Management of post-operative pain by placement of an intraoperative intercostal catheter after single port video-assisted thoracoscopic surgery: a propensity-score matched study
Introduction
For early stage postoperative pain control, regional nerve blockade has been shown to provide effective pain relief. Thoracic epidural analgesia is useful in thoracotomy patients, and could result in earlier mobilization and minimize post operation complications. However, epidural analgesia carries the considerable risk of epidural hematoma, dural perforation, and urinary retention (1,2). Alternatively, use of a single intercostal catheter (ICC) might avoid such complications while providing comparable analgesia effect (3). Single-incision thoracoscopic surgery has been reported since 2004 (4), however it remains controversial in regard to postoperative pain outcome as compared with conventional video-assisted thoracoscopic surgery (VATS) (5), despite producing a smaller intercostal wound incision and intercostal nerve injury area. We hypothesized that a targeted local analgesic technique aimed at the location of pain generation could alleviate postoperative pain. Continued intercostal analgesia may be useful in uniport VATS patients. In this study, we try to evaluate post-operative analgesia in single port patients with or without continual intercostal nerve blockade.
Methods
A retrospective database was used to identify patients who underwent single port VATS between March 2014 and August 2015. In total, there were 235 recorded cases, including various kinds of operations, such as wedge resection, anatomic resection, mediastinal tumor resection, empyema decortication and pericardial-pleural window. Since May 2015, we began to place ICC post operation for intercostal nerve blockade. A total of 50 patients consecutively received single port VATS with intercostal nerve blockade at Chang Gung Memorial Hospital. After excluding patients who had (I) empyema decortication (II) tumor over the pleural surface (III) allergy history to amide-type local anesthetic, 185 patients were finally included in this study. The study was approved by the institutional review board of Chang Gung Memorial Hospital (IRB No: 104-1204B). Due to its retrospective characteristic, the need to obtain written informed consent from each patient was waived. All participants had similar pre operation workup, including chest radiography, chest computed tomography (CT), pulmonary function test, complete blood counts and so on. Age, gender, body mass index, diagnosis, surgical approach (wedge, anatomic resection, or mediastinal tumor resection), operative time, blood loss, postoperative complications, daily visual analog scale (VAS), were collected from the hospital information system. Surgical mortality was defined as death occurring during the same hospitalization or within 30 days after the operation.
Surgical technique
The operative technique for single port VATS lung resection was as described by Gonzalez-Rivas et al. (6). Briefly speaking, patients were put in the standard lateral position, a 2–3 cm wound was created at the pivot of the fourth or fifth intercostal space and anterior axillary line. If the target lesion is located at the upper lobe or extended thymectomy is needed, the fourth is better than the fifth intercostal space; otherwise, the fifth intercostal space is the location of choice (7,8). Lung isolation was obtained with a double lumen endotracheal tube ventilation. A 30-degree 10 mm thoracoscope was then placed at the top of the incision wound. Rib resection or rib spreading was not used in our clinical practice. All procedures were performed under thoracoscope. The specimen was retrieved by a plastic bag through the incision wound, whereby if the tumor was larger than the wound, we gradually enlarged the wound until the tumor could be retrieved and we recorded the final wound length in our operation record.
Analgesia techniques
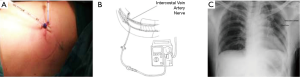
At the end of operation, a dilator catheter (8 Fr Angiotech) was used to dissect the subpleural space under thoracoscope. With the help of thoracoscope and dilator catheter, we could dissect the sub pleural space cautiously without penetrating parietal pleural. After we removed the dilator catheter, a 7 Fr, 20-cm-length radio-opaque polyurethane catheter with side holes was then passed over the guidewire (Figure 1A) and injected levobupivacaine 10 mL into the intercostal space (Figure 1B). For patients without ICC, we also injected 10 mL levobupivacaine over the uniport affected intercostal sub pleural space. When the patients were sent to ordinary ward or intensive care unit, continual local anesthetic infusion was given (levobupivacaine 0.2%) through sub pleural catheters with 2.5 mL/h, which was suggestive by anesthesiologists and the dose is about one third of maximum dose (Figure 2A,B). After operation, all of the patients took chest X-ray to check the position of chest tube and ICC (Figure 2C). If the chest tubes were still in place, the infusion continued up to chest tube removal after operation. In addition, the standard postoperative oral anodyne regimes consisted of acetaminophen 500 mg Q6H, ibuprofen 600 mg Q8H, and Ultracet (Tramadol 37.5 mg + Acetaminophen 325 mg) Q6 H. IV form morphine (0.1 mg/Kg) would be given on request if patients’ numerical rating scale (NRS) >3 at rest or NRS >5 during activity
Numerical rating scale (NRS) or visual analog scale (VAS) score
Patients’ pain was assessed by a numeric rating scale .A chart card which has a 10-cm horizontal line with word anchors at each end, ranging from 0= “no pain” to 10= “worst pain” was used. If patients have difficulty communicating with us directly, we use the same chart card with pictures showing scaled facial pain to evaluate the pain severity. We put a high value on pain evaluation in our hospital. Each nursing staff was trained to evaluate patient’s pain with consistent manner and method. The pain score is recorded by nursing staff in in our hospital information system.
Statistical analysis
Continuous data are expressed as mean value with a range of one standard deviation (SD). Before comparison, two groups’ continuous variables were examined by Levene test. If the variances is not equal, we will use Brown-Forsythe test to see whether significant difference between two groups. If the variances is equal, one way ANOVA was used to examine the variables. In order to get more rigorous conclusion, age, gender, BMI, FEV1, type of surgery, drainage tube and post-operative oral anodyne were used to propensity scores matching (1:1). Continuous variables were examined the same as above. Two-tailed P values of 0.05 were considered statistically significant. All calculations were performed using the SPSS statistical package, version 19.0 (SPSS Inc., Chicago, IL, USA) and R 2.12.
Results
There were 185 patients enrolled in our study with 100 patients participating in propensity matching comparison (50 cases and 50 propensity-matched controls).
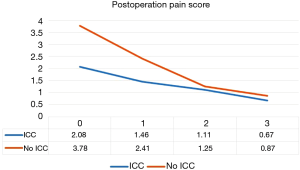
The demographic data are presented in Table 1. No 30 days mortality occurred in our study. We compared the two groups’ operative time, postoperative day 0, 1, 2, 3, discharge day pain score, IV form morphine dosage and frequency, drainage duration and post operation hospital stay. There was no difference in operative time (P=0.588) and discharge pain score (P=0.656). Mean post-operative drainage duration and hospital stay was 3.83 and 5.04 days for the single port VATS without ICC group and 3.12 days and 4.32 days for single port VATS with ICC group respectively (P=0.099, P=0.143). However, we found patients with ICC had better postoperative day 0 and day 1 pain score (2.08 vs. 3.78, P<0.001 and 1.46 vs. 2.41, P<0.001 respectively, Figure 3). In addition, we also found patients with continual intercostal nerve blockade have lower IV form morphine use frequency and dosage (0.5 vs. 1.0, P=0.003, 3.04 vs. 5.85 mg, P=0.004).

Full table
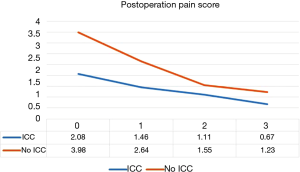
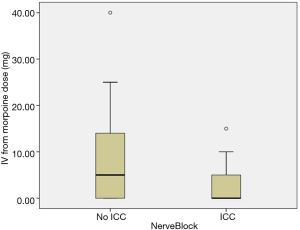
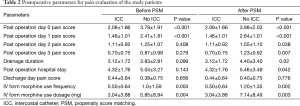
In order to draw a more rigorous conclusion, propensity score matching (PSM) was used to create a more balanced cohort comparison. Postoperative day 0, 1, 2, 3 pain score (P<0.001, P<0.001, P=0.038, P=0.007, Figure 4), IV form morphine dosage (P=0.003, Figure 5) and frequency, drainage duration and postoperative hospital stay were all better in patients with ICC groups, except discharge day pain score. A detailed comparison of postoperative parameters is given in Table 2.
Full table
Of the 185 patients, seven suffered from subcutaneous emphysema or prolonged air leakage (>7 days). Three patients needed another operation for air leakage repair. One patient had chylothorax and conservative treatment failed. Finally, the patients received thoracic duct ligation. No levobupivacaine related acute toxicity or catheter related complication was observed in our cohort study.
Discussion
Acute postoperative pain management is important in thoracic surgery. Adequate pain control can lower the possibility of pulmonary complications, such as atelectasis or pneumonia and improve the quality of life for patients. Currently, no golden standard exists with regard to single port VATS postoperative pain management. Various kinds of postoperative analgesia have been tried by thoracic surgeons and anesthesiologists (9-15). Regional analgesia techniques seem to be effective in postoperative management. Since more than 90% of our patients had chest tube drain for more than one day, single shot levobupivacaine is insufficient to control acute postoperative pain. Epidural analgesia, though rare, might bring considerable risk of undesirable compilations, and liposomal bupivacaine is not available everywhere. Continual levobupivacaine infusion through ICC might be a more accessible and safe option for acute pain control. Otherwise, uniport VATS is a good candidate for continual intercostal nerve block on account of its single wound with smaller intercostal nerve injury area. In addition, advantages of intraoperative ICC placement include direct visualization under thoracoscope, which can help to avoid intercostal vessels injury, chest wall hematoma, and preserve the integrity of parietal pleura to make sure the levobupivacaine infusion diffuses throughout the intercostal space without leakage.
Our cohort showed that postoperative day 0, 1, 3 pain score, average IV form morphine usage frequency and dosage, drainage duration, and postoperative hospital stay were obviously lower in patients with continual intercostal nerve blockade in propensity matching comparison. This at least implied that patients with continual intercostal blockade might have a better quality of life, better activity, and faster recovery post operation. However, if continual intercostal blockade to alleviate patients’ pain sensation is insufficient, oral anodyne is indispensable (15). Although we try to eliminate the difference between groups with or without ICC by PSM, multimodal regimen might produce bias in our study. Future prospective study should be conducted with unity of anodyne regimen.
That our average operative time was slightly shorter in the ICC group and showed no difference in statistics might be the result of (I) being more familiar with the single port VATS techniques; (II) very little time consumed for the placement of the ICC into the intercostal space. In fact, placing the ICC intraoperatively was a simple and quick technique. Otherwise, compared with previous reports of single port VATS (16-19), our preliminary result is at least not inferior to others’ reports in our control group patients. This strengthens the reliability of our study (Table 3).

Full table
The major limitation of our study lies in its retrospective design. Nevertheless, we try to present a well-balanced observation cohort, which might serve as the basis for further prospective, randomized study. Second, our assessment of analgesia was based on the nursing staff’s record. Although pain assessment were done by different staff members, uniform nursing staff training, timing of pain assessment and recording method in the hospital information system might minimize individual bias in our study. Furthermore, with the help of matching study, the intergroup difference could be reduced to some extent. Third, owing to the short follow up duration, our study could not assess the long term effect on neuralgia and paresthesia, and further surveillance is warranted.
Conclusions
In conclusion, for patients post single port VATS, continual intercostal levobupivacaine infusion appears in our study to be a safe, effective and easily practiced technique, associated with a shorter hospital stay and less post-operative pain. Further prospective trials are needed to assess the long term outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- de Leon-Casasola OA, Parker B, Lema MJ, et al. Postoperative epidural bupivacaine-morphine therapy. Experience with 4,227 surgical cancer patients. Anesthesiology 1994;81:368-75. [Crossref] [PubMed]
- Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology 1995;82:1474-506. [Crossref] [PubMed]
- van Kleef JW, Logeman EA, Burm AG, et al. Continuous interpleural infusion of bupivacaine for postoperative analgesia after surgery with flank incisions: a double-blind comparison of 0.25% and 0.5% solutions. Anesth Analg 1992;75:268-74. [PubMed]
- Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [Crossref] [PubMed]
- Young R, McElnay P, Leslie R, et al. Is uniport thoracoscopic surgery less painful than multiple port approaches? Interact Cardiovasc Thorac Surg 2015;20:409-14. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: initial results. J Thorac Cardiovasc Surg 2012;143:745-7. [Crossref] [PubMed]
- Wu CF, Gonzalez-Rivas D, Wen CT, et al. Single-port video-assisted thoracoscopic mediastinal tumour resection. Interact Cardiovasc Thorac Surg 2015;21:644-9. [Crossref] [PubMed]
- Wu CF, Gonzalez-Rivas D, Wen CT, et al. Comparative short-term clinical outcomes of mediastinum tumor excision performed by conventional VATS and single-port VATS: is it worthwhile? Medicine (Baltimore) 2015;94:e1975. [Crossref] [PubMed]
- Demmy TL, Nwogu C, Solan P, et al. Chest tube-delivered bupivacaine improves pain and decreases opioid use after thoracoscopy. Ann Thorac Surg 2009;87:1040-6; discussion 1046-7. [Crossref] [PubMed]
- Milone L, Edmondson D, Lebenthal A, et al. Multiple nerve blocks after video-assisted thoracic surgery (VATS). Surg Endosc 2011;25:2731-2. [Crossref] [PubMed]
- Kamiyoshihara M, Nagashima T, Ibe T, et al. Is epidural analgesia necessary after video-assisted thoracoscopic lobectomy? Asian Cardiovasc Thorac Ann 2010;18:464-8. [Crossref] [PubMed]
- Hill SE, Keller RA, Stafford-Smith M, et al. Efficacy of single-dose, multilevel paravertebral nerve blockade for analgesia after thoracoscopic procedures. Anesthesiology 2006;104:1047-53. [Crossref] [PubMed]
- Rice DC, Cata JP, Mena GE, et al. Posterior intercostal nerve block with liposomal bupivacaine: an alternative to thoracic epidural analgesia. Ann Thorac Surg 2015;99:1953-60. [Crossref] [PubMed]
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. [Crossref] [PubMed]
- Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol 2009;22:588-93. [Crossref] [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [Crossref] [PubMed]
- Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [Crossref] [PubMed]
- Hsu PK, Lin WC, Chang YC, et al. Multiinstitutional analysis of single-port video-assisted thoracoscopic anatomical resection for primary lung cancer. Ann Thorac Surg 2015;99:1739-44. [Crossref] [PubMed]
- Ocakcioglu I, Alpay L, Demir M, et al. Is single port enough in minimally surgery for pneumothorax? Surg Endosc 2016;30:59-64. [Crossref] [PubMed]


