
Effective omalizumab treatment influenced eosinophil function in severe allergic asthmatics
Highlight box
Key findings
• Effective omalizumab treatment downregulated surface expression of co-stimulatory molecules cluster of differentiation (CD) 40, CD80, and CD86 on eosinophils from peripheral blood and the concentration of serum eotaxin-1 in subjects with allergic asthma.
What is known and what is new
• Omalizumab is an effective anti-immunoglobulin E (IgE) treatment for allergic asthma and with multiple complications (allergic rhinitis, cough, anxiety). Eosinophil function including antigen presenting ability plays a critical role in the pathogenesis of allergic airway inflammation.
What is the implication, and what should change now
• The function of eosinophils as secondary antigen-presenting cells (APCs) might have a subsidiary role in the mechanisms of anti-IgE treatment for asthma, and omalizumab treatment improved clinical manifestation in allergic asthma patients with multiple complications.
Introduction
Approximately afflicting up to 300 million people worldwide, asthma has been becoming a global health problem (1). In China, one latest epidemiological study revealed that the asthmatic population had reached 45 million and the prevalence of asthma was around 4.2% among Chinese adults aged 20 years or older (2). Allergic asthma is the most well-studied asthma phenotype, accounting for more than 50% of adult asthmatics (3).
Omalizumab, a recombinant highly-humanized monoclonal anti-immunoglobulin E (IgE) antibody preventing the crosslinking between IgE and the high affinity IgE receptor FcεRⅠ, was the first licensed biologic agent to treat allergic asthma (4). In addition, omalizumab inhibits allergic inflammation through downregulating the expression of FcεRⅠ on mast cells (5), basophils (6) and dendritic cells (7), regulates immune responses through restoring generation of regulatory T cells (Tregs) (8) and reducing the number of membrane IgE+ B cells (9). Many large randomized clinical trials have proved the efficacy of omalizumab in patients with allergic asthma (10-14). In China, omalizumab was approved to treat severe allergic asthma in 2017 and a nationwide, real-world study conducted in severe allergic asthmatics treated with omalizumab indicated that 91.5% of patients reported response to omalizumab treatment after week 16 (15).
Eosinophils are pleiotropic cells that have the capability to amplify immune response after allergen challenges through the release of both already contained and newly synthesized granular proteins, cytokines, lipid mediators, and growth factors (16). In addition to the capacity to act as effector cells in T helper 2 cell (Th2) response, eosinophils have also been recognized as non-professional antigen-presenting cells (APCs) to initiate Th2 immune response in the early stage of allergic reactions (17). Multiple kinds of research revealed that eosinophils could express major histocompatibility complex (MHC) class Ⅱ and costimulatory molecules cluster of differentiation (CD) 80 and CD86 (18,19). However, whether omalizumab influences the function of eosinophils is not known yet.
In this study, we aimed to explore the influence of omalizumab on eosinophil function in severe allergic asthma. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1818/rc).
Methods
Study subjects
In this prospective clinical observational study, allergic asthmatics were recruited from January 2020 to March 2022 at Ruijin Hospital (Figure 1). The inclusion criteria were as follows. Firstly, Asthma was diagnosed according to the Global Initiative for Asthma (GINA) (20). Secondly, patients whose asthma was not well-controlled despite step 4–5 treatment recommended by GINA (20). Thirdly, allergic asthma was diagnosed, which was defined as asthmatic patients had elevated total IgE levels (≥60 kU/L, ImmunoCap, Pharmacia Diagnostics AB, Uppsala, Sweden) and asthma associated with sensitization to aeroallergens, which leads to asthma symptoms and airway inflammation (3). Lastly, patients were treated with omalizumab for at least 16 weeks. Omalizumab was administrated subcutaneously at 2- or 4-week intervals. Bodyweight and total serum IgE levels were considered to warrant omalizumab administration of at least 0.016 mg/kg/IgE (IU/mL) every 4 weeks. Response to omalizumab was assessed by each patient and specialist physician according to the specialist’s global evaluation of treatment effectiveness (GETE) (21) at week 16. Patients with a GETE rating of “excellent” or “good” were regarded as responders, while those with a GETE rating of “moderate”, “poor”, or “worsening” were classified as non-responders. Patients with severe comorbid diseases such as acute respiratory infection, bronchiectasis, sarcoidosis, or lung cancer and a treatment course of fewer than 16 weeks were excluded from the study. Each participant signed an informed consent form before the study. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Ruijin Hospital (No. 2019-YK061). Each participant signed an informed consent form before the study.
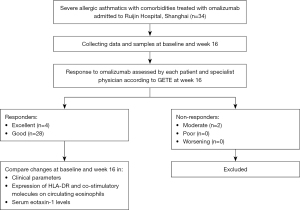
Data and samples collection
The following demographic characteristics and clinical parameters (Table 1), including blood eosinophil count and ratio and spirometry tests, etc., were collected at baseline and week 16. ACT and mini-AQLQ for asthma-related clinical characteristics, mini-RQLQ for allergic rhinitis (AR)-related clinical characteristics, VAS for allergic symptoms involving noses, eyes, airways and skin, LCQ for cough and self-rating anxiety scale (SAS) for anxiety were collected at baseline and week 16. For each study participant, 10 mL of peripheral venous blood was harvested at baseline and week 16. The serum was separated by centrifugation and kept frozen at −80 ℃ for subsequent assays.
Table 1
| Characteristics | Values |
|---|---|
| Age (years) | 51.66±16.91 |
| Female | 20 (62.5) |
| Height (m) | 1.64±0.09 |
| Body weight (kg) | 63.96 (59.41, 68.50) |
| BMI (kg/m2) | 23.71±3.40 |
| Smoking history | 4 (12.5) |
| Family history of asthma | 11 (34.4) |
| Pet ownership history | 10 (31.3) |
| Age of asthma onset (years) | 32.97 (24.92, 41.02) |
| Duration of asthma (years) | 18.71 (12.34, 25.07) |
| Exacerbations in preceding year, n | 2.2±1.3 |
| Comorbidities | |
| Allergic rhinitis | 26 (81.3) |
| Nasal polyp | 6 (18.8) |
| Chronic urticaria | 8 (25.0) |
| Allergic dermatitis | 14 (43.8) |
| Allergic conjunctivitis | 13 (40.6) |
| Blood eosinophil count (cells/μL) | 412.61 (241.52, 583.90) |
| Blood eosinophil ratio (%) | 5.37±4.32 |
| Total serum IgE (kU/L) | 1,048.25 (630.24, 1,466.25) |
| FVC, % predicted | 87.10±16.75 |
| FEV1, % predicted | 76.61±22.96 |
| FEV1/FVC, % predicted | 86.18±15.96 |
| MEF75% | 55.53±33.34 |
| MEF50% | 47.49±31.93 |
| MEF25% | 46.60±30.00 |
| MEF75/25 | 46.20 (33.52, 58.88) |
| FeNO (ppb) | 50.81 (35.49, 66.12) |
| ACT | 17.56 (15.10, 20.01) |
| ICS/LABA grouped by dose | |
| High dose | 16 (50.0) |
| Moderate dose | 16 (50.0) |
| Concomitant OCS use | 11 (34.3) |
Data are presented as n (%) or mean ± SD or median (IQR). SD, standard deviation; IQR, interquartile range; BMI, body mass index; IgE, immunoglobulin E; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; MEF, maximal expiratory flow; FeNO, fractional exhaled nitric oxide; ACT, asthma control test; ICS, inhaled corticosteroid; LABA, long-acting β2 agonist; OCS, oral corticosteroids.
Eosinophil expression of HLA-DR, CD40, CD80, and CD86
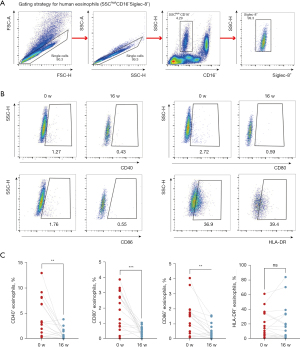
One mL ethylene diamine tetraacetic acid (EDTA)-treated whole blood samples freshly collected at baseline and week 16 were incubated with APC/Cy7 anti-human CD16 antibody (Clone: 3G8, BD sciences), APC anti-human Siglec-8 antibody (Clone: 7C9, Biolegend), PE anti-human CD40 antibody (Clone: HB14, Biolegend), PE/Cyanine7 anti-human human leukocyte antigen (HLA)-DR antibody (Clone: L243, Biolegend), Brilliant Violet 421 anti-human CD80 antibody (Clone: L307.4, BD sciences), BB515 anti-human CD86 antibody (Clone: FUN-1, BD sciences).for 20 minutes at room temperature in the dark, then red blood cells were lysed using 1× Red Blood Cell Lysis buffer (BD sciences). After erythrocyte lysis, cells were washed, fixed with Fixation Buffer (Biolegend) and resuspended in Cyto-last Buffer (Biolegend) until analysis. Flow cytometry was performed to examine the expression of HLA-DR, CD40, CD80, and CD86 on eosinophils. Eosinophils were gated as SSChigh, siglec-8+, and CD16−. Data were analyzed using Cytexpert (Beckman) and flowjo 10 software.
Measurement of serum eotaxin-1 concentrations in asthma patients
Serum eotaxin-1 concentrations were quantitatively measured by solid phase sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, MN, USA) before and after 16 weeks of omalizumab therapy, according to the manufacturer’s instructions.
Statistical analyses
Normally distributed continuous variables were presented as mean and standard deviations (SD), and non-normally distributed variables were expressed as the median and interquartile range (IQR). Categorical variables were presented as count and percentage. Continuous effectiveness outcomes were analyzed using the paired Student’s t-test (change from post- to pre-treatment levels) or the Wilcoxon signed-rank test. For correlation analysis, the Pearson correlation was applied. Analyses were performed using SPSS version 26.0 statistical software or GraphPad Prism 9.3.0 (GraphPad Software, USA). A two-tailed P value less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of patients at baseline
Totally 32 allergic asthma patients who responded positively to omalizumab treatment were included in the study. Demographic and clinical characteristics of asthma patients at baseline are shown in Table 1.
Patients in this study had increased total serum IgE levels [1,048.25 (630.24, 1,466.25) kU/L], high blood eosinophil count [412.61 (241.52, 583.90) cells/µL] and fractional exhaled nitric oxide [FeNO, 50.81 (35.49, 66.12) ppb], low ACT scores [17.56 (15.10, 20.01)], damaged pulmonary function [forced vital capacity (FVC) % predicted 87.10±16.75 and forced expiratory volume in the first second (FEV1) % predicted 76.61±22.96]. Besides, there were 81.3% of asthma patients with concomitant AR. Regarding inhaled corticosteroid (ICS)/long-acting β2 agonist (LABA) treatment, 50% of patients were taking a high dose and a moderate dose respectively, which was categorized by GINA guidelines (20). There were 34.3% of asthmatics receiving oral corticosteroids (OCS) concomitantly.
The expression of co-stimulatory molecules on eosinophils changes after successful omalizumab treatment
The influence of omalizumab on the expression of molecules related to antigen-presenting function on eosinophils after 16 weeks of omalizumab therapy is shown in Figure 2 and Table S1. After 16 weeks of omalizumab therapy, responders showed a remarkable fall in the expression of co-stimulatory molecules CD40, CD80, and CD86 on circulating eosinophils. There was no noticeable change in HLA-DR expression on eosinophils after omalizumab therapy.
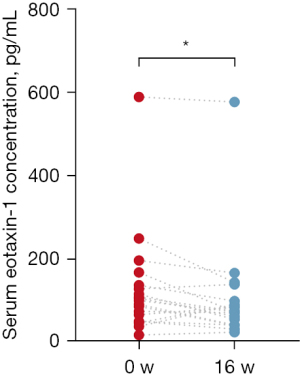
Change in eotaxin-1 in serum after omalizumab therapy
The change in serum eotaxin-1 concentrations after omalizumab therapy is shown in Figure 3 and Table S1. Omalizumab responders exhibited a significant decline in serum eotaxin-1 levels after therapy.
Changes in symptom scores after 16 weeks of omalizumab treatment
The improvements in symptoms of allergic asthmatics after omalizumab therapy are shown in Table 2. There were significant improvements in ACT scores (4.22, P<0.001), mini-AQLQ scores (−14.44, P=0.019), LCQ scores for cough (3.03, P=0.009), and VAS scores for allergic symptoms (−13.00, P=0.001) after 16 weeks of omalizumab treatment. For allergic asthma complicating AR or anxiety, the scores of mini-RQLQ (−8.50, P=0.047) or SAS (−5.08, P=0.040) statistically decreased compared with baseline, respectively.
Table 2
| Characteristics | Pre-treatment | Post-treatment | Compared with baseline | P value |
|---|---|---|---|---|
| ACTa | 17.56±6.22 | 21.78±3.25 | 4.22±4.95 | <0.001*** |
| Mini-AQLQa | 72.00±17.14 | 57.56±19.19 | −14.44±21.87 | 0.019* |
| LCQa | 13.97±3.97 | 17.00±2.87 | 3.03±2.63 | 0.009** |
| VASb | 37.00 (20.00, 64.00) | 17.00 (10.00, 27.00) | −13.00 (−26.79, 4.50) | 0.001** |
| Mini-RQLQa | 27.00±22.73 | 18.50±13.43 | −8.50±13.19 | 0.047* |
| SASa | 40.90±15.03 | 35.82±10.12 | −5.08±8.69 | 0.040* |
a, mean ± SD, paired Student’s t-test; b, median (IQR), Wilcoxon signed-rank test; *, P<0.05; **, P<0.01; ***, P<0.001. ACT, asthma control test; Mini-AQLQ, mini asthma quality of life questionnaire; LCQ, Leicester cough questionnaire; Mini-RQLQ, mini rhino-conjunctivitis quality of life questionnaire; VAS, visual analogue scale; SAS, Self-rating Anxiety Scale; SD, standard deviation; IQR, interquartile range.
Changes in clinical biomarkers after omalizumab treatment
The effects of omalizumab on clinical biomarkers are shown in Table 3. After 16 weeks of omalizumab therapy, total serum IgE levels elevated significantly (568, P<0.001). In addition, all responders exhibited a significant improvement in FEV1/FVC% predicted (3.88, P=0.033) and FeNO (−22.24, P=0.028). However, there was no significant decline in the peripheral blood eosinophil count and ratio after omalizumab therapy.
Table 3
| Characteristics | Pre-treatment | Post-treatment | Compared with baseline | P value |
|---|---|---|---|---|
| Blood eosinophil count, cells/μLa | 403.46±374.48 | 471.15±488.43 | 67.69±339.62 | 0.319 |
| Blood eosinophil ratio, %a | 6.15±4.39 | 6.66±5.23 | 0.51±4.74 | 0.666 |
| Total serum IgE, kU/Lb | 698.0 (259.0, 1,250.0) | 1,178.0 (715.0, 2,383.5) | 568.0 (58.0, 1,322.8) | <0.001*** |
| FVC, % predicteda | 86.43±17.30 | 85.58±19.42 | −0.85±10.56 | 0.697 |
| FEV1, % predicteda | 74.42±23.12 | 76.78±23.18 | 2.36±9.07 | 0.215 |
| FEV1/FVC, % predictedb | 88.20 (74.90, 97.10) | 89.55 (77.03, 98.73) | 3.88±9.04 | 0.033* |
| MEF75%b | 53.10 (29.45, 79.80) | 57.30 (26.88, 80.90) | 2.20 (−1.88, 15.58) | 0.097 |
| MEF50%b | 39.00 (24.9, 67.50) | 47.15 (26.03, 77.75) | 4.10 (−5.03, 14.80) | 0.100 |
| MEF25%a | 43.86±26.76 | 50.42±28.74 | 6.56±20.18 | 0.125 |
| MEF75/25a | 43.58±29.24 | 48.74±29.53 | 5.16±15.33 | 0.113 |
| FeNO, ppba | 60.76±40.50 | 38.53±30.51 | −22.24±37.82 | 0.028* |
The data are shown as mean ± SD or median (IQR). a, paired Student’s t-test; b, Wilcoxon signed-rank test; *, P<0.05; ***, P<0.001. IgE, immunoglobulin E; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; MEF, maximal expiratory flow; FeNO, fractional exhaled nitric oxide; SD, standard deviation; IQR, interquartile range.
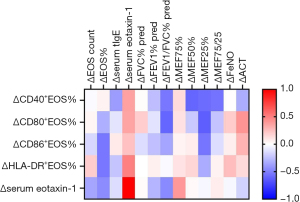
Correlations between change in molecules related to eosinophil and change in clinical biomarkers
After 16 weeks of omalizumab treatment, a correlation matrix for change in molecules related to eosinophil with change in clinical biomarkers is displayed in Figure 4. After omalizumab therapy, the change in FEV1/FVC% (r=−0.61, P=0.048) and maximal expiratory flow (MEF) 25% (r=−0.63, P=0.05) both had a negative correlation with the change in CD80+ eosinophils significantly, which indicated that more decline in expression of CD80 on eosinophils related to more improvement in FEV1/FVC% and MEF25%.
Discussion
To our knowledge, this is the first study that has demonstrated effective anti-IgE treatment could downregulate co-stimulatory molecules expression on circulating eosinophil and serum eotaxin-1 levels in patients with severe allergic asthma. Additionally, we conducted a variety of questionnaires according to individual characteristics to assess the effectiveness of omalizumab on allergic asthmatics thoroughly and proved that anti-IgE treatment could alleviate multiple asthma-related and allergic symptoms.
Eosinophils are a type of granulated innate immune cells that are involved in diverse inflammatory responses. In asthma, eosinophils are typically recruited to tissues during type 2 inflammatory responses and play an essential role in airway inflammation (16). In 2006, Noga et al. found that omalizumab could induce eosinophil apoptosis (22). Likewise, one post hoc analysis analyzed data from 8 randomized trials of omalizumab and concluded that omalizumab treatment was associated with circulating eosinophil count reduction (23). Hanania et al. found that the subgroup with a peripheral blood eosinophil count of more than 260 cells/µL had lower exacerbation frequency after 48 weeks of omalizumab therapy (24). On the contrary, another prospective real-world study suggested that omalizumab initiation in asthmatics resulted in fewer exacerbations, lower hospitalizations, and higher ACT scores regardless of blood eosinophil count (25). Our results showed that blood eosinophil count and the ratio at the end of 16 weeks of omalizumab therapy were similar to those measured at baseline. Therefore, we supposed that it was not a key issue that the number of circulating eosinophils changed, but rather their functions altered after omalizumab treatment.
Previously, it has been demonstrated that eosinophils could express MHCⅡ and co-stimulatory molecules CD40, CD80, and CD86, which are necessary for antigen uptake and process (26). Besides, our prior study identified that eosinophils perform a function for antigen uptake and Th2 cell differentiation (27). One study demonstrated that the blockade of CD86 could reduce the production of IgE and Th2 cytokines and inhibit the recruitment of eosinophils to the lung in animal experiments (28). This study showed that the expression of co-stimulatory molecules CD40, CD80, and CD86 on eosinophils decreased statistically after omalizumab treatment. In addition, the improvements in FEV1/FVC% predicted and MEF25% after omalizumab treatment were positively associated with the decline in CD80+ eosinophils. These findings suggested that the function of eosinophils as secondary APCs might have a subsidiary role in the mechanisms of anti-IgE treatment for asthma, and omalizumab may alter the ability of eosinophils in antigen-presenting.
Eotaxin-1, a member of the C-C motif chemokine ligand (CCL) family, also called CCL11, was first discovered by Jose et al. in 1994 and demonstrated to play a crucial role in eosinophil accumulation in allergic airways in vivo (29). Subsequently, human eotaxin was cloned and found to be an effective and selective eosinophil chemoattractant by binding to and activating its high-affinity C-C chemokine receptor (CCR) 3 expressed on eosinophils (30,31). Afterward, it was demonstrated that the expression of eotaxin was higher in airways and the induced sputum in allergic asthmatics than in normal controls (32,33). Moreover, asthma patients with exacerbations presented significantly higher plasma eotaxin levels than stable asthmatics (34). Our study discovered that the serum eotaxin-1 levels decreased significantly in omalizumab responders after 16-week omalizumab treatment for the first time. Taken together, the experimental results confirmed that the multifaceted functions of eosinophils altered after effective treatment with omalizumab. Presumably, the changes in eosinophil may be important in the comprehensive assessment of the efficacy of omalizumab treatment.
This study observed a significant improvement in FEV1/FVC%, FeNO, ACT, and mini-AQLQ scores, consistent with previous studies (11,15). We also conducted VAS and LCQ to evaluate the effectiveness of omalizumab on multiorgan allergy symptoms and cough, respectively, and a significant improvement in VAS and LCQ was observed. There were 81.3% allergic asthmatics complicated from AR in our study. Patients with asthma and co-existing AR in our study benefiting from omalizumab therapy also experienced a significant improvement in rhinitis-specific quality of life, which has also been confirmed in prior studies (35,36).
Asthmatics with anxiety have enhanced perception of bronchoconstriction, and accurate perception, in turn, increases anxiety (37). One randomized controlled trial indicated that interventions reducing anxiety could improve asthmatic children’s health (38). In this study, we evaluated the effects of omalizumab on anxiety in allergic asthmatics using a SAS questionnaire and omalizumab was found to reduce anxious feelings in allergic asthmatics for the first time. These combined results of the studies suggested that anxiety and asthma are not mutually exclusive, and managing anxiety may improve asthma management and vice versa. Additionally, omalizumab improved anxiety, and anxiety improvements were also indicated in chronic rhinosinusitis (39) and chronic spontaneous urticaria (40) treated with omalizumab. Alexithymia, another cognitive disorder, has been shown to be improved by omalizumab (41). The correlation of eosinophil loss of function induced by omalizumab on cognitive disorders should be further investigated as the involvement of eosinophils in the brain has been shown (42).
There existed several limitations in our study. Firstly, the sample size was relatively small, and a larger sample size is required to verify these findings. Secondly, as omalizumab has been applied to treat severe asthma clinically for many years and patients with a high degree of compliance with the indications tend to be treated very well, we didn’t recruit enough non-responders to the omalizumab treatment as a control group. Thirdly, detailed in vitro experiments need to be designed to further validate the effect of omalizumab on eosinophil chemotaxis and antigen-presenting function.
Conclusions
In conclusion, this study elucidated that effective omalizumab treatment downregulated surface expression of co-stimulatory molecules CD40, CD80, and CD86 on peripheral eosinophils and concentration of serum eotaxin-1 in allergic asthma. Omalizumab treatment improved multiple allergies related clinical parameters and biomarkers in severe allergic asthmatics.
Acknowledgments
We thank all subjects who volunteered for this study. We thank Bo Peng (Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Institute of Respiratory Diseases, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases, Shanghai, China) for her assistance in writing-review and editing.
Funding: This study was supported by grants from the Chinese National Natural Science Foundation (No. 82170022); Shanghai Municipal Key Clinical Specialty (No. shslczdzk02202); Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases (No. 20dz2261100).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1818/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1818/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1818/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Ruijin Hospital (No. 2019-YK061). Each participant signed an informed consent form before the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol 2020;42:5-15. [Crossref] [PubMed]
- Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019;394:407-18. [Crossref] [PubMed]
- Akar-Ghibril N, Casale T, Custovic A, et al. Allergic Endotypes and Phenotypes of Asthma. J Allergy Clin Immunol Pract 2020;8:429-40. [Crossref] [PubMed]
- Omalizumab: anti-IgE monoclonal antibody E25, E25, humanised anti-IgE MAb, IGE 025, monoclonal antibody E25, Olizumab, Xolair, rhuMAb-E25. BioDrugs 2002;16:380-6. [Crossref] [PubMed]
- Beck LA, Marcotte GV, MacGlashan D, et al. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol 2004;114:527-30. [Crossref] [PubMed]
- MacGlashan DW Jr, Bochner BS, Adelman DC, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997;158:1438-45. [Crossref] [PubMed]
- Prussin C, Griffith DT, Boesel KM, et al. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol 2003;112:1147-54. [Crossref] [PubMed]
- López-Abente J, Benito-Villalvilla C, Jaumont X, et al. Omalizumab restores the ability of human plasmacytoid dendritic cells to induce Foxp3(+)Tregs. Eur Respir J 2021;57:2000751. [Crossref] [PubMed]
- Chan MA, Gigliotti NM, Dotson AL, et al. Omalizumab may decrease IgE synthesis by targeting membrane IgE+ human B cells. Clin Transl Allergy 2013;3:29. [Crossref] [PubMed]
- Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001;108:184-90. [Crossref] [PubMed]
- Buhl R, Hanf G, Solèr M, et al. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J 2002;20:1088-94. [Crossref] [PubMed]
- Corren J, Casale T, Deniz Y, et al. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol 2003;111:87-90. [Crossref] [PubMed]
- Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011;154:573-82. [Crossref] [PubMed]
- Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol 2017;140:162-169.e2. [Crossref] [PubMed]
- Zhang M, Jin M, Zhou X, et al. Effectiveness of omalizumab in patients with severe allergic asthma: A retrospective study in China. Respir Med 2021;186:106522. [Crossref] [PubMed]
- Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov 2013;12:117-29. [Crossref] [PubMed]
- Schuijs MJ, Hammad H, Lambrecht BN. Professional and 'Amateur' Antigen-Presenting Cells In Type 2 Immunity. Trends Immunol 2019;40:22-34. [Crossref] [PubMed]
- Rodolpho JMA, Camillo L, Araújo MSS, et al. Robust Phenotypic Activation of Eosinophils during Experimental Toxocara canis Infection. Front Immunol 2018;9:64. [Crossref] [PubMed]
- Wang HB, Ghiran I, Matthaei K, et al. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol 2007;179:7585-92. [Crossref] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2022. Available online: www.ginasthma.org
- Humbert M, Taillé C, Mala L, et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J 2018;51:1702523. [Crossref] [PubMed]
- Noga O, Hanf G, Brachmann I, et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J Allergy Clin Immunol 2006;117:1493-9. [Crossref] [PubMed]
- Busse WW, Humbert M, Haselkorn T, et al. Effect of omalizumab on lung function and eosinophil levels in adolescents with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol 2020;124:190-6. [Crossref] [PubMed]
- Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013;187:804-11. [Crossref] [PubMed]
- Casale TB, Luskin AT, Busse W, et al. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence From PROSPERO, A Prospective Real-World Study. J Allergy Clin Immunol Pract 2019;7:156-164.e1. [Crossref] [PubMed]
- Shi HZ. Eosinophils function as antigen-presenting cells. J Leukoc Biol 2004;76:520-7. [Crossref] [PubMed]
- Peng B, Sun L, Zhang M, et al. Role of IL-25 on Eosinophils in the Initiation of Th2 Responses in Allergic Asthma. Front Immunol 2022;13:842500. [Crossref] [PubMed]
- Tsuyuki S, Tsuyuki J, Einsle K, et al. Costimulation through B7-2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J Exp Med 1997;185:1671-9. [Crossref] [PubMed]
- Jose PJ, Griffiths-Johnson DA, Collins PD, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med 1994;179:881-7. [Crossref] [PubMed]
- Ponath PD, Qin S, Ringler DJ, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest 1996;97:604-12. [Crossref] [PubMed]
- Daugherty BL, Siciliano SJ, DeMartino JA, et al. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med 1996;183:2349-54. [Crossref] [PubMed]
- Ying S, Robinson DS, Meng Q, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol 1997;27:3507-16. [Crossref] [PubMed]
- Yamada H, Yamaguchi M, Yamamoto K, et al. Eotaxin in induced sputum of asthmatics: relationship with eosinophils and eosinophil cationic protein in sputum. Allergy 2000;55:392-7. [Crossref] [PubMed]
- Lilly CM, Woodruff PG, Camargo CA Jr, et al. Elevated plasma eotaxin levels in patients with acute asthma. J Allergy Clin Immunol 1999;104:786-90. [Crossref] [PubMed]
- Humbert M, Bousquet J, Bachert C, et al. IgE-Mediated Multimorbidities in Allergic Asthma and the Potential for Omalizumab Therapy. J Allergy Clin Immunol Pract 2019;7:1418-29. [Crossref] [PubMed]
- Just J, Thonnelier C, Bourgoin-Heck M, et al. Omalizumab Effectiveness in Severe Allergic Asthma with Multiple Allergic Comorbidities: A Post-Hoc Analysis of the STELLAIR Study. J Asthma Allergy 2021;14:1129-38. [Crossref] [PubMed]
- Spinhoven P, van Peski-Oosterbaan AS, Van der Does AJ, et al. Association of anxiety with perception of histamine induced bronchoconstriction in patients with asthma. Thorax 1997;52:149-52. [Crossref] [PubMed]
- Chiang LC, Ma WF, Huang JL, et al. Effect of relaxation-breathing training on anxiety and asthma signs/symptoms of children with moderate-to-severe asthma: a randomized controlled trial. Int J Nurs Stud 2009;46:1061-70. [Crossref] [PubMed]
- Vogt F, Sahota J, Bidder T, et al. Chronic rhinosinusitis with and without nasal polyps and asthma: Omalizumab improves residual anxiety but not depression. Clin Transl Allergy 2021;11:e12002. [Crossref] [PubMed]
- Patella V, Zunno R, Florio G, et al. Omalizumab improves perceived stress, anxiety, and depression in chronic spontaneous urticaria. J Allergy Clin Immunol Pract 2021;9:1402-4. [Crossref] [PubMed]
- Liotta M, Liotta M, Saitta S, et al. Severe allergic asthma: Does alexithymia interfere with omalizumab treatment outcome? Asian Pac J Allergy Immunol 2023;41:53-9. [PubMed]
- Manti S, Brown P, Perez MK, et al. The Role of Neurotrophins in Inflammation and Allergy. Vitam Horm 2017;104:313-41. [Crossref] [PubMed]


