of RT; and laboratory results including hematology, blood biochemistry, and liver and kidney function tests.
PD-L1 expression on tumor cells was assessed through immunohistochemistry by utilizing the anti-PD-L1 clone 22C3 from Dako®. Histologic slides containing a minimum of 100 tumor cells. In total, 67.4% (29/43) patients had EGFR sensitizing mutations (such as exon 19 deletion, exon 21 L858R, L861Q or L861R, exon 20 S786I, and T790M mutations).
Treatments
Of the total patients, 155 were treated with anti-PD-(L)1 alone (ICI-alone group) and 202 were treated with anti-PD-(L)1 in combination with RT (ICI + RT group), 209 received chemotherapy. The types of ICIs are shown in Table S1. Of the 202 (56.6%) patients who received combination therapy, 160 (79.2%) were treated with RT prior to immunotherapy, 42 (20.8%) were treated concomitantly with RT and immunotherapy. Of the 155 (43.4%) patients who received immunotherapy alone, treatment was given until disease progression, severe toxicity, or death.
The patients received conventional fractionated RT or stereotactic RT. The single dose of conventional fractionated RT ranged from 1.23 to 4 Gy, and the number of fractions ranged from 10 to 38 fractions. The single dose of stereotactic RT ranged from 5 to 8 Gy, and the number of fractions ranged from 3 to 10 fractions. Treatment was given once daily, five times a week. The median biologically effective dose (BED) was 72 Gy, ranging from 12 to 175 Gy, with a total dose of 21–70 Gy. The median planning target volume (PTV) size was 213 mL, ranging from 9.9–2,148.0 mL. The duration of RT ranged from 5–49 days. The BED was calculated using the basic BED model in the literature (26), which takes into account the beam on time and the prescribed dose, and different alpha/beta ratio for different histological classes.
Outcomes
All patients were followed-up from September 2018 to March 2020 for survival until death or loss to follow-up. PFS and OS were measured as the time period between the start of treatment and documented disease progression and death owing to any cause, respectively. Tumor control included complete response (CR) and partial response (PR), both of which were classified as achieving objective response rate (ORR). We also used the disease control rate (DCR) included stable disease (SD), PR, and CR. The rates of in-field objective response (ifORR) and in-field disease control (ifDCR) were defined in irradiated lesions only. In contrast, the rates of out-of-field objective response [distant response rate (DRR)] and out-of-field disease control [distant control rate (DCRt)] were assessed in unirradiated lesions.
Treatment efficacy was evaluated through imaging every 6 weeks during the course of treatment. Patients underwent imaging evaluations after completing RT but while still on immunotherapy. According to Response Evaluation Criteria In Solid Tumours (RECIST; v. 1.1), magnetic resonance imaging (MRI) or computed tomography (CT) scans of the brain, neck, chest, and abdomen for all patients were assessed by at least two associate chief physicians or higher-level imaging specialists and one oncologist for a comprehensive evaluation of the patient’s overall health status.
The enrolled patients underwent laboratory assessments (including hematology, blood biochemistry, and liver and kidney function tests) before each treatment cycle. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 5.0). Assessments were performed by at least three independent medical professionals.
Statistical analyses
Associations between variables and PFS or OS were analyzed using Kaplan-Meier survival curves, the log-rank test, and univariate or multivariate Cox regression models. Multivariate hazard ratios (HRs) were estimated using a stratified Cox regression model, and 95% confidence intervals (CIs) were calculated using the Brookmeyer-Crowley method. A Spearmen correlation was performed firstly to make sure that all the factors included in the multivariate analysis were not strongly correlated each other. Subgroup analyses were performed by estimating unstratified HRs using a Cox proportional hazards model. Univariate and multivariate analyses were conducted using logistic regression models to assess the risk factors for immune-related pneumonitis. Analyses were performed using IBM SPSS Statistics 23.0 software. Categorical variables were compared by using the chi-square test. A two-tailed P value of <0.05 was considered significant.
Results
Clinicopathologic features and outcomes
In total, 1,213 patients who treated with immunotherapy between September 2018 and March 2020 were screened in a procedure. Of the 1,213 patients, 439 were diagnosed with lung cancer, 45 were diagnosed with SCLC, and 37 who did not have complete treatment records were excluded from the study. A total of 357 patients were ultimately enrolled in this study. The 357 patients were divided into the ICI + RT group (n=202) and the ICI alone group (n=155).
Table 1 presents the baseline characteristics of the 357 patients enrolled in the study. Median PFS was 6.0 months with immunotherapy alone and 12.0 months with immunotherapy plus RT (HR, 0.535; 95% CI: 0.402–0.713; P<0.0001) (Figure 1A). A trend of prolonging OS with combination therapy was found; however, the difference was not statistically significant between the groups (ICI + RT vs. ICI alone: HR, 0.746; 95% CI: 0.494–1.128; P=0.130) (Figure 1B). The ICI + RT group exhibited a significantly higher ORR than the ICI alone group (30.2% vs. 18.7%; P=0.014). DCR also differed significantly between the groups and was higher in the ICI + RT group (82.2% vs. 71.0%; P=0.015) (Figure 2A).
Table 1
| Characteristics | ICI + RT (n=202) | ICI alone (n=155) | P value |
|---|---|---|---|
| Age, mean ± SD (years) | 57.54±10.00 | 58.50±11.46 | 0.950 |
| Gender, n (%) | 0.543 | ||
| Male | 153 (75.7) | 113 (72.9) | |
| Female | 49 (24.3) | 42 (27.1) | |
| Smoking status, n (%) | 0.241 | ||
| Never-smoker | 103 (51.0) | 69 (44.5) | |
| Former/active smoker | 99 (49.0) | 86 (55.5) | |
| Tumor histology, n (%) | 0.490 | ||
| Adenocarcinoma | 110 (54.5) | 96 (61.9) | |
| Squamous carcinoma | 88 (43.6) | 55 (35.5) | |
| NSCLC | 3 (1.5) | 3 (1.9) | |
| Others† | 1 (0.5) | 1 (0.6) | |
| Metastatic sites, n (%) | 0.105 | ||
| Bone | 50 (24.8) | 43 (27.7) | |
| Lung/pleura | 39 (19.3) | 43 (27.7) | |
| Brain | 39 (19.3) | 20 (12.9) | |
| Local lymph nodes | 27 (13.4) | 17 (11.0) | |
| Adrenal glands | 21 (10.4) | 11 (7.1) | |
| Liver | 23 (11.4) | 19 (12.3) | |
| Others‡ | 13 (6.4) | 6 (3.9) | |
| EGFR, n (%) | 0.556 | ||
| Mutation | 14 (6.9) | 15 (9.7) | |
| Wild-type | 9 (4.5) | 5 (3.2) | |
| Unknown | 179 (88.6) | 135 (87.1) | |
| PD-L1, n (%) | 0.056 | ||
| <1% | 7 (3.5) | 6 (3.9) | |
| 1–49% | 9 (4.5) | 6 (3.9) | |
| ≥50% | 5 (2.5) | 16 (10.3) | |
| Unknown | 181 (89.6) | 127 (81.9) | |
| Line of ICI treatment | 0.002 | ||
| 1 | 38 (18.8) | 63 (40.6) | |
| ≥2 | 164 (81.2) | 92 (59.4) |
†, 1 adenosquamous carcinoma (ICI alone); 1 sarcomatoid carcinoma (ICI + RT); ‡, 6 soft tissues, 2 peritoneum, 2 kidney, 2 pericardium, 1 spleen (ICI alone); combination: 2 pancreases, 2 kidneys,1 oophoron, 1 pericardium (ICI + RT). ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death 1 ligand 1; RT, radiotherapy; SD, standard deviation.

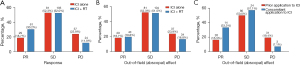
No significant differences were observed in the DRR and DCRt between the ICI + RT group and ICI alone group (19.8% vs. 18.7%, P=0.893 and 71.3% vs. 71.0%, P=1.000, respectively) (Figure 2B). Regarding the distant effect of the RT group, the effects of prior ICI application and concomitant ICI application in terms of DRR and DCRt was compared; results are presented in Figure 2C. Best DRR was significantly higher with concomitant ICI application than with prior ICI application (33.3% vs. 16.3%; OR, 2.58; 95% CI: 1.20–5.55; P=0.018). Best DCRt was also significantly higher with concomitant ICI application than with prior ICI application (90.5% vs. 66.2%; OR, 4.84: 95% CI: 1.64–14.27; P=0.002).
Univariate and multivariate analyses for PFS
In univariate analysis, sex (male and female: HR, 0.72; P=0.042), Karnofsky Performance Status (KPS) (≤80 and >80: HR, 0.72; P=0.029), treatment (RT and non-RT, HR: 0.54; P<0.001), regional lymph node metastasis (HR, 1.86; P=0.001), and local recurrence (recurrence and non-recurrence: HR, 1.56; P=0.032) were identified as the significant factors (Table 2). Spearmen correlation coefficient among variables ranges from −0.045 to 0.439, and there is no strong correlation between any pair of independent variables.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | – | – | |||
| <60 | 1.00 (ref.) | ||||
| ≥60 | 0.87 (0.65–1.15) | 0.323 | |||
| Gender | |||||
| Female | 1.00 (ref.) | 1.00 (ref.) | |||
| Male | 0.72 (0.53–0.99) | 0.042 | 0.78 (0.57–1.07) | 0.12 | |
| Never-smoker | – | – | |||
| Never smoker | 1.00 (ref.) | ||||
| Current/former | 0.94 (0.71–1.25) | 0.668 | |||
| KPS | |||||
| ≤80 | 1.00 (ref.) | 1.00 (ref.) | |||
| >80 | 0.72 (0.54–0.97) | 0.029 | 0.65 (0.49–0.88) | 0.005 | |
| Histological features | – | – | |||
| Squamous cell carcinoma | 1.00 (ref.) | ||||
| Adenocarcinoma | 1.08 (0.34–3.44) | 0.891 | |||
| Others | 1.30 (0.41–4.10) | 0.652 | |||
| Treatment regimen | |||||
| ICI | 1.00 (ref.) | 1.00 (ref.) | |||
| ICI + RT | 0.54 (0.40–0.71) | <0.001 | 0.52 (0.39–0.70) | <0.001 | |
| Line of ICI treatment | – | – | |||
| 1 | 1.00 (ref.) | ||||
| ≥2 | 1.13 (0.83–1.54) | 0.442 | |||
| Metastatic sites | – | – | |||
| Multiple metastasis | 1.00 (ref.) | ||||
| Oligometastasis | 1.01 (0.68–1.48) | 0.958 | |||
| Local recurrence | |||||
| Yes | 1.00 (ref.) | 1.00 (ref.) | |||
| No | 1.56 (1.04–2.35) | 0.032 | 1.46 (1.26–2.83) | 0.1 | |
| Local lymph nodes | |||||
| Metastasis | 1.00 (ref.) | 1.00 (ref.) | |||
| Non-metastasis | 1.86 (1.29–2.68) | 0.001 | 1.89 (1.26–2.83) | 0.002 | |
| PD-L1 status (%) | – | – | |||
| <1 | 1.00 (ref.) | ||||
| ≥1 | 0.81(0.35–1.86) | 0.619 | |||
CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; KPS, Karnofsky Performance Status; PD-L1, programmed cell death 1 ligand 1; RT, radiotherapy.
The multivariate Cox regression analysis of PFS showed that combination with RT (HR, 0.52; 95% CI: 0.39–0.70; P<0.001), KPS (HR, 0.65; 95% CI: 0.49–0.88; P=0.005), and regional lymph node metastasis before treatment (HR, 1.89; 95% CI: 1.26–2.83; P=0.002) were the significant factors (Table 2).
Univariate and multivariate analyses for PFS in the RT subgroup
Of the 357 patients, 202 (56.6%) received immunotherapy in combination with RT, including pre-immune or intra-immune RT. The characteristics of RT are shown in Table 3. A statistical analysis of the RT subgroup was performed to compare the outcomes of patients with different baseline values of BED, number of irradiated lesions, PTV size, and RT interval below or above the respective pre-established cut-off value. PFS was obtained for each RT characteristic (Figure 3).
Table 3
| Characteristics | Radiotherapy (n=202) |
|---|---|
| Age (years) | |
| >60 | 109 (54.0) |
| ≤60 | 93 (46.0) |
| Male gender | |
| Male | 153 (75.7) |
| Female | 49 (24.3) |
| Smoking status | |
| Never smoker | 103 (51.0) |
| Current/former | 99 (49.0) |
| Histological features | |
| Adenocarcinoma | 110 (54.5) |
| Squamous cell carcinoma | 88 (43.6) |
| Others | 4 (2.0) |
| PD-L1 status | |
| <1 | 7 (3.5) |
| ≥1 | 14 (6.9) |
| Unknown | 181 (89.6) |
| KPS | |
| ≤80 | 114 (56.4) |
| >80 | 88 (43.6) |
| BED (Gy) | 72 [12–175] |
| PTV size (Gy) | 213 [9.9–2,148.0] |
| Total dose (Gy) | 54 [10–70] |
| Number of fractions (Gy) | 25 [3–38] |
| Dose per fraction (Gy) | 2 [1.23–8] |
| Duration of radiotherapy (day) | 32 [5–49] |
| Irradiated lesion | |
| Singe irradiated site | 133 (65.8) |
| Multiples irradiated site | 59 (29.2) |
| Time interval between radiotherapy and immunotherapy | |
| >6 months | 98 (48.5) |
| ≤6 months | 100 (49.5) |
| Timing of radiotherapy | |
| Prior application to ICI | 160 (79.2) |
| Concomitant with ICIs | 42 (20.8) |
| Type of radiation | |
| Traditional RT | 191 (94.6) |
| SBRT | 11 (5.4) |
Data are presented as n (%) or median [range]. BED, biological effective dose; ICI, immune checkpoint inhibitor; KPS, Karnofsky Performance Status; PD-L1, programmed cell death 1 ligand 1; PTV, planning target volume; RT, radiotherapy; SBRT, stereotactic body radiotherapy.

In the univariate analysis, a high BED (≥72 vs. <72: HR, 0.78; 95% CI: 0.51–1.19; P=0.25) and a low RT-immunotherapy interval (≤6 vs. >6 months: HR, 0.84; 95% CI: 0.56–1.27; P=0.415) showed better efficacy but without statistical significance. The number of irradiated lesion (singe irradiated site vs. multiples irradiated site: HR, 0.54; 95% CI: 0.35–0.84; P=0.006), PTV volume (≥213.7 vs. <213.7 mL: HR, 1.97; 95% CI: 1.14–3.39; P=0.015), gender (male vs. female: HR, 1.51; 95% CI: 0.95–2.4; P=0.083) were significantly correlated with PFS (Table 4). Values of correlation coefficient range between −0.138 and 0.049, and there is no strong correlation between any pair of independent variables.
Table 4
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | – | – | |||
| ≥60 | 1.00 (ref.) | ||||
| <60 | 0.76 (0.51–1.13) | 0.169 | |||
| Gender | |||||
| Male | 1.00 (ref.) | 1.00 (ref.) | |||
| Female | 1.51 (0.95–2.41) | 0.083 | 0.89 (0.44–1.84) | 0.766 | |
| Smoking status | – | – | |||
| Never smoker | 1.00 (ref.) | ||||
| Current/former | 0.85 (0.57–1.28) | 0.44 | |||
| Histological features | – | – | |||
| Adenocarcinoma | 1.00 (ref.) | ||||
| Squamous cell carcinoma | 0.75 (0.50–1.12) | 0.159 | |||
| Others | 0.85 (0.12–6.15) | 0.869 | |||
| PD-L1 status (%) | – | – | |||
| <1 | 1.00 (ref.) | ||||
| ≥1 | 0.76 (0.49–1.20) | 0.246 | |||
| KPS | – | – | |||
| ≤80 | 1.00 (ref.) | ||||
| >80 | 0.85 (0.57–1.27) | 0.42 | |||
| PTV size (mL) | |||||
| <213.7 | 1.00 (ref.) | 1.00 (ref.) | |||
| ≥213.7 | 1.97 (1.14–3.39) | 0.015 | 1.89 (1.04–3.42) | 0.035 | |
| BED (Gy) | – | – | |||
| <72 | 1.00 (ref.) | ||||
| ≥72 | 0.78 (0.51–1.19) | 0.25 | |||
| No. of irradiated lesion | |||||
| Multiples irradiated site | 1.00 (ref.) | 1.00 (ref.) | |||
| Singe irradiated site | 0.54 (0.35–0.84) | 0.006 | 0.63 (0.35–1.11) | 0.628 | |
| Time interval between radiotherapy and immunity | – | – | |||
| >6 months | 1.00 (ref.) | ||||
| ≤6 months | 0.84 (0.56–1.27) | 0.415 | |||
| Type of radiation | – | – | |||
| Traditional RT | 1.00 (ref.) | ||||
| SBRT | 0.98 (0.40–2.43) | 0.979 | |||
BED, biological effective dose; CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; KPS, Karnofsky Performance Status; PD-L1, programmed cell death 1 ligand 1; PTV, planning target volume; RT, radiotherapy; SBRT, stereotactic body radiotherapy.
Subsequently, three variables with P values <0.1 in the univariate analysis were included in the multivariate analysis, and it was found that only PTV volume (≥213.7 vs. <213.7 mL: HR, 1.89; 95% CI: 1.04–3.42; P=0.035) could serve as an independent predictor for immunotherapy PFS (Table 4).
Onset, frequency, and CTCAE grade of AEs
Details of treatment-related AEs are presented in Table 5. Immune-related AEs were observed in 137 (38.4%) patients, of which 86 (42.6%) were in the ICI + RT group and 51 (32.9%) were in the ICI alone group (P=0.079). The main AEs included pneumonitis [36 (17.8%) patients in the ICI + RT group vs. 12 (7.7%) patients in the ICI alone group; P=0.007], hepatic insufficiency [24 (11.9%) vs. 9 (5.8%); P=0.064], hypothyroidism (13 (6.4%) vs. 10 (6.5%); P=1.000), and renal insufficiency [4 (2.0%) vs. 1 (0.6%); P=0.393] (Table 4). Overall, 35 (9.8%) patients experienced grade 3 or worse AEs [25 (12.4%) in the ICI + RT group and 10 (6.5%) in the ICI alone group; P=0.062].
Table 5
| Characteristics | Any grade, n (%) | Grade 3+, n (%) | |||||
|---|---|---|---|---|---|---|---|
| ICI + RT (n=202) | ICI alone (n=155) | P value | ICI + RT (n=202) | ICI alone (n=155) | P value | ||
| All AEs | 86 (42.6) | 51 (32.9) | 0.079 | 25 (12.4) | 10 (6.5) | 0.073 | |
| Pneumonitis | 36 (17.8) | 12 (7.7) | 0.007 | 11 (5.4) | 5 (3.2) | 0.440 | |
| Hepatic insufficiency | 24 (11.9) | 9 (5.8) | 0.064 | 7 (3.5) | 2 (1.3) | 0.309 | |
| Renal insufficiency | 4 (2.0) | 1 (0.6) | 0.393 | – | – | – | |
| Hypothyroidism | 13 (6.4) | 10 (6.5) | 1.000 | 3 (1.5) | 3 (1.9) | 1.000 | |
| Myocarditis | 1 (0.5) | 1 (0.6) | 1.000 | – | – | – | |
| Diarrhea | 8 (4.0) | 10 (6.5) | 0.333 | 3 (1.5) | 1 (0.6) | 0.636 | |
| Fatigue | 11 (5.4) | 6 (3.9) | 0.619 | – | – | – | |
| Decreased appetite | 6 (3.0) | 1 (0.6) | 0.144 | – | – | – | |
| Rash/pruritus | 1 (0.5) | 2 (1.3) | 0.583 | 0 (0.0) | 1 (0.6) | 0.434 | |
| CCEP | 1 (0.5) | 1 (0.6) | 1.000 | – | – | – | |
AEs, adverse events; CCEP, cutaneous capillary endothelial proliferation; ICI, immune checkpoint inhibitor; RT, radiotherapy.
Among all AEs, the incidence of only pneumonia differed significantly between the groups (P=0.007). However, the incidence of CTCAE ≥3 pneumonia [11 (5.4%) vs. 5 (3.2%); P=0.440] did not differ significantly (Table 4). In the pneumonitis cohort (n=48), 36 (75.0%) patients received RT, of which 22 (45.8%) received thoracic RT and 14 (29.2%) received non-thoracic RT (Table 6). The incidence of pneumonitis in the ICI + RT group was higher than that in the ICI alone group (P=0.007). According to our observation, non-thoracic RT increased the risk of pneumonitis compared with immunotherapy alone (P=0.006).
Table 6
| Pneumonitis | All patients (n=357), n (%) |
ICI + RT (n=202), n (%) | ICI alone (n=155), n (%) | ||
|---|---|---|---|---|---|
| Total (n=202) | Thoracic radiotherapy (n=130) | Non-thoracic radiotherapy (n=65) | |||
| Total | 48 (13.4) | 36 (17.8) | 22 (16.9) | 14 (21.5) | 12 (7.7) |
| Grade 1 | 19 (39.6) | 15 (41.7) | 9 (40.9) | 6 (42.9) | 4 (33.3) |
| Grade 2 | 13(27.1) | 10 (27.8) | 5 (22.7) | 5 (35.7) | 3 (25.0) |
| Grade 3 | 15 (31.3) | 10 (27.8) | 7 (31.8) | 3 (21.4) | 5 (41.7) |
| Grade 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Grade 5 | 1 (2.1) | 1 (2.8) | 1 (4.5) | 0 (0.0) | 0 (0.0) |
ICI, immune checkpoint inhibitor; RT, radiotherapy.
Risk factors for immune-related pneumonitis
The results of the logistic regression analysis for immune-related pneumonitis (IRP) are presented in Table 7. In univariate analysis, age (≥60 vs. <60 years: HR, 2.04; 95% CI: 1.08–3.87; P=0.029) and prior or concomitant application of RT (yes vs. no: HR, 2.58; 95% CI: 1.30–5.16; P=0.007) were identified as the significant factors for IRP. Values of correlation coefficient is −0.049 and there is no strong correlation between any pair of these two independent variables.
Table 7
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | |||||
| <60 | 1.00 (ref.) | 1.00 (ref.) | |||
| ≥60 | 2.04 (1.08–3.87) | 0.029 | 1.98 (1.04–3.79) | 0.038 | |
| Gender | – | – | |||
| Female | 1.00 (ref.) | ||||
| Male | 0.74 (0.35–1.56) | 0.428 | |||
| Never-smoker | – | – | |||
| Never smoker | 1.00 (ref.) | ||||
| Current/former | 1.01 (0.55–1.86) | 0.969 | |||
| KPS | – | – | |||
| ≤80 | 1.00 (ref.) | ||||
| >80 | 1.50 (0.81–2.77) | 0.197 | |||
| Histological features | – | – | |||
| Squamous cell carcinoma | 1.00 (ref.) | ||||
| Adenocarcinoma | 1.10 (0.13–9.75) | 0.906 | |||
| Others | 1.01 (0.13–8.92) | 0.960 | |||
| Treatment regimen | |||||
| ICI | 1.00 (ref.) | 1.00 (ref.) | |||
| ICI + RT | 2.58 (1.30–5.16) | 0.007 | 2.53 (1.26–5.06) | 0.009 | |
| Line of ICI treatment | – | – | |||
| 1 | 1.00 (ref.) | ||||
| ≥2 | 0.85 (0.44–1.64) | 0.625 | |||
CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; KPS, Karnofsky Performance Status; RT, radiotherapy.
In multivariate analysis, age (HR, 1.98; 95% CI: 1.04–3.79; P=0.038) and prior or concomitant application of RT (HR, 2.53; 95% CI: 1.26–5.06; P=0.009) were found to be independently associated with IRP (Table 7).
Discussion
Our results suggest that the patients who received RT prior to or concurrent with ICIs for advanced NSCLC had significantly higher PFS and tumor response rates than those who had not received RT, which also increased the incidence rate of grade 1–2 IRP in the patients.
Abscopal effect is the most significant systemic immune response caused by radiation therapy, wherein radiation to a metastatic tumor site can cause anti-tumor immune response in distant nonirradiated tumor sites. Previous studies have shown that RT combined with immunotherapy can increase the abscopal effect (27). This special effect has rarely been reported in patients with thoracic tumor treated with RT alone, as well as with the combination of RT and other types of immunotherapy (28). Data from large randomized trials are lacking to date. Abscopal effect refers generally to distant effect related to the immune impact of RT, and we cannot distinguish between abscopal effects and tumor regression caused by immunotherapy in a retrospective study. Consequently, we replace “abscopal” with “distant” in the entire manuscript. This analysis, which included RT for intrathoracic diseases and metastasis, proved that the distant effect is related to the combination of RT with immunotherapy.
In the PEMBRO-RT trial (20), no differences in response rates were observed between the pembrolizumab plus RT group and pembrolizumab alone group (36% vs. 18%; P=0.07), and subgroup analyses indicated that the patients with PD-L1-negative tumors received maximum benefits from the addition of RT (22% vs. 4%; P=0.14). In the MDACC trial (13), no differences in outcomes were noted between groups (median PFS: 5.1 months with immunotherapy alone vs. 9.1 months with immunotherapy plus RT; P=0.52); however, immunotherapy in combination with RT of 50 Gy in four fractions seemed more effective in terms of response rate (38% vs. 10%; P=0.11) and PFS (20.8 vs. 6.8 months; P=0.03). Herrera et al. also discussed that a combination of low-dose radiation therapy and immunotherapy can activate both the innate and adaptive immune system, when applied to all lesions (29). The results of a randomized phase I/II trial investigating the treatment of lung and liver lesions in NSCLC patients have demonstrated that combining pembrolizumab with hypofractionated stereotactic body RT (SBRT) (50 Gy in 4 fractions) yields better out-of-field ORRs and longer PFS than combining pembrolizumab with traditional RT (45 Gy in 15 fractions). These findings suggest that hypofractionated RT may be more effective in coordinating the effects of immunotherapy (13). The findings of the present retrospective study are in concordance with those of a pooled analysis of two randomized trials by Theelen et al., which found that RT in combination with pembrolizumab immunotherapy could significantly improve the response rate and prognosis of patients with metastatic NSCLC (12). A retrospective study in Japan (n=146) failed to detect the effect of RT in combination with nivolumab on PFS (HR, 0.65; 95% CI: 0.37–1.14) (30). However, two retrospective studies with a small sample size (n=35 and n=85) have shown that the combination of RT and nivolumab can improve OS and PFS without increasing acute toxicity in patients with advanced NSCLC (22,23).
The current study found that the combination of immunotherapy and RT was linked to OS prolongation for up to 1 and 2 years, PFS prolongation for up to 1 and 2 years, and improvement of the ORR and DCR. The higher survival benefits and better tumor response rates with combination radio-immunotherapy can be attributed to the following mechanisms: RT can induce immunogenic cell death to produce T cell-specific tumor-associated antigen (TAA), increase antigen presentation, release chemokines that increase T-cell infiltration in the tumor, and improve the recognition and killing effect of cytotoxic T lymphocytes and natural killer cells on tumor cells (31). On the other hand, after fractionated RT, interferon-γ (IFN-γ) produced by CD8+ T cells can upregulate PD-L1 expression in tumor cells (32,33). So far, numerous clinical studies have demonstrated or are investigating the synergistic effect of RT and ICIs.
The effects of different RT locations, doses, and segmentation schemes on immune enhancement are poorly understood. Thus, determining the treatment with highest efficacy and safety is challenging. To balance the discrepancy and the multiple confounding factors, Cox proportional analysis was performed in RT subgroups. As there are different fractions among patients, we test the median BED rather than the median dose cut-off. The results of multivariate analysis showed that the single-site and high BED irradiation groups had better PFS. There is no consensus on the optimal dose of RT for metastatic NSCLC. Single-site RT combined with immunotherapy was found to be more beneficial for patients with oligometastatic NSCLC who had 1 to 2 metastases, according to a recent study (34).
In addition, PTV size of RT was demonstrated as an independent predictor of immunotherapy efficacy, where a higher PTV size leads to worse PFS. It has been shown in multiple studies that RT could decrease lymphocyte count (35). Lymphocytes in the peripheral blood circulation are sensitive to radiation, even at low doses. Shiraishi et al. (36) found through comparative research that concurrent chemoradiotherapy significantly reduced peripheral blood lymphocyte counts compared to chemotherapy alone, suggesting that lymphocyte reduction during anti-tumor treatment is mainly related to radiation. In a retrospective analysis, absolute lymphocyte count (ALC) was identified as the most relevant baseline parameters associated with OS in NSCLC patients treated with ICI (37). In a cohort of patients with NSCLC treated with nivolumab in routine practice, pretreatment neutrophil to lymphocyte ratio (NLR) ≥5 was associated with inferior outcomes (38). Therefore, it is possible that the volume of radiation may impact lymphocyte blood count, thereby influencing the effectiveness of ICI therapy. In the real world, the choice of radiation therapy is based on the clinician’s subjectivity and safety concerns (considering the size, location, or both of the irradiated lesions). More prospective and external cohort validations are required to confirm the results of the present study.
The sequence of RT and immunotherapy has been controversial, and the outcomes of current studies suggest that immunotherapy should be initiated as early as possible after RT. The phase I trial revealed that concurrent therapy with SBRT in stage IV NSCLC patients is as safe as sequential therapy, allowing for earlier systemic treatment without increased toxicity (39). In the PEMBRO-RT study, PFS and OS were significantly prolonged and ORR increased by a factor of 1 (P<0.10) when pembrolizumab was administered 1 week after SBRT (3×8 Gy) (23). However, Schapira et al. (40) found in a retrospective study of advanced NSCLC with brain metastases that synchronous PD-1 inhibitors in brain stereotactic radiosurgery (SRS) provided better OS and local control rate than sequential treatment. The best combination of RT and immunotherapy remains to be further studied to guide clinical diagnosis and treatment.
Conclusions drawn regarding the pulmonary toxicity of iRT in patients with NSCLC have been conflicting. Some studies have shown that concurrent iRT may not increase toxicity (41,42). However, the findings of this retrospective study are in concordance with those of a secondary analysis of phase I KEYNOTE-001 study, which found that a large proportion of patients who had previously received any RT experienced pulmonary toxicity after ICIs (43). Results from the current study demonstrated that the incidences of mild (grade 1–2) pneumonia were significantly higher in patients receiving a combination of PD-1/PD-L1 inhibitors and RT than in those who received ICI treatment alone. Our subgroup analysis showed no significant difference in the incidence of pneumonia between the patients who received radiation to the chest and those who received radiation for metastasis. Nevertheless, striking a balance between the safety and efficacy of iRT remains to be achieved in the future.
This study has some limitations. First, AEs may have been underestimated, which can be attributed to the retrospective nature of the study. Second, expression data of PD-L1 were unavailable for 86% of the patients in this study, which play a significant role in tumor development by suppressing immune system. Biomarkers such as tumor mutation burden, NLR, and microsatellite instability (MSI) are considered useful in predicting the outcomes. To sum up, PD-L1 together with other biomarkers may help predict the outcomes of immunotherapy. Third, some patients were at later line, while this is the characteristic of the real-world setting. A Cox proportional analysis was performed in the overall patient population, taking into account the number of treatment lines as the confounding factor, to balance differences and multiple confounding factors. Multivariate analysis showed that PFS was better in the ICI + RT group. Additional prospective and external cohort validations are needed to confirm the results. Lastly, OS did not reach the ideal value, because there was a long gap between the diagnosis of the disease and administration of immunotherapy, which may have affected the efficacy of the drug.
Conclusions
Combination therapy using ICIs and radiation may improve PFS and tumor response rates in advanced NSCLC patients. When compared with RT applied prior to ICI, the application of RT concomitant with ICI led to a higher DRR. However, combination therapy using radiation and immunotherapy may increase the incidence of immune-related pneumonitis. In addition, an excessive volume of PTV in RT may negatively affect the efficacy of immunotherapy.
Acknowledgments
We are grateful to all patients and their families and all staff at the study center.
Funding: This work was supported by grants from the National Natural Science Foundation of China (Nos. 82172866 and 82030082); the Department of Science & Technology of Shandong Province (No. 2021CXGC011102); and the Shandong Provincial Natural Science Foundation (No. ZR2019LZL019).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1685/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1685/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1685/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1685/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the ethics board of Shandong Cancer Hospital Affiliated to Shandong First Medical University (No. SDTHEC2022011004) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol 2021;39:2339-49. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Kon E, Benhar I. Immune checkpoint inhibitor combinations: Current efforts and important aspects for success. Drug Resist Updat 2019;45:13-29. [Crossref] [PubMed]
- Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845-51. [Crossref] [PubMed]
- Yi M, Jiao D, Qin S, et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 2019;18:60. [Crossref] [PubMed]
- Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol 2019;20:1670-80. [Crossref] [PubMed]
- Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16:860-7. [Crossref] [PubMed]
- Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol 2021; Epub ahead of print. [Crossref] [PubMed]
- Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467-75. [Crossref] [PubMed]
- Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer 2020;8:e001001. [Crossref] [PubMed]
- Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 2017;544:250-4. [Crossref] [PubMed]
- Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60-5. [Crossref] [PubMed]
- Najafi M, Jahanbakhshi A, Gomar M, et al. State of the Art in Combination Immuno/Radiotherapy for Brain Metastases: Systematic Review and Meta-Analysis. Curr Oncol 2022;29:2995-3012. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Liu Y, Dong Y, Kong L, et al. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol 2018;11:104. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276-82. [Crossref] [PubMed]
- Gray JE, Villegas A, Daniel D, et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020;15:288-93. [Crossref] [PubMed]
- Ratnayake G, Shanker M, Roberts K, et al. Prior or concurrent radiotherapy and nivolumab immunotherapy in non-small cell lung cancer. Asia Pac J Clin Oncol 2020;16:56-62. [Crossref] [PubMed]
- Fiorica F, Belluomini L, Stefanelli A, et al. Immune Checkpoint Inhibitor Nivolumab and Radiotherapy in Pretreated Lung Cancer Patients: Efficacy and Safety of Combination. Am J Clin Oncol 2018;41:1101-5. [Crossref] [PubMed]
- Li M, Gan L, Song A, et al. Rethinking pulmonary toxicity in advanced non-small cell lung cancer in the era of combining anti-PD-1/PD-L1 therapy with thoracic radiotherapy. Biochim Biophys Acta Rev Cancer 2019;1871:323-30. [Crossref] [PubMed]
- Bang A, Wilhite TJ, Pike LRG, et al. Multicenter Evaluation of the Tolerability of Combined Treatment With PD-1 and CTLA-4 Immune Checkpoint Inhibitors and Palliative Radiation Therapy. Int J Radiat Oncol Biol Phys 2017;98:344-51. [Crossref] [PubMed]
- Jones B, Hopewell JW. Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: derivation of "protective" dose modification factors. Br J Radiol 2019;92:20180111. [PubMed]
- Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol 2018;39:644-55. [Crossref] [PubMed]
- Barsky AR, Cengel KA, Katz SI, et al. First-ever Abscopal Effect after Palliative Radiotherapy and Immuno-gene Therapy for Malignant Pleural Mesothelioma. Cureus 2019;11:e4102. [Crossref] [PubMed]
- Herrera FG, Romero P, Coukos G. Lighting up the tumor fire with low-dose irradiation. Trends Immunol 2022;43:173-9. [Crossref] [PubMed]
- Kataoka Y, Ebi N, Fujimoto D, et al. Prior radiotherapy does not predict nivolumab response in non-small-cell lung cancer: a retrospective cohort study. Ann Oncol 2017;28:1402. [Crossref] [PubMed]
- Vanpouille-Box C, Formenti SC, Demaria S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin Cancer Res 2018;24:259-65. [Crossref] [PubMed]
- Takamori S, Toyokawa G, Takada K, et al. Combination Therapy of Radiotherapy and Anti-PD-1/PD-L1 Treatment in Non-Small-cell Lung Cancer: A Mini-review. Clin Lung Cancer 2018;19:12-6. [Crossref] [PubMed]
- Gong X, Li X, Jiang T, et al. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1085-97. [Crossref] [PubMed]
- Wang P, Yin T, Zhao K, et al. Efficacy of single-site radiotherapy plus PD-1 inhibitors vs PD-1 inhibitors for oligometastatic non-small cell lung cancer. J Cancer Res Clin Oncol 2022;148:1253-61. [Crossref] [PubMed]
- Stover DG, Reddy VK, Shyr Y, et al. Long-term impact of prior rituximab therapy and early lymphocyte recovery on auto-SCT outcome for diffuse large B-cell lymphoma. Bone Marrow Transplant 2012;47:82-7. [Crossref] [PubMed]
- Shiraishi Y, Fang P, Xu C, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: A propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol 2018;128:154-60. [Crossref] [PubMed]
- Huemer F, Lang D, Westphal T, et al. Baseline Absolute Lymphocyte Count and ECOG Performance Score Are Associated with Survival in Advanced Non-Small Cell Lung Cancer Undergoing PD-1/PD-L1 Blockade. J Clin Med 2019;8:1014. [Crossref] [PubMed]
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. [Crossref] [PubMed]
- Bestvina CM, Pointer KB, Karrison T, et al. A Phase 1 Trial of Concurrent or Sequential Ipilimumab, Nivolumab, and Stereotactic Body Radiotherapy in Patients With Stage IV NSCLC Study. J Thorac Oncol 2022;17:130-40. [Crossref] [PubMed]
- Schapira E, Hubbeling H, Yeap BY, et al. Improved Overall Survival and Locoregional Disease Control With Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients With Brain Metastases. Int J Radiat Oncol Biol Phys 2018;101:624-9. [Crossref] [PubMed]
- Amin NP, Zainib M, Parker SM, et al. Multi-institutional report on toxicities of concurrent nivolumab and radiation therapy. Adv Radiat Oncol 2018;3:399-404. [Crossref] [PubMed]
- Verma V, Cushman TR, Selek U, et al. Safety of Combined Immunotherapy and Thoracic Radiation Therapy: Analysis of 3 Single-Institutional Phase I/II Trials. Int J Radiat Oncol Biol Phys 2018;101:1141-8. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]


