Physiologic assessment before video thoracoscopic resection for lung cancer in patients with abnormal pulmonary function
Introduction
Preoperative assessment before surgical resection of non-small cell lung cancer (NSCLC) is intended to identify patients with high risk of postoperative complications and to reduce mortality and morbidity. Different algorithms for risk assessment before lung resection have been published (1-4). However, all these algorithms are based on estimations of postoperative morbidity and mortality in conventional thoracotomy. These recommendations do not take into account current data on minimally invasive surgery.
In fact, surgical removal of NSCLC by minimally invasive video thoracoscopy seems particularly suitable for fragile patients (5-7). Scott et al. (8) showed in a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial that patients undergoing video-assisted lobectomy had fewer respiratory complications and shorter length of stay versus open lobectomy. In a retrospective study, Garzon et al. (6) reported 25 patients with altered function [expiratory volume in one second (FEV1) <0.8 L or <50% of the predicted value]. Thirteen of the 25 patients underwent lobectomy by video-assisted thoracoscopic surgery (VATS). No perioperative deaths were observed and postoperative morbidity was 29%. Moreover, to our knowledge, no study has specifically evaluated the association between the settings provided by cardiopulmonary exercise testing (CPET), other than peak oxygen volume (VO2peak) and the occurrence of postoperative cardiopulmonary complications in a cohort of patients undergoing lung resection by VATS. Given the very poor prognosis of lung cancer in absence of surgical treatment, preoperative evaluation should be optimised in patients with borderline respiratory function in order to provide them with a potentially curative intervention with acceptable risk.
This study aimed to evaluate the relation between preoperative pulmonary function and exercise tests and the occurrence of postoperative complications after VATS pulmonary resection in patients with abnormal pulmonary function. We also attempted to determine predictive FEV1 threshold value complications in VATS.
Methods
Study population
We analysed the clinical and surgical data of all consecutive patients who underwent lung resection surgery by VATS in the Thoracic Surgery department of Rouen University Hospital, France between September 2008 and April 2014 (prospective surgical database declared to the CNIL: the French Data Protection Authority; 1690770 v 0). All patients with the following criteria were included: (I) surgery for NSCLC performed during the study period; and (II) impaired preoperative respiratory function defined by a FEV1, and/or diffusing capacity of carbon monoxide (DLCO) <80% of the predicted value. Clinical data, surgical data and perioperative outcome were prospectively recorded for each patient by a senior surgeon. Written informed consent was obtained from all patients, and the study was conducted according to the principles of the Declaration of Helsinki.
Pulmonary function tests, calculation of predicted postoperative values and cardiopulmonary exercise testing (CPET)
Spirometry, plethysmography and measurement of DLCO were carried out according to European standards (Jaeger-Masterlab, Stuttgart, Germany). Forced vital capacity (FVC) and FEV1 were measured. Residual volume (RV), total lung capacity (TLC), DLCO and transfer coefficient of carbon monoxide (KCO) were calculated. These values were expressed in absolute terms (litres, litres per second) and as a percentage of the theoretical values established for a European population (9).
We calculated predicted postoperative values (ppo), ppoFEV1, ppoDLCO and ppoVO2peak for segmentectomy and lobectomy using the following formula: ppoFEV1=preoperative FEV1 × [(19− a) − b]/(19− a)] where a and b are the number of unobstructed and obstructed segments to be respectively resected, obstructed segments being identified by bronchoscopy. Patients underwent an incremental exercise test on an electromagnetic cycle ergometer (Ergoline 900, Sensor Medics, Anaheim, CA, USA). Oxygen pulse saturation (SpO2) was measured by a pulse oximeter (type Biox 3700, Ohmeda, Louisville, CO, USA). According to the protocol, patients started with a warm-up period of 3 minutes, and then work rate was increased each minute to obtain a 10-minute exercise duration.
The theoretical maximum heart rate (HR) was calculated from the patient’s age according to the equation HRmax theoretical = 210–0.65× age. The maximum work rate corresponded to the highest work rate maintained for 1 minute. The VO2peak corresponded to the value of the highest VO2 during the test. The theoretical VO2max and the theoretical maximum work rate were calculated from equations including age and sex. Reference values for maximum parameters of CPET were those of the study by Hansen et al. (10). Maximality was assessed on physiological and clinical criteria as recommended by the Société de Pneumologie de Langue Française (11).
Surgery by video thoracoscopy
Indication for lung resection and extend of resection was discussed in our multidisciplinary meeting. All stages were accepted for resection. We used VATS mainly in intent to treat with conversion if needed. Bulky or proximal tumour with indication of bronchial or vascular plasty was a relative contra-indication for VATS resection. Pre-operative CT-scan images were analysed and 3D-reconstruction was done for complex resections. Systematic dissection of mediastinal lymph nodes was performed for all tumours and radical for all lesions more than 2 cm. The technique used for VATS major resection was the anterior approach described by Hansen et al. (12).
Postoperative events
Operative mortality was calculated taking into account all deaths up to 30 days after the operation or during hospitalisation. Postoperative complications were classified according to Ginsberg (13): (I) major respiratory complications: atelectasis requiring bronchoscopy, pneumonia, respiratory failure, empyema and bronchopleural fistula; (II) minor respiratory complications: atelectasis requiring no fibro-aspiration, prolonged drainage (more than 5 days) and pneumothorax without clinical repercussions; (III) cardiovascular complications: cardiac arrest, myocardial infarction, heart failure, pulmonary embolism, cardiac conduction disorder and atrial fibrillation (AF); and (IV) various complications: postoperative bleeding, chylothorax etc.
Statistical analysis
We first conducted a univariate analysis. Quantitative variables were described by their mean and median. Dispersion was evaluated by standard deviation. Categorical variables were described by their frequency. Means were compared with a two-tailed Student t-test and percentages by Chi2 test. A P<0.05 was considered as statistically significant. The Fisher exact test was used when conditions for applying Chi2 test were not met. To identify independent predictors of postoperative complications, significant variables with P<0.20 in univariate analysis were included in the multivariate analysis. Survival was estimated by Kaplan-Meier method. Significant differences in the probability of survival between strata were assessed by log-rank. A P value less than 0.05 was considered significant. The α risk was controlled by the method of Holm for ANOVA and Tukey HSD for Chi2.
Results
Demographics and clinical data
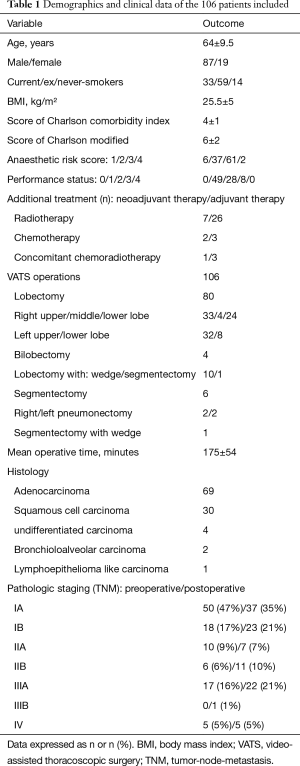
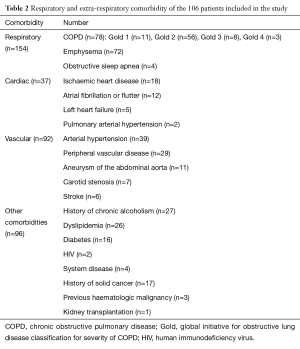
One hundred and six consecutive patients were included in the study. The average age at diagnosis was 64±9.5 years (range, 43–84 years). There were 87 men (82%). Ninety-seven percent of the patients had a history of smoking (Table 1). Table 2 summarises respiratory and extra-respiratory comorbidities. Eighty-one patients (77%) had chronic obstructive pulmonary disease (COPD). Three patients were treated by long-term oxygen therapy. No COPD patient had FEV1 less than the 30% predicted. Four patients had a history of chest radiation and 10 had previous thoracic surgery including 3 pulmonary resections.
Full table
Full table
The NSCLC lesion was located in the upper lobes in 61% of cases [right (n=33) and left (n=32)]. Five patients had a lesion involving two lobes. Tumours were classified according to TNM stages (Table 1). Ten patients had neoadjuvant therapy: radiotherapy (n=7), chemotherapy (n=2) or concomitant chemoradiotherapy (n=1). Procedures included: lobectomy (n=80), lobectomy associated with wedge resection (n=10) or segmentectomy (n=1), segmentectomy (n=6), bilobectomy (n=4), and pneumonectomy (n=4) or segmentectomy associated with wedge resection (n=1). One hundred and two patients underwent lymphadenectomy: radical dissection for 64 and sampling for 38 patients. The resection margins (R1) were invaded in four patients.
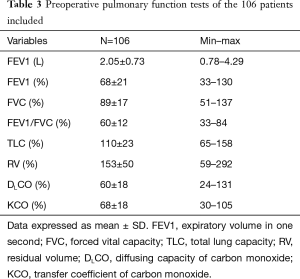
PFT were performed on average 62±57 days (median, 42 days) before surgery. Mean preoperative FEV1 and DLCO were 68%±21% and 60%±18% respectively. Data on preoperative PFT are presented in Table 3.
Full table
Short-term surgery outcomes
The average operation time was 175±54 minutes (range, 63–330 min). Sixteen intraoperative complications (15%) were identified. No deaths were noted. The most frequently observed complication was intraoperative vascular tear (n=10). The overall conversion rate to thoracotomy was 17%. Mean and median drainage times were 5±3 days (range, 1–5 days) and 4 days respectively. Five patients required revision surgery: one lobectomy for necrotizing infectious pneumonitis, three re-drainages (two for prolonged bubbling and one for empyema) and one bubble resection with talc pleurodesis. Median hospital stay was 7 days (range, 1–67 days).
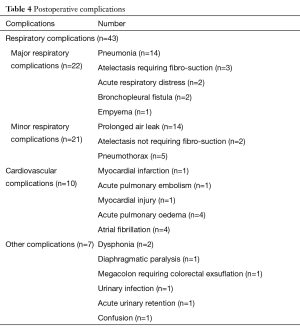
Thirty-seven patients had at least one postoperative complication. Overall morbidity was 34.9%. The number of complications was 0.6 per patient. Postoperative complications are summarized in Table 4. Operative mortality from all resections was 1.9%, two deaths occurred within 30 days following surgery. The first death was due to a cardiac arrest on myocardial infarction in a patient who had undergone left upper lobectomy and the second to a massive pulmonary embolism, on day 5 after right upper lobectomy.
Full table
Prognosis factors for outcomes
In univariate analysis, FEV1, expressed as a percentage of the predicted value, was associated with major postoperative respiratory complications (mean FEV1 equal to 59% in presence of complications vs. 70% in absence of complications; P=0.03). Figure 1A represents the predicted probability of major respiratory complications based on preoperative FEV1.
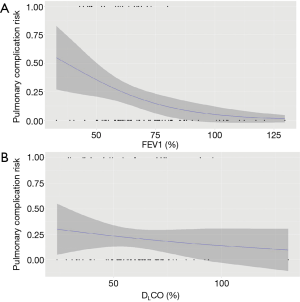
FEV1, expressed in absolute values, did not differ significantly according to the occurrence or non-occurrence of complications (1.74 vs. 2.12 L; P=0.06).
Contrary to FEV1, DLCO was not related to major pulmonary complications (Figure 1B). In fact, mean DLCO was 57% and 61% respectively in the groups of patients presenting and not presenting major pulmonary complications (P=0.41).
ppo values were calculated for 89 patients (84%) for lobectomy and segmentectomy. ppoFEV1 (L), ppoFEV1 (%) and ppoDLCO (%) were respectively 1.65±0.58 L (range, 0.57–3.02 L), 55%±17% (range, 31–116%) and 49%±15% (range, 20–97%). ppoFEV1 (L) and ppoFEV1 (%) were associated with major pulmonary complications. As with preoperative DLCO, ppoDLCO was not associated with postoperative morbidity.
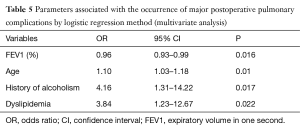
Four predictive parameters were identified by multivariate analysis (Table 5). The most accurate logistic regression model performed best with the most contrasting odds ratio (OR) (4.16 and 0.96) and included a pulmonary function parameter FEV1. It was independently associated with major postoperative respiratory complications. OR are summarised in Table 5. Morbidity was less common when FEV1 was higher. However, the area under the ROC curve was 0.72, indicating average discrimination ability. We were unable to determine the FEV1 threshold that allowed staging of patients. Indeed, whatever the chosen FEV1 value, the capacity (sensitivity, specificity and Youden index) of FEV1 to identify patients at risk of major respiratory complications remained identical.
Full table
Cardiopulmonary exercise testing (CPET)
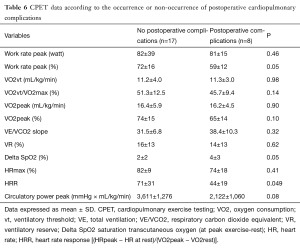
Twenty-five patients (24%) performed incremental CPET, on average 39±18 days before surgery. Mean FEV1 and mean DLCO were respectively 60%±14% and 54%±17%. The 25 tests conducted responded to maximum criteria. Six patients were treated with beta blockers, two patients in the group with complications and five in the group without postoperative complications. The patients presenting postoperative cardiovascular complications (n=8) were compared to patients not presenting complications (n=17) (Table 6). The aerobic work rate at peak exercise was significantly lower in the group with complications (59% vs. 72%, P=0.05). VO2peak tended to be lower in the group with complications (65% vs. 74%; P=0.14). Desaturation during exercise [OR, 0.462; 95% confidence interval (CI), 0.191–0.878, P=0.039] and heart rate response (HRR) (OR, 0.953; 95% CI, 0.895–0.993, P=0.05) were associated with postoperative complications.
Full table
Discussion
We found that FEV1 was independently correlated with postoperative pulmonary complications in major pulmonary resection by VATS, unlike DLCO. However, we were not able to determine a consistent FEV1 threshold above which the risk of postoperative morbidity was lower, partly due to a weak association between FEV1 and postoperative pulmonary complications. We found that ppoFEV1 was associated with major postoperative pulmonary complications, unlike ppoDLCO, but its value did not exceed FEV1 in terms of predictability of postoperative morbidity. Desaturation during exercise and HRR were associated with the occurrence of postoperative cardiopulmonary complications.
Our finding that DLCO was not predictive of postoperative pulmonary complications was similar to that of Berry et al. (14). Indeed the latter determined the impact of preoperative pulmonary function on postoperative morbidity after VATS lobectomy in a retrospective analysis of 340 patients with pre-lobectomy FEV1 and/or DLCO below or equal to 60%. The authors found that FEV1 and DLCO were predictive of pulmonary complications when lobectomy was performed by thoracotomy but not by VATS. Other studies have shown similar results and did not report FEV1 and DLCO as independent predictors of postoperative pulmonary complications (15,16).
We choice cut-off of 80% because current recommendations give a threshold of 80% for FEV1 and DLCO, below which the risk of postoperative complications is high thereby justifying further explorations. Licker et al. (17) found that the best FEV1 threshold value for predicting respiratory complications was 60% in patients undergoing thoracotomy. In the study by Win et al. (18), the authors showed that FEV1 threshold may be lower (45–50% predicted). The literature also suggests that patients with poor respiratory function have perioperative outcomes similar to those with normal lung function when lung resection was performed by VATS (19-21). In view of data in the literature and the results of this work, the FEV1 threshold below which further explorations are indicated could probably be lowered.
VO2peak was lower in patients with postoperative cardiopulmonary complications but not significantly. This result is probably due to a lack of power. Indeed, many studies have shown that VO2max strongly correlated with postoperative morbidity and mortality (22,23). Desaturation during exercise was associated with the occurrence of postoperative cardiopulmonary complications as has already been shown for thoracotomy (24-26).
To date, no study has evaluated CPET parameters other than VO2max in patients operated by VATS lung resection. HRR was significantly higher for patients who presented postoperative cardiovascular complications. HRR corresponded to myocardial consumption of oxygen. Its elevation beyond 50 reflects the probable existence of a cardiomyopathy. This result highlights the importance of cardiac evaluation in preoperative assessment. HRR seems to be a promising prognostic marker of cardiopulmonary morbidity after lung resection.
The operative mortality in our study was 1.9%, which was similar to that reported in other series with mortality rates ranging from 0.6% to 5%. Morbidity was 34.9% and conversion rate 17% in our cohort which may seem high compared to current data in the literature (10% to 30%) (27-30), but the functional parameters of our study population were lower and we included patients who underwent supra-lobar lung resections.
Our study has limitations due primarily to its retrospective nature, but data collection was prospective, which implies no loss of information. Furthermore, we included all types of lung resection in our study population. Nevertheless, it comprised a majority of lobectomies and at least 1 segmentectomy. Finally, only 25 CPETs were performed in our cohort resulting in a lack of power.
In conclusion, FEV1 but not DLCO was a significant predictor of pulmonary complications after VATS pulmonary resection for lung cancer in patients with abnormal pulmonary function. However FEV1 have little ability to predict pulmonary complications. CPET seems more discriminating, but a larger population of patients is necessary to support this hypothesis. Our results question the current decision-making algorithms and the necessity to validate in studies that take into account advances in thoracic surgery.
Acknowledgements
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Garzon JC, Ng CS, Sihoe AD, et al. Video-assisted thoracic surgery pulmonary resection for lung cancer in patients with poor lung function. Ann Thorac Surg 2006;81:1996-2003. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [Crossref] [PubMed]
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5-40. [Crossref] [PubMed]
- Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 1984;129:S49-55. [Crossref] [PubMed]
- Aguilaniu B, Richard R, Costes F, et al. Cardiopulmonary exercise testing. Rev Mal Respir 2007;24:2S111-60.
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [Crossref] [PubMed]
- Ginsberg RJ. Lung cancer surgery: acceptable morbidity and mortality, expected results and quality control. Surg Oncol 2002;11:263-6. [Crossref] [PubMed]
- Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51; discussion 1051-2. [Crossref] [PubMed]
- Wang J, Olak J, Ultmann RE, et al. Assessment of pulmonary complications after lung resection. Ann Thorac Surg 1999;67:1444-7. [Crossref] [PubMed]
- Wang JS, Abboud RT, Evans KG, et al. Role of CO diffusing capacity during exercise in the preoperative evaluation for lung resection. Am J Respir Crit Care Med 2000;162:1435-44. [Crossref] [PubMed]
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [Crossref] [PubMed]
- Win T, Jackson A, Sharples L, et al. Relationship between pulmonary function and lung cancer surgical outcome. Eur Respir J 2005;25:594-9. [Crossref] [PubMed]
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13. [Crossref] [PubMed]
- Endoh H, Tanaka S, Yajima T, et al. Pulmonary function after pulmonary resection by posterior thoracotomy, anterior thoracotomy or video-assisted surgery. Eur J Cardiothorac Surg 2010;37:1209-14. [Crossref] [PubMed]
- Oparka J, Yan TD, Ryan E, et al. Does video-assisted thoracic surgery provide a safe alternative to conventional techniques in patients with limited pulmonary function who are otherwise suitable for lung resection? Interact Cardiovasc Thorac Surg 2013;17:159-62. [Crossref] [PubMed]
- Benzo R, Kelley GA, Recchi L, et al. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med 2007;101:1790-7. [Crossref] [PubMed]
- Loewen GM, Watson D, Kohman L, et al. Preoperative exercise Vo2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol 2007;2:619-25. [Crossref] [PubMed]
- Toker A, Ziyade S, Bayrak Y, et al. Prediction of cardiopulmonary morbidity after resection for lung cancer: stair climbing test complications after lung cancer surgery. Thorac Cardiovasc Surg 2007;55:253-6. [Crossref] [PubMed]
- Rao V, Todd TR, Kuus A, et al. Exercise oximetry versus spirometry in the assessment of risk prior to lung resection. Ann Thorac Surg 1995;60:603-8; discussion 609. [Crossref] [PubMed]
- Brunelli A, Refai M, Xiumé F, et al. Oxygen desaturation during maximal stair-climbing test and postoperative complications after major lung resections. Eur J Cardiothorac Surg 2008;33:77-82. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; discussion 1001-2. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5. [PubMed]


