
Analysis of heart displacement during thoracic radiotherapy based on electrocardiograph-gated 4-dimensional magnetic resonance imaging
Highlight box
Key findings
• The extent of movement of the heart and its substructures during one cardiac cycle was approximately 4.0–26.1 mm, and compensatory margins should be applied to the planning CT by extending the margins.
What is known and what is new?
• The locations of the heart and its substructures determined by planning CT do not represent the true positions due to cardiac movement.
• Our research applied 4D-MRI to evaluate the dynamic changes during one cardiac cycle and calculated compensatory margins for planning CT that could improve the resolution of the heart and its substructures.
What is the implication, and what should change now?
• It is inappropriate to directly apply the delineations from planning CT to assess heart exposure during radiotherapy. Compensatory margins should be applied to the planning CT.
Introduction
Radiotherapy is an important treatment for thoracic tumours but carries the disadvantage of radiation damage to surrounding organs, including the heart (1). The heart was once considered a relatively radioresistant organ unlikely to be damaged when exposed to an irradiation dose of <30 Gy, although the risk increased when exposure >40 Gy (2). However, recent research suggests damage occurs even at moderate doses, including the estimate that the rate of cardiac disease increased after the atomic bomb following an average irradiation dose of 0.5–2 Gy (3). Nonetheless, the specific threshold dose below which the heart receives no damage is unknown (4). Irradiation damage to the heart frequently occurs during thoracic radiotherapy for conditions such as oesophageal cancer, lung cancer, Hodgkin’s disease, and breast cancer (2,5). As the survival time of cancer patients is prolonged after radiotherapy, radiation-induced heart disease (RIHD) has attracted increasing attention because of its influence on survival. RIHD encompasses a series of effects on the heart, from subclinical histopathological findings to clinical disease, including damage to the pericardium, myocardium, valves, conduction system, and coronary arteries, and the exposure dose and volume are major factors in determining the extent of damage (6). Dose-volume parameters based on planning computed tomography (CT) scans are currently the main method to predict RIHD.
In addition to respiratory movement, the heart is especially influenced by periodic cardiac movement (7,8). According to previous studies (9-14), planning CT that displayed images of the heart and its substructures failed to represent its real morphology, volume, and location, and volume-dose parameters based on planning CT were inaccurate due to these displacements. Tong et al. calculated the displacements of 1.2±0.9, 0.6±0.5, and 0.6±0.5 mm for the heart, 0.5±0.4, 0.4±0.3, and 0.8±0.6 mm for the pericardium, and 1.0±0.8, 4.1±2.8, and 1.9±1.2 mm for the left ventricular muscle (LVM) in the left-right, ventral-dorsal, and caudal-cranial directions by 4-dimensional CT (4D-CT) (9). Li et al. also found the displacements of CA bifurcations in the LR, CC, and AP directions were 6, 6, and 5 mm (left) and 6, 8, and 7 mm (right) (10). At present, studies of the movement of the heart and its substructures are mostly based on cone-beam CT (CBCT), 4D-CT, and other CT techniques (9-12). However, it is challenging to delineate soft tissues with these methods compared with magnetic resonance imaging (MRI) (6). Considering MRI had the advantage of discriminating soft tissues, we aimed to conduct research on the displacement and the compensatory extension of the pericardium, heart, interatrial septum, interventricular septum, LVM, antero-lateral papillary muscle (ALPM), and postero-medial papillary muscle (PMPM) with 4D-MRI. With the application of the breath-hold technique, the influence of respiratory movement was offset. We present this article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-447/rc).
Methods
Patients
The inclusion criteria were patients arranged for thoracic radiotherapy, especially for who had mediastinal enlarged lymph node. The exclusion criteria were as follows: (I) patients without sufficient lung function; (II) patients with claustrophobia or metallic implants failed to received MRI examination; (III) patients with baseline heart disease. Eventually, 15 patients with oesophageal or lung cancers were enrolled, including one female and nine males aged from 59 to 77 years. The pre-treatment 4D-MRI data and planning CT images from December 10th, 2018, to March 4th, 2020 were made available to the MIM workstation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved the by Ethics Committee of Shandong Cancer Hospital (No. SDTHEC2019008013), and individual consent for this retrospective analysis was waived.
ECG-gated 4D-MRI
Breath-hold ECG-gated 4D-MRI images were obtained with a GE Discovery MR750W device (General Electric Company, USA). The scan ranged from the level of the aortic arch to the bottom of the pericardium, the slice thickness was 5 mm, and the spacing was 0 mm. Radio-frequency excitation and signal acquisition were performed during MRI of the whole cardiac cycle, and ECG information was simultaneously integrated into the MRI system. MR signals were used to reconstruct images in different phases, so the contraction and relaxation of the atria and ventricles could be observed in a “cine” mode. In this study, every 5% of the cardiac cycle was reconstructed into one phase.
Planning CT
Patients were scanned by a Philips CT simulator in the supine position and immobilized with their hands above their heads holding handles. Magnification markers were applied on the chest and both sides of the body. Plain and enhanced CT images were then transported to a Varian Eclipse 8.6.15 system (Varian Medical Systems, USA) for radiation planning and design. Contrast-enhanced CT (CECT) images were used to contour the target volume, while plain CT images were used for planning and dose calculation. The planning CT images were then transported to a Varian Trilogy linear accelerator to facilitate radiation treatment after verification by clinicians.
Delineation principles
The pericardium, heart, interatrial septum, interventricular septum, LVM, ALPM, and PMPM were assessed. The superior boundary of the pericardium started from the left superior pulmonary vein, and the inferior boundary ended where the pericardium fused with the diaphragm, while the heart started from the presence of the left atrium and ended when the signals of the muscle tissue vanished, excluding the superior and inferior vena cava. The boundary between the LVM and interventricular septum was defined as the left margin of the right coronary anterior descending branch. The ALPM and PMPM were contoured separately. All images were contoured by the same clinician and verified by another clinician.
Data acquisition
Both planning CT and 4D-MRI images were transported to a commercial MIM Maestro 6.7.6 workstation (MIM Software Inc., Cleveland, OH, USA) for margin delineation. The boundaries of the heart and its substructures were delineated at 20 phases which included the whole heart (pericardium included), the heart (pericardium not included), the atrial septum, the ventricular septum, the LVM, the ALPM, and the PMPM. The heart and its substructure boundaries in 20 phases were fused, and the fusion volume was generated on the base of the “fusion” function of MIM workstation. Planning CT and the 4D-MRI images were registered based on the frame assistant. Displacement of the heart and its substructures was measured in the anterior-posterior (AP), left-right (LR), and cranial-caudal (CC) axes. The compensatory expansion range was calculated by expending the boundary of the heart and its substructures on the planning CT to cover more than 95% of the fusion volume.
Statistical analysis
All data from the 15 patients (representing a relatively small sample size) were presented as the mean ± standard deviation. The measurement errors were tried to be minimized by measured through two different researchers, and adopted the average. The differences of groups which were composed of the displacement of each substructure (pericardium, heart, interatrial septum, interventricular septum, LVM, ALPM and PMPM) in each direction (AP, LR and CC axes) were tested with the Kruskal-Wallis H test. Differences were considered significant at two-side P value of <0.05. Statistical analysis was conducted with SPSS 23.0 software (SPSS Inc., Chicago, IL).
Results
Displacement of the heart and its substructures
The displacements of the heart and its substructures in the AP, LR, and CC directions were as follows (Table 1): interatrial septum: 18.4±6.9, 16.4±6.5, and 4.0±3.2 millimetre mm; interventricular septum: 20.4±7.6, 16.1±5.2, and 4.5±5.0 mm; LVM: 17.6±5.9, 26.1±5.8, and 4.0±5.2 mm; ALPM: 17.3±4.2, 14.3±6.9, and 5.0±6.2 mm; and PMPM: 19.7±7.5, 9.8±5.7, and 8.0±7.5 mm, respectively. The displacements in the AP and LR directions were 9.9±3.7 and 15.9±6.7 mm for the pericardium and 19.5±4.4 and 20.9±5.5 mm for the heart, respectively, while movements of the pericardium and heart in the CC direction failed to be observed due to the limited imaging range of 4D-MRI. According to our data, the displacement of the heart and other substructures ranged from 4 to 20 mm, implying the margin displacement influenced by periodic cardiac movement was nonnegligible (as showed in Figure 1). Interestingly, although the size of the left ventricular papillary muscles was smaller than the other structures, the motion amplitude showed no significant differences among structures (P=0.423, 0.423, 0.406 respectively in AP, LR, and CC axes). Movement of the pericardium was significantly milder in the AP direction than that of other structures. The most obvious movement was for the LVM in the LR direction, which might be related to LVM systolic and diastolic deformation during blood ejection.
Table 1
| Structures | Pericardium, mm |
Heart, mm | Interatrial septum, mm | Interventricular septum, mm | LVM, mm | ALPM, mm | PMPM, mm |
|---|---|---|---|---|---|---|---|
| AP | 9.9±3.7 (4.8–15.5) |
19.5±4.4 (13.7–27.9) |
18.4±6.9 (10.4–29.0) |
20.4±7.6 (12.2–32.3) |
17.6±5.9 (11.3–32.2) |
17.3±4.2 (12.4–25.3) |
19.7±7.5 (12.0–35.8) |
| LR | 15.9±6.7 (5.7–29.0) |
20.9±5.5 (12.0–28.0) |
16.4±6.5 (5.9–27.9) |
16.1±5.2 (10.7–27.4) |
26.1±5.8 (19.1–35.1) |
14.3±6.9 (6.3–25.5) |
9.8±5.7 (2.3–22.2) |
| CC | – | – | 4.0±3.2 (0.0–10.0) |
4.5±5.0 (0.0–15.0) |
4.0±5.2 (0.0–15.0) |
5.0±6.2 (0.0–20.0) |
8.0±7.5 (0.0–25.0) |
Data are represented as mean ± standard deviation (range). Movement of the pericardium and heart in the CC direction was not observable due to the imaging range of 4D-MRI. AP, anterior-posterior; LR, left-right; CC, cranial-caudal; LVM, left ventricular muscle; ALPM, antero-lateral papillary muscle; PMPM, postero-medial papillary muscle; 4D-MRI, 4-dimensional magnetic resonance imaging.
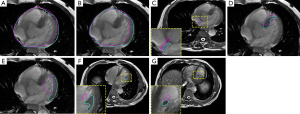
Compensatory extension range for planning CT
Images showed the edge of the fusion volume of each structure was located outside that on the planning CT. The mean fusion volume was larger than the planning CT volume (Table 2) in the pericardium: 743.39 vs. 726.62 mL, heart: 547.94 vs. 546.53 mL, interatrial septum: 11.93 vs. 3.71 mL, interventricular septum: 59.79 vs. 28.45 mL, LVM: 99.96 vs. 51.25 mL, ALPM: 6.8 vs. 1.01 mL, and PMPM: 5.09 vs. 0.62 mL, respectively. The compensatory extension range would extend the margin of the planning CT in six directions (anterior, posterior, left, right, cranial, and caudal) by the following distances (Table 3): pericardium: 1.7±0.9, 3.6±1.6, 1.8±1.6, 3.0±1.7, 2.1±3.5, and 2.9±2.7 cm; heart: 1.2±1.0, 2.5±1.7, 1.0±0.7, 2.8±1.2, 1.8±3.4, and 3.3±2.9 cm; interatrial septum: 3.8±2.6, 3.4±2.9, 3.1±1.7, 2.8±1.6, 0.9±1.8, and 2.0±3.9 cm; interventricular septum: 3.3±1.7, 4.9±2.4, 2.0±1.9, 4.1±1.9, 1.1±1.8, and 2.9±3.7 cm; LVM: 2.2±1.6, 3.0±1.6, 1.1±0.8, 5.3±2.0, 1.8±2.4, and 2.4±3.5 cm; ALPM: 5.9±3.5, 3.4±2.5, 2.1±1.5, 6.1±2.4, 5.4±4.2, and 3.6±3.2 cm; and PMPM: 6.6±2.7, 2.9±2.2, 2.6±2.5, 6.6±2.7, 3.9±4.1, and 4.8±5.1 cm, respectively. The compensatory margins for the ALPM (mean value =4.42 cm) and PMPM (mean value =4.57 cm) were larger than those for the pericardium (mean value =2.52 cm), heart (mean value =2.10 cm), and LVM (mean value =2.63 cm), with P<0.05, reflecting the heterogeneity in the motion of the heart and its structures, and reminding us that for patients with high risk, the left ventricular muscle or other substructures should be contoured as organs at risk (OAR) separately. The compensatory extension was also larger in the caudal direction than in the cranial direction except for the ALPM, with the extent of the compensatory extension range almost 2- to 5- fold higher in the right direction than in the left direction except for the interatrial septum. According to the data, planning CT cannot represent the true location and volume of the heart (as showed in the Figure 2), and a compensatory extension range should be applied to assess volume-dose parameters.
Table 2
| Structures | Pericardium, mL | Heart, mL | Interatrial septum, mL | Interventricular septum, mL | LVM, mL | ALPM, mL | PMPM, mL |
|---|---|---|---|---|---|---|---|
| ITV | 743.39 | 547.94 | 11.93 | 59.79 | 99.96 | 6.8 | 5.09 |
| Planning CT | 726.62 | 546.53 | 3.71 | 28.45 | 51.25 | 1.01 | 0.62 |
CT, computed tomography; ITV, internal target volume; LVM, left ventricular muscle; ALPM, antero-lateral papillary muscle; PMPM, postero-medial papillary muscle.
Table 3
| Structures | Pericardium, cm | Heart, cm | Interatrial septum, cm | Interventricular septum, cm | LVM, cm | ALPM, cm | PMPM, cm |
|---|---|---|---|---|---|---|---|
| Anterior | 1.7±0.9 (0.0–3.0) | 1.2±1.0 (1.0–3.0) | 3.8±2.6 (0.0–7.0) | 3.3±1.7 (0.0–6.0) | 2.2±1.6 (0.0–4.0) | 5.9±3.5 (2.0–12.0) | 6.6±2.7 (2.0–9.0) |
| Posterior | 3.6±1.6 (0.0–5.0) | 2.5±1.7 (0.0–6.0) | 3.4±2.9 (0.0–8.0) | 4.9±2.4 (1.0–9.0) | 3.0±1.6 (1.0–6.0) | 3.4±2.5 (0.0–9.0) | 2.9±2.2 (0.0–6.0) |
| Left | 1.8±1.6 (0.0–4.0) | 1.0±0.7 (0.0–2.0) | 3.1±1.7 (1.0–5.0) | 2.0±1.9 (1.0–7.0) | 1.1±0.8 (0.0–2.0) | 2.1±1.5 (0.0–5.0) | 2.6±2.5 (0.0–7.0) |
| Right | 3.0±1.7 (0.0–4.0) | 2.8±1.2 (1.0–5.0) | 2.8±1.6 (1.0–6.0) | 4.1±1.9 (1.0–6.0) | 5.3±2.0 (3.0–9.0) | 6.1±2.4 (3.0–10.0) | 6.6±2.7 (0.0–9.0) |
| Cranial | 2.1±3.5 (0.0–5.0) | 1.8±3.4 (0.0–4.0) | 0.9±1.8 (0.0–5.0) | 1.1±1.8 (0.0–4.0) | 1.8±2.4 (0.0–6.0) | 5.4±4.2 (0.0–12.0) | 3.9±4.1 (0.0–12.0) |
| Caudal | 2.9±2.7 (0.0–7.0) | 3.3±2.9 (0.0–7.0) | 2.0±3.9 (0.0–12.0) | 2.9±3.7 (0.0–10.0) | 2.4±3.5 (0.0–5.0) | 3.6±3.2 (0.0–8.0) | 4.8±5.1 (0.0–12.0) |
Data are represented as mean ± standard deviation (range). LVM, left ventricular muscle; ALPM, antero-lateral papillary muscle; PMPM, postero-medial papillary muscle.
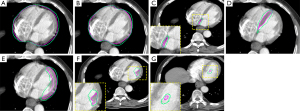
Discussion
Radiotherapy has led to survival benefits for thoracic cancers, such as breast cancer, Hodgkin's lymphoma (HL), oesophageal cancer, and lung cancer. However, the longer survival period has resulted in an increased risk of RIHD. A study involving 1,474 HL patients reported the relative risk (RR) in post-radiation patients ranged from 3 to 5, and 66–80% of patients suffered from RIHD induced by mediastinal radiation (15). Cardiac-induced mortality was the leading cause of non-cancer-induced death in both HL and breast cancer after radiotherapy (16). Relatively few studies have focused on RIHD of oesophageal or lung cancer in comparison to HL and breast cancer because the survival period of oesophageal and lung cancer is obviously shorter. However, the dose to the heart during oesophageal radiotherapy is elevated because of the anatomical location. The mean dose received by the heart in breast cancer is commonly 10–15 Gy, while in distal oesophageal radiotherapy, this might reach or exceed 50 Gy (16). Beukema et al. collected articles published from 1970 to 2013 related to cardiotoxicity after oesophageal cancer radiotherapy and found the rate of RHID was approximately 10.8% (5–44%) (17). Ogino et al. conducted a retrospective study of 343 oesophageal patients who received concurrent radiotherapy or radiotherapy alone with a median follow-up period of 79 months (range, 48–127 months) and who achieved long-term survival (more than 4 years). The end point of the study was symptomatic heart disease, with a 5-year incidence of 13.8% (18).
The clinical spectra of heart disease caused by radiotherapy includes pericardial disease, myocarditis, valvular disease, coronary artery disease (CAD), and conduction abnormalities. Pericardium effusion and pericarditis are the most common RIHDs, and the former is usually asymptomatic. Acute pericarditis mostly occurs during or after radiotherapy, while delayed chronic pericarditis usually occurs 1 year after treatment (19). The main mechanism of myocardial damage is fibrosis, which might lead to congestive insufficiency, while most chronic heart failure occurs decades after treatment (1). CAD is rare in RIHD but is fatal, with a latent period of approximately 10 years, while radiation-induced valvular disease mainly influences the left ventricle (5). Valvular disease involves valvular contraction and regurgitation, and symptoms of the latter are more common and usually occur 10 years post-radiation. In a study by Lund et al., the incidence of left ventricular valve regurgitation in patients with HL after radiotherapy was 6–40%, compared with 2% in patients without radiotherapy (20). The pathological basis of conduction system disorder is also fibrosis induced by irradiation and manifests as ECG abnormalities, although approximately 70% of patients return to normal ECG readings without intervention. Other than acute pericarditis, most RIHDs have a relatively long incubation period, and the incidence increases with time.
The two major risk factors of RIHD include irradiation dose and volume (6). According to a long-term follow-up of 4,414 post-radiation breast cancer patients by Hooning et al., the rate of RIHD was related to the mean dose to the heart during radiotherapy (21). Another study of RIHD in breast cancer put forward a specific dosimetric relationship between delivered dose and rate of cardiac mortality, which increased by 3% for every additional 1 Gy of radiation dose (4). Carmel et al. proved that the rate of pericarditis induced by entire heart irradiation was decreased by blocking the left ventricle and inferior pericardium region (22). The results of these studies demonstrate the importance of evaluating the displacement of the heart and its substructures, which influences the irradiation dose and volume. Factors including fraction dose, radiotherapy techniques, chemotherapeutic agents (mainly anthracyclines and trastuzumab), and patient risk factors such as age are also related to RIHD. With the development of radiotherapy technology, the cardiotoxicity of radiotherapy has been significantly reduced. According to Lin et al., compared with 3-dimensional conformal radiotherapy (3D-CRT), intensity modulated radiation therapy (IMRT) remarkably reduced cardiac mortality (72.6% vs. 52.9%, P<0.001), but no significant difference in cancer-specific mortality was seen between methods (P=0.86) (23). However, the risk of cardiotoxicity has not been eliminated by current techniques (24). Anthracyclines are commonly used as chemotherapy schemes in the treatment of breast cancer and HL patients, and the most common regimen of concurrent radiochemotherapy for oesophageal cancer is 5-FU and cisplatin, which have also been proven to be slightly cardiotoxic (25,26).
It is necessary to accurately evaluate the displacement of the heart and its substructures caused by periodic cardiac activity and calculate the compensatory extension range that could be directive in clinical practice when creating a radiotherapy plan. Many studies have proven that delineation of the pericardium, heart, LVM, and CA system based on planning CT fails to show the real margin of the substructures mentioned above during the cardiac cycle (9-14), and a compensatory margin should be applied. Tong et al. calculated the displacements of 1.2±0.9, 0.6±0.5, and 0.6±0.5 mm for the heart, 0.5±0.4, 0.4±0.3, and 0.8±0.6 mm for the pericardium, and 1.0±0.8, 4.1±2.8, and 1.9±1.2 mm for the LVM in the left-right, ventral-dorsal, and caudal-cranial directions by 4D-CT (9), while Kataria et al. suggest radial and cranio-caudal margins of 7 mm and 4 mm, respectively, would cover the range of motions of the coronary artery (CA) on CECT (11). Li et al. found the maximum compensatory margins in the LR, CC, and AP directions for the CA bifurcations were 6, 6, and 5 mm (left) and 6, 8, and 7 mm (right) on 4D-CT, respectively (10). Therefore, the volume-dose parameters used to evaluate the dose to the heart might not truly reflect the dose received during oesophageal radiotherapy and might not provide accurate protection for the heart.
Based on our results, the displacement of the whole heart and its substructures caused by cardiac activity was non-negligible, ranging from 4 to 26.1 mm. The most significant motion was in the LR direction for the LVM (26.1 mm), and the amplitude of pericardium motion was slightly less than the motion of the LVM, interatrial septum, and interventricular septum. These reminded us that the dose-volume parameters of heart substructures especially the LVM should be limited separately to reduce the rate of RIHD. For example, extending a certain margin as the compensatory extension to represent the OAR and then limiting the dose-volume parameters could be performed in clinical practice. As position, volume, and morphology change during the cardiac cycle, we then reconstructed the fusion volume, which reflected the actual locations based on 20 phases of 4D-MRI. Radiotherapy plans are still mainly designed and evaluated by CT images, so we hoped to provide guidance of compensatory extension range for planning CT to cover all the motion scope of the heart and its substructures. The fusion volume boundary of each structure was larger than that contoured on the planning CT, and the compensatory extension ranged from 0.9 to 6.6 mm. The volume differences between fusion volume reconstructed by 4D-MRI and the planning CT of the pericardium (743.39 vs. 726.62 mL) and heart (547.94 vs. 546.53 mL) were moderate, while for other structures, the largest difference was as much as an eight-fold difference seen in the ALPM and PMPM. These results were consistent with the trend that the motion amplitude of the left ventricular papillary muscles was similar to that of other structures (P=0.423, 0.423, 0.406 respectively in AP, LR, and CC axes), even though these muscles have a much smaller volume. The largest compensatory distance was for the left ventricular papillary muscle. There were no significant differences among the motion amplitudes of the involved substructures, but the compensatory extension range were significantly different (P=0.044). Through further analysis, disparities mainly existed between the heart and ALPM (P=0.008), heart and PMPM (P=0.008), LVM and ALPM (P=0.039), LVM and PMPM (P=0.038), pericardium and ALPM (P=0.041), and pericardium and PMPM (P=0.039). This may be because the left ventricular papillary muscles are more active and that it is more difficult to assess the potential exposure to these muscles with the dose-volume parameters based on planning CT.
Our research has the advantage of applying the breath-hold ECG-gated 4D-MRI technique, as well as focusing on substructures of the heart. However, the research was also limited by the sample size, which led to a relatively large deviation, a larger sample should be studied in the future to make the results more convincing. As a result of a narrow imaging range, the inferior margin of the pericardium and the whole heart were not completely displayed on 4D-MRI, which made it impossible to measure their motion amplitude, and their compensatory margins might be smaller than those required. Further, directly adding the extension measured through the boundary displacement is imprecise and requires more accurate algorithms to calculate the compensatory margins.
Conclusions
Cardiac periodic motion causes obvious margin displacement of the heart and its substructures, making it inappropriate to directly apply delineations from planning CT to assess heart exposure during thoracic radiotherapy, especially for patients with underlying heart disease. According to the present research, a compensatory extension range should be applied to the planning CT.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-447/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-447/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-447/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-447/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved the by Ethics Committee of Shandong Cancer Hospital (No. SDTHEC2019008013), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang H, Wei J, Zheng Q, et al. Radiation-induced heart disease: a review of classification, mechanism and prevention. Int J Biol Sci 2019;15:2128-38. [Crossref] [PubMed]
- Selwyn AP. The cardiovascular system and radiation. Lancet 1983;2:152-4. [Crossref] [PubMed]
- Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ 2010;340:b5349. [Crossref] [PubMed]
- Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656-65. [Crossref] [PubMed]
- Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart 2009;95:252-8. [Crossref] [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [Crossref] [PubMed]
- Topolnjak R, Borst GR, Nijkamp J, et al. Image-guided radiotherapy for left-sided breast cancer patients: geometrical uncertainty of the heart. Int J Radiat Oncol Biol Phys 2012;82:e647-55. [Crossref] [PubMed]
- Qi XS, Hu A, Wang K, et al. Respiration induced heart motion and indications of gated delivery for left-sided breast irradiation. Int J Radiat Oncol Biol Phys 2012;82:1605-11. [Crossref] [PubMed]
- Tong Y, Yin Y, Lu J, et al. Quantification of heart, pericardium, and left ventricular myocardium movements during the cardiac cycle for thoracic tumor radiotherapy. Onco Targets Ther 2018;11:547-54. [Crossref] [PubMed]
- Li Q, Tong Y, Yin Y, et al. Definition of the margin of major coronary artery bifurcations during radiotherapy with electrocardiograph-gated 4D-CT. Phys Med 2018;49:90-4. [Crossref] [PubMed]
- Kataria T, Bisht SS, Gupta D, et al. Quantification of coronary artery motion and internal risk volume from ECG gated radiotherapy planning scans. Radiother Oncol 2016;121:59-63. [Crossref] [PubMed]
- Tan W, Xu L, Wang X, et al. Estimation of the displacement of cardiac substructures and the motion of the coronary arteries using electrocardiographic gating. Onco Targets Ther 2013;6:1325-32. [Crossref] [PubMed]
- Omidi A, Weiss E, Wilson JS, et al. Effects of respiratory and cardiac motion on estimating radiation dose to the left ventricle during radiotherapy for lung cancer. J Appl Clin Med Phys 2023;24:e13855. [Crossref] [PubMed]
- Habatsch M, Schneider M, Requardt M, et al. Movement assessment of breast and organ-at-risks using free-breathing, self-gating 4D magnetic resonance imaging workflow for breast cancer radiation therapy. Phys Imaging Radiat Oncol 2022;22:111-4. [Crossref] [PubMed]
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878-86. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Beukema JC, van Luijk P, Widder J, et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol 2015;114:85-90. [Crossref] [PubMed]
- Ogino I, Watanabe S, Iwahashi N, et al. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther Onkol 2016;192:359-67. [Crossref] [PubMed]
- Madan R, Benson R, Sharma DN, et al. Radiation induced heart disease: Pathogenesis, management and review literature. J Egypt Natl Canc Inst 2015;27:187-93. [Crossref] [PubMed]
- Lund MB, Ihlen H, Voss BM, et al. Increased risk of heart valve regurgitation after mediastinal radiation for Hodgkin's disease: an echocardiographic study. Heart 1996;75:591-5. [Crossref] [PubMed]
- Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365-75. [Crossref] [PubMed]
- Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin's disease. An analysis of technique, tumor eradication, and complications. Cancer 1976;37:2813-25. [Crossref] [PubMed]
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078-85. [Crossref] [PubMed]
- Boero IJ, Paravati AJ, Triplett DP, et al. Modern Radiation Therapy and Cardiac Outcomes in Breast Cancer. Int J Radiat Oncol Biol Phys 2016;94:700-8. [Crossref] [PubMed]
- Schlitt A, Jordan K, Vordermark D, et al. Cardiotoxicity and oncological treatments. Dtsch Arztebl Int 2014;111:161-8. [PubMed]
- Patanè S. Cardiotoxicity: cisplatin and long-term cancer survivors. Int J Cardiol 2014;175:201-2. [Crossref] [PubMed]
(English Language Editor: B. Draper)


