
Effectiveness and feasibility of transcatheter aortic valve replacement in treating combined aortic and mitral regurgitation: a retrospective observational study
Highlight box
Key findings
• Transcatheter aortic valve replacement (TAVR) significantly reduced mitral regurgitation (MR) secondary to aortic regurgitation (AR), which shows highly effective and feasible of TAVR in treating combined aortic and mitral regurgitation.
What is known and what is new?
• Functional mitral regurgitation secondary to aortic valve disease might be improved after aortic valve replacement. TAVR was effective for aortic valvular disease in a less invasive approach, however it’s not recommended for patients with combined valvular disease.
• Dilated ventricle and atrial fibrillation were common in elder patients with chronic AR, which resulted in functional mitral regurgitation in most cases. Mitral regurgitation once confirmed as functional combined with AR would be significantly improved after TAVR.
What is the implication, and what should change now?
• TAVR should be taken into consideration of treatment options for combined valvular disease after thoroughly evaluation.
Introduction
Transcatheter aortic valve replacement (TAVR), which is mainly applied in the treatment of aortic stenosis (AS), has become a safe and effective procedure for high surgical–risk populations or patients who cannot tolerate surgical aortic valve replacement (SAVR) (1-5). With the evolution of surgical techniques and the expansion of indications, TAVR is playing an increasingly important role in the treatment of aortic regurgitation (AR) (6). Notably, concomitant mitral regurgitation is more likely to happen in patients with aortic valve dysfunction. Combined aortic and mitral value dysfunction raises the challenge to either surgical or transcatheter treatment. Studies showed that mitral regurgitation would be improved in 40–100% AS patients who receiving SAVR, however, the improvement was less (12–80%) in those who receiving TAVR (7). Real-world data of applying TAVR for AR is increasing, which supports TAVR for appropriate AR patients (8). While mitral valve regurgitation is also common in AR patients, whether TAVR would be efficacy for combined aortic and mitral regurgitation remains unclear. In this study, we analyzed the outcomes of TAVR in patients with combined severe aortic and mitral regurgitation at the Zhongnan Hospital of Wuhan University to investigate the clinical effectiveness and feasibility of TAVR in treating combined valvular diseases. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-505/rc).
Methods
Clinical data
Patients with severe AR and moderate/severe mitral regurgitation (MR) diagnosed at the Zhongnan Hospital of Wuhan University and treated with TAVR from December 2021 to November 2022 were enrolled in this retrospective clinical observational study. Patients with following conditions were suggested for TAVR: (I) severe AR confirmed by echocardiogram; (II) New York Heart Association (NYHA) functional class III or IV; (III) surgical contraindications with society of thoracic surgeons score ≥8; (IV) desire to receive TAVR. The inclusion criteria for study were as follows: (I) receiving TAVR for AR combined with moderate or severe MR; and (II) functional MR diagnosed by echocardiogram. Patients were excluded if they met any of the followings: (I) mild MR; (II) having serious complications or failure of TAVR, which resulted in surgical valve replacement or death during operation; and/or (III) having missing or incomplete data. The TAVR team consisted of the medical staffs at the Structural Heart Disease Center, specialists from the Department of Medical Imaging, Department of Anesthesiology, Intensive Care Unit, and operating room. The ratio of the regurgitant jet area (RJA) to the left atrial area (LAA) (RJA/LAA) was used to assess the degree of mitral regurgitation.
Complete preoperative data were collected for all patients. The imaging data were analyzed and reconstructed using the 3MENSIO software (Pie Medical Imaging, Netherlands). The patients were fully informed of the treatment-related risks and signed informed consent forms. TAVR procedures were performed in a hybrid operating room by the TAVR team. Follow-up data were collected 1 month after operation for comparisons. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved and filed by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2023046K). Informed written consent was obtained from all patients while recruiting.
Statistical analysis
The normally distributed measurement data are expressed as the mean ± standard deviation and were analyzed using the paired-sample t-test and an analysis of variance. The numeration data are presented as the number (percentage), and the ranked data were analyzed using the Chi square (Z) test. The statistical analysis was performed using the SPSS 23.0. A P value <0.05 was considered significantly different.
Results
Baseline data
Of the 40 patients who underwent TAVR during the retrospective observation period, 11 (9 male and 2 female) were enrolled in this study (Figure 1). The patients had a mean age of 74.7±2.7 years and a mean preoperative Society of Thoracic Surgeons score of 8.5±1.2. All the patients presented WHO functional class III–IV at admission, most of whom had enlarged left ventricle, and reduced left heart function. Data are presented in Table 1.
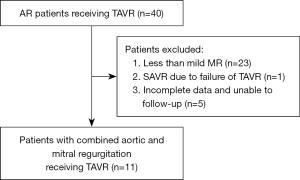
Table 1
| Variables | Value |
|---|---|
| Baseline data | |
| Age (years) | 74.7±2.7 |
| Males, n (%) | 9 (81.8) |
| Females, n (%) | 2 (18.2) |
| Body mass index (kg/m2) | 22.2±1.1 |
| Co-existing diseases, n (%) | |
| Hypertension | 10 (90.1) |
| Diabetes | 2 (18.2) |
| Coronary heart disease | 4 (36.4) |
| Cerebral infarction | 2 (18.2) |
| Tumors | 1 (9.0) |
| Chronic obstructive pulmonary disease | 7 (63.6) |
| Renal insufficiency | 8 (72.7) |
| Hepatic insufficiency | 8 (72.7) |
| Atrial fibrillation | 5 (45.4) |
| Tricuspid regurgitation | 8 (72.7) |
| STS score | 8.5±1.2 |
| Laboratory tests | |
| Creatinine (mmol/L) | 116.2±60.2 |
| Albumin (g/L) | 33.0±4.3 |
| ALT (U/L) | 19.1±9.9 |
| TBIL (μmol/L) | 17.8±6.2 |
| HGB (g/L) | 111.2±23.1 |
| NT-Pro BNP (pg/mL) | 6,799.7±1,928.5 |
| Ultrasound indicators | |
| AAo (mm) | 39.3±3.9 |
| LVEDD (mm) | 65.5±10.7 |
| Mitral annulus diameter (mm) | 36.7±5.4 |
| LVEF (%) | 46.4±14.3 |
| RJA/LAA (%) | 42.4±6.8 |
Data are presented as No. (%) or mean ± standard deviation. STS, Society of Thoracic Surgeons; ALT, alanine aminotransferase; TBIL, total bilirubin; HGB, hemoglobin; NT-ProBNP, N-terminal prohormone of brain natriuretic peptide; AAo, ascending aorta; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; RJA, regurgitant jet area; LAA, left atrial area.
Intra- and postoperative conditions
No perioperative deaths were recorded. Retrievable self-expanding valve grafts were used in all the patients, and the grafts were implanted via the transfemoral route in 8 patients and via the transapical route in 3 patients. After the operation, 2 patients underwent permanent pacemaker implantations due to high-grade atrioventricular blocks. During the transfemoral TAVR, 1 patient developed descending aortic dissection due to the presence of severe aortic atherosclerosis and underwent concurrent intracavitary aortic stenting. Three of the 5 patients with atrial fibrillation had their rhythm converted to sinus rhythm post-operation. No new cases of persistent atrial fibrillation were noted during the perioperative period.
Left heart was significantly retrieved after TAVR as there was an average reduction of about 7mm in the left ventricular end-diastolic diameter (LVEDD), along with the mitral annular diameter reduced nearly 5 mm after TAVR. No significant improvements of ejection fraction (EF) were seen within 24 hours post-operation (44.6%±9.3% post-operation vs 46.4%±14.3% at admission, P=0.529). Importantly, 50% of MR diminished after TAVR (Table 2). All the patients recovered well and were successfully discharged from hospital.
Table 2
| Variables | Before TAVR | After TAVR | t/χ2/Z | P value |
|---|---|---|---|---|
| LVEDD (mm) | 65.5±10.7 | 58.6±8.8 | 6.009 | <0.001 |
| Mitral annulus diameter (mm) | 36.7±5.4 | 31.5±2.8 | 5.131 | <0.001 |
| RJA/LAA | 42.4±6.8 | 24.7±11.5 | 6.057 | <0.001 |
Data are presented as mean ± standard deviation. TAVR, transcatheter aortic valve replacement; LVEDD, left ventricular end-diastolic diameter; RJA, regurgitant jet area; LAA, left atrial area.
Follow-up
No serious cardiovascular events were reported during the 1-month follow-up. One patient underwent retroperitoneal sarcoma resection after TAVR and recovered well. LVEF increased by nearly 5% (44.6%±9.3% post-operation vs. 50.0%±9.4% 1-month follow-up, P=0.022).
Discussion
TAVR is widely recognized as a safe and effective option for the treatment of AS. AR used to be considered a relative contraindication to TAVR because the aortic annulus fails to provide sufficient radial force (9,10). With technical developments and innovations, TAVR has become much more promising in the treatment of AR, and some patients with AR have benefited significantly from TAVR (11,12). However, few studies have explored the role of TAVR in critical patients with AR combined with MR.
Because of the severe over-load of left ventricle in AR, patients often suffered from reduced intra-aortic antegrade flow, and systemic multisystemic insufficiencies during decompensation. In AR patients with cardiac decompensation, the 1-year mortality rate can reach 20% for those who with LVEF ≤30%, and only 5% of these patients would choose conventional surgical treatment (13). Moreover, about 75% of AR patients presented MR with characteristics of a dilated mitral annulus, the abnormal position and function of papillary muscle, and poor leaflet coaptation, which further increased risk of conventional surgery in addition to their older age. TAVR was not offered for patients with combined valvular disease for a long time as it resolves aortic disease only. However, with the theory that most MR secondary to AR were due to changes in left ventricular structure and function (14), it is believed that functional MR can be improved after resolving the etiology of aortic valve disease. TAVR increase intra-aortic antegrade flow, reduce left ventricular end-diastolic pressure, increase effective cardiac output, and improve systemic tissue perfusion when resolve AR. It can be an optimal option for patients with combined valvular disease. MR was significantly improved in our study as mitral annular diameter reduced. However, more data, such as in mitral annular area/shape, may further illustrate the details of improvement.
In the present study, we observed clinical outcomes of those who had combined aortic and mitral regurgitation received TAVR. All MR were confirmed to be functional and secondary to AR without organic changes. After TAVR, a remarkable improvement in mitral regurgitation was observed together with a significant reduction in the LVEDD and reversal LV remodeling. Though the LVEF slightly decreased post-operation, cardiac function was significantly improved as LVEF increased at 1-month follow-up. There was no statistic improvements of LVEF between at admission and follow-up, which may be related to the small sample size and the overestimation of EF values due to the presence of MR before TAVR.
TAVR is a less invasive but high challenging procedure with multiple fatal complications especially for AR patients (15-17). Some of the patients didn’t have the opportunity for rescue surgery during TAVR because of their critical illness. Thus, preoperative assessment is particularly important. The anatomy of the valvar annuli is one of the key factors for successful TAVR in AR patients, especially for those who with combined valve disease.
In our experience, AR resulted in severe dilation of aortic ring and had higher chances of failure due to unable to stabilize the prothesis. Therefore, the anatomy of aortic root should be carefully assessed, including the sizes of the aortic annulus, the angle of the annular plane, the height of the coronary openings, and the morphologies of the left ventricular outflow tract, sinotubular junction, and ascending aorta. Transfemoral TAVR is not recommended if: (I) the diameters of either the plane or 4 mm below aortic annulus exceed 28 mm; (II) the angle of aortic annular plane exceeds 60°; (III) the ascending aorta is significantly dilated (>4.5 cm) within 5 cm above the aortic annular plane; (IV) the angle between the aortic annulus and mitral annulus is <90°; and (V) the height of the coronary opening is less than 1 cm, or the valve leaflet is too long to cover the coronary opening. Transapical route could be applied for those who were not suitable for transfemoral route as there were three stabilization arms in prothesis for transapical route. We included TAVR of both transfemoral and transapical routes in our study, and no differences were observed between them.
In addition to the anatomy of the aortic root, an echocardiographic assessment of MR is also important before TAVR. The diagnostic criteria for functional MR must exclude significant rheumatic valve disease, infective endocarditis, and significant organic pathologies of the leaflets and subvalvular structures (e.g., severe mitral stenosis, significant calcification of the valve, and structural abnormalities of the subvalvular tendon cords, and papillary muscles). Since the annulus is usually large in AR patients, insufficient structural support might lead to valve displacement and perivalvular leakage (18). An oversize valve ratio of 15–20% is recommended to provide adequate support of the annulus, however, increased oversize adds the risk of annular tears and high-grade atrioventricular blocks (19).
Due to the difficulty in intraoperative positioning and anchoring, a retrievable self-expanding valve graft is recommended for adjustment during procedure. After the valve is released, observation should last for at least 30 min. Radiography and transesophageal cardiography can also be used to assesses the position of the prosthetic valve and the improvement of mitral regurgitation. Stabilized blood pressure and heart rate also provides additional support for successful TAVR.
There are few limitations in our current study. Firstly, the design of a single-center retrospective observational study might bring bias in conclusions. Secondly, there were only 11 patients included in the analysis. Thirdly, the follow-up data was obtained in 1-month after TAVR. More patients and longer follow-up are needed to develop better implementation criteria and assessment systems.
In summary, TAVR is a feasible in critical patients with combined aortic and mitral regurgitation and can lead to an improvement of MR. In patients with significant residual MR, moreover, a staged procedure on the mitral valve could be speculated.
Conclusions
TAVR is effective and feasible for high-risk patients with combined aortic and mitral regurgitation.
Acknowledgments
We would like to thank all colleagues in center of structural heart disease for their dedicated work for every patient. We also would like to thank all staff from different department for their contributions in every operation.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-505/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-505/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-505/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-505/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved and filed by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2023046K). Informed written consent was obtained from all the patients while recruiting.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019;380:1695-705. [Crossref] [PubMed]
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med 2019;380:1706-15. [Crossref] [PubMed]
- Writing Committee Members. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:450-500. Erratum in: J Am Coll Cardiol 2021;77:1276. [Crossref] [PubMed]
- Testa L, Latib A, Rossi ML, et al. CoreValve implantation for severe aortic regurgitation: a multicentre registry. EuroIntervention 2014;10:739-45. [Crossref] [PubMed]
- Didier R, Eltchaninoff H, Donzeau-Gouge P, et al. Five-Year Clinical Outcome and Valve Durability After Transcatheter Aortic Valve Replacement in High-Risk Patients. Circulation 2018;138:2597-607. [Crossref] [PubMed]
- Nombela-Franco L, Ribeiro HB, Urena M, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol 2014;63:2643-58. [Crossref] [PubMed]
- Markham R, Ghodsian M, Sharma R. TAVR in Patients with Pure Aortic Regurgitation: Ready to Use? Curr Cardiol Rep 2020;22:98. [Crossref] [PubMed]
- De Backer O, Pilgrim T, Simonato M, et al. Usefulness of Transcatheter Aortic Valve Implantation for Treatment of Pure Native Aortic Valve Regurgitation. Am J Cardiol 2018;122:1028-35. [Crossref] [PubMed]
- Jiang J, Liu X, He Y, et al. Transcatheter Aortic Valve Replacement for Pure Native Aortic Valve Regurgitation: A Systematic Review. Cardiology 2018;141:132-40. [Crossref] [PubMed]
- Roy D, Sharma R, Brecker SJ. Native aortic valve regurgitation: transcatheter therapeutic options. EuroIntervention 2013;9:S55-62. [Crossref] [PubMed]
- Moazami N, Diodato MD, Moon MR, et al. Does functional mitral regurgitation improve with isolated aortic valve replacement? J Card Surg 2004;19:444-8. [Crossref] [PubMed]
- Takagi H, Hari Y, Kawai N, et al. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation. Heart Lung Circ 2020;29:729-41. [Crossref] [PubMed]
- Schmitto JD, Lee LS, Mokashi SA, et al. Functional mitral regurgitation. Cardiol Rev 2010;18:285-91. [Crossref] [PubMed]
- Sawaya FJ, Deutsch MA, Seiffert M, et al. Safety and Efficacy of Transcatheter Aortic Valve Replacement in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study. JACC Cardiovasc Interv 2017;10:1048-56. [Crossref] [PubMed]
- Hansen JW, Foy A, Yadav P, et al. Death and Dialysis After Transcatheter Aortic Valve Replacement: An Analysis of the STS/ACC TVT Registry. JACC Cardiovasc Interv 2017;10:2064-75. [Crossref] [PubMed]
- Yoon SH, Schmidt T, Bleiziffer S, et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J Am Coll Cardiol 2017;70:2752-63. [Crossref] [PubMed]
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585-95. [Crossref] [PubMed]
- Rodés-Cabau J, Ellenbogen KA, Krahn AD, et al. Management of Conduction Disturbances Associated With Transcatheter Aortic Valve Replacement: JACC Scientific Expert Panel. J Am Coll Cardiol 2019;74:1086-106. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


