
Clinical pharmacokinetic study of tacrolimus in continuous intravenous administration for lung transplantation
Introduction
Tacrolimus is a potent calcineurin inhibitor (CNI), which functions as the cornerstone of immunosuppression after lung transplantation and other types of solid organ transplantation (1). However, tacrolimus therapy is complicated by highly variable pharmacokinetic parameters and a narrow therapeutic index that necessitates concentration monitoring (2). The high variation of tacrolimus bioavailability in the early phase of lung transplantation may result in several conditions, including high fluctuation in the whole blood concentrations, increased tacrolimus toxicity, and decreasing tacrolimus efficacy (3). Therefore, the route and dose of tacrolimus administration in the early phase of lung transplantation have been issued (4). Administration of tacrolimus orally or through a nasogastric tube has been suggested; however, many patients remain intubated regarding the significant potential for coexistent impairment of gastrointestinal, hepatic, and renal functions (5). Although sublingual administration may be useful in patients on ventilators and those with significant gastrointestinal difficulties, however, intravenous administration may be preferable in the early postoperative period (6). Although a 24-hour continuous intravenous administration is recommended by the manufacturers after transplantation (4), twice daily bolus intravenous administration had recently been shown to be safe and beneficial (7,8). Nevertheless, there are some concerns regarding neurotoxicity and nephrotoxicity due to increased peak levels of the drug during bolus intravenous administration (9). Therefore, continuous intravenous tacrolimus administration might be useful for maintaining safe drug levels in critically ill recipients. However, there are no clear guidelines on how to monitor this narrow therapeutic window agent and the duration needed to achieve the therapeutic range with continuous intravenous administration in the early phase of lung transplantation. In the present study, we report our experience of continuous intravenous tacrolimus administration in the early post-lung transplant period at our institution to guide the timeline to achieve a therapeutic range of tacrolimus in the early phase of lung transplantation. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1760/rc).
Methods
This is a single-center cohort study of adult patients who had lung transplantation at Samsung Medical Center from January 2011 to July 2021. The patients were 18 years or older, had first-time lung transplantation, and received tacrolimus following lung transplantation. All lung transplant patients received basiliximab to postoperatively induce immunosuppression between days 0 and 4. Additionally, our standard immunosuppressive regimen was based on a triple-drug combination of tacrolimus, mycophenolate mofetil, and corticosteroids. All patients received 500-mg intravenous methylprednisolone before reperfusion, followed by intravenous administration of 0.5 mg/kg for 14 days. Then, we gradually tapered the dose every two weeks to 0.125 mg/kg. When patients are tolerating oral intake, intravenous methylprednisolone was converted to oral prednisolone. One 1,000-mg mycophenolate mofetil was administered twice daily unless it resulted in leukopenia or liver dysfunction, in which case the dose was lowered or discontinued. Tacrolimus administration started immediately after transplantation with an initial continuous intravenous infusion, which was dosed at 0.01 mg/kg/day. Tacrolimus concentrations while on the continuous intravenous infusion were measured every 24 h by the liquid chromatography-mass spectrometry (LC-MS/MS) using an Agilent 6460 LC-MS/MS (Agilent Technologies, Santa Clara, CA, USA), to adjust tacrolimus doses to achieve an exposure target of 10–15 ng/mL. After reaching a steady-state, tacrolimus dosing was switched to an oral equivalent daily dose in patients assured of adequate oral intake and tacrolimus trough concentrations were measured once daily. Assuming an oral bioavailability of 10%, the oral equivalent dose was administered twice daily. Subsequent changes in immunosuppression were based on adverse effects, renal function, and clinical judgment of the clinical pharmacist.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board at Samsung Medical Center approved the study (IRB No. 2022-03-062) and waived the requirement for informed consent because of the retrospective nature of the study. All patient records and data were anonymized and de-identified before analysis.
The tacrolimus time in the therapeutic range (TTRin, %) is defined as the number of day in therapeutic ranges over days of tacrolimus administration. It was calculated using Rosendaal’s linear interpolation method (10). This method assumes that a linear relationship exists between each measured value and then assigns a specific value for each day between the levels. The time to therapeutic range (TTRto, days) is defined as the number of days of tacrolimus administration before the level coincides with the therapeutic range for two consecutive days. The coefficient of variation (CoV) of tacrolimus trough concentrations (expressed as a percent) is calculated as the (standard deviation of tacrolimus trough concentrations/mean of tacrolimus trough concentrations) × 100% (11,12).
Acute kidney injury (AKI) was defined as an increase of the serum creatinine by 0.3 mg/dL or more within 48 hours, to 1.5 times baseline or more within 7 days, or urine output less than 0.5 mL/kg/h for 6 hours according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria (13). Neurotoxicity was defined as posterior reversible encephalopathy syndrome or minor neurotoxic effects, including headache, tremor, or change in mental status (14), observed for one month of the postoperative period.
Results
During the study period, a total of 74 patients underwent lung transplantation. Of these, three patients aged under 18 years old, three patients underwent repeated transplantation, and one patient died after one measure of tacrolimus trough concentration was excluded from the analysis. Finally, 67 patients aged 18 years or older and received first-time lung transplantation were included in the study. The baseline characteristics of these patients are summarized in Table 1. The median age of the patients was 57.0 (interquartile range, 51.5–62.0) years. Additionally, the majority (68.7%) were male, and all but one underwent bilateral lung transplantation (98.5%). The most common indication for lung transplantation was idiopathic pulmonary fibrosis (61.2%), which is followed by connective tissue disease-related interstitial lung disease (25.4%).
Table 1
| Characteristics | No. of patients (%) or median [IQR] |
|---|---|
| Age, years | 57.0 [51.5–62.0] |
| Sex, male | 46 (68.7) |
| Body mass index, kg/m2 | 20.6 [18.1–22.6] |
| Primary diagnosis | |
| Idiopathic pulmonary fibrosis | 41 (61.2) |
| CTD-related interstitial lung disease | 17 (25.4) |
| Acute respiratory distress syndrome | 7 (10.4) |
| Bronchiolitis obliterans after HSCT | 2 (3.0) |
| Wait-list duration, day | 99.5 [23.0–234.5] |
| Intra-operation | |
| Bilateral lung transplantation | 66 (98.5) |
| Operation time, min | 560 [504–640] |
| Post-transplantation outcomes | |
| Acute kidney injury by RIFLE criteria | 23 (34.3) |
| Requiring RRT | 10 (14.9) |
| ECMO | 4 (6.0) |
| Postoperative duration of ECMO, days | 3 [2.0–4.3] |
| Total ICU length of stay, days | 8.0 [6.0–22.8] |
| Time from transplantation to discharge, days | 45.0 [22.0–103.0] |
| Hospital mortality | 16 (23.9) |
IQR, interquartile range; CTD, connective tissue disease; HSCT, hematopoietic stem cell transplantation; RIFLE, Risk, Injury, Failure, Loss, and End-stage Kidney; RRT, renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
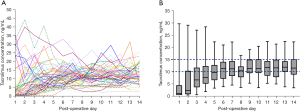
The individual trend lines of tacrolimus trough concentration showed substantial variability over time, with a wide deviation from the therapeutic range of 10–15 ng/mL, although it trended toward the therapeutic range over time (Figure 1A). However, tacrolimus dosing was switched to an oral equivalent daily dose within a median of 6 days (5–8 days), and the tacrolimus trough concentration was maintained as shown in Figure 1B. The median percentage of tacrolimus TTRin was 35.7% (21.4–42.9%) for the 2-week postoperative period. The median day of TTRto was 7 days (5–9 days), and the median tacrolimus trough concentration was 10.02 ng/mL (7.87–12.26 ng/mL) for the 2-week postoperative period (Figure 1B). The median CoV of tacrolimus was 49.7% (40.8–61.6%).
AKI following tacrolimus infusion occurred in 23 (34.3%) patients, with most (18/23, 78%) injuries occurring during the first postoperative week. Furthermore, renal replacement therapy was required in only 10 (14.9%) patients, all were discontinued before discharge from the intensive care unit. Furthermore, neurotoxicity of tacrolimus was not observed in patients participating in this study. However, there was no acute cellular rejection that occurred within one month of the postoperative period.
Discussion
This study described tacrolimus monitoring parameters, including TTRin, TTRto, and CoV, with continuous intravenous administration occurring within the first 2 weeks after lung transplantation. Although tacrolimus pharmacokinetic parameters varied significantly over time, most patients achieved the therapeutic range of tacrolimus within 1 week without severe adverse events occurring throughout the daily measure and dose titration of tacrolimus trough concentrations.
The standard approach to tacrolimus dosing after lung transplantation is largely reactive. Therefore, the initial dosing and titration are personalized based on therapeutic drug monitoring. However, there are no clear guidelines on the duration to achieve the therapeutic range with continuous intravenous administration in the early phase of lung transplantation. Additionally, the impact of the faster TTRto or the higher TTRin on graft function during the immediate postoperative period of lung transplantation could not be evaluated, given the high CoV of tacrolimus (3). Moreover, early initiation of high doses of CNIs could be associated with worsening renal function and increased risk of infection. Therefore, it might be justifiable to allow time for the patient to recover from surgery and then gradually increase CNI doses. In the present study, tacrolimus administration commenced at a low dose of 0.01 mg/kg/day immediately after transplantation, which was not expected to increase the risk of infection. Nonetheless, the therapeutic range of tacrolimus was achieved for most patients within 1 week without severe adverse events, which supports the suggested initial dose of 0.01–0.05 mg/kg/day through continuous infusion (4).
Despite the clinical pharmacist’s daily intervention in this study, the pharmacokinetic profile of tacrolimus was found to be variable, which is consistent with findings from a previous study. This study demonstrated that tacrolimus was highly variable, particularly in the early postoperative period (11). Recently, the variability of tacrolimus in trough concentrations has been recognized as a novel marker to identify at-risk transplant recipients for poor outcomes in other solid organ transplantation (12). However, there is no suggested cutoff value of CoV to predict patients with an increased risk of negative outcomes. This study evaluated tacrolimus variability using CoV, which was measured by tacrolimus trough concentrations. However, the association between the variability of tacrolimus and the lung transplantation outcome could not be evaluated due to the absence of cellular rejection events during the hospitalization.
Although this study contributes to providing additional information on the pharmacokinetic profiles of tacrolimus administered by continuous intravenous infusion in the early post-lung transplant period, there are potential limitations that should be acknowledged. First, given its observational nature, there could be a selection bias that possibly influences the significance of our findings. However, the data were collected from all consecutive patients who received lung transplantation at our center. Patients requiring extracorporeal membrane oxygenation support immediately after the transplant operation, which might be associated with drug sequestration, were available. However, all (n=4) were removed within a median of 3 days (2.0–4.3 days). The designated clinical pharmacist managed the dose of tacrolimus, according to our institutional protocol. Accordingly, our findings may have limited generalizability. Therefore, additional external validations with larger samples are warranted. Second, because of the small number of patients and no rejection event, it was difficult to evaluate the effect of the therapeutic range of tacrolimus on graft function during the immediate postoperative period of lung transplantation.
In summary, continuous intravenous administration with the daily measure and dose titration of tacrolimus trough concentrations allowed the therapeutic range of tacrolimus to be reached within 1 week without significant adverse events, although the pharmacokinetic parameters were highly variable over time.
Acknowledgments
Funding: This work was supported by a Samsung Medical Center Grant (No. OTA2002901).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1760/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1760/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1760/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nelson J, Alvey N, Bowman L, et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy 2022;42:599-633. [Crossref] [PubMed]
- Venkataramanan R, Swaminathan A, Prasad T, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 1995;29:404-30. [Crossref] [PubMed]
- Sikma MA, van Maarseveen EM, van de Graaf EA, et al. Pharmacokinetics and Toxicity of Tacrolimus Early After Heart and Lung Transplantation. Am J Transplant 2015;15:2301-13. [Crossref] [PubMed]
- Garrity ER Jr, Hertz MI, Trulock EP, et al. Suggested guidelines for the use of tacrolimus in lung-transplant recipients. J Heart Lung Transplant 1999;18:175-6. [PubMed]
- Raviv Y, D'Ovidio F, Pierre A, et al. Prevalence of gastroparesis before and after lung transplantation and its association with lung allograft outcomes. Clin Transplant 2012;26:133-42. [Crossref] [PubMed]
- Monchaud C, Marquet P. Pharmacokinetic optimization of immunosuppressive therapy in thoracic transplantation: part I. Clin Pharmacokinet 2009;48:419-62. [Crossref] [PubMed]
- Snell GI, Ivulich S, Mitchell L, et al. Evolution to twice daily bolus intravenous tacrolimus: optimizing efficacy and safety of calcineurin inhibitor delivery early post lung transplant. Ann Transplant 2013;18:399-407. [Crossref] [PubMed]
- Hirano Y, Sugimoto S, Mano T, et al. Prolonged Administration of Twice-Daily Bolus Intravenous Tacrolimus in the Early Phase After Lung Transplantation. Ann Transplant 2017;22:484-92. [Crossref] [PubMed]
- Snell GI, Westall GP. Immunosuppression for lung transplantation: evidence to date. Drugs 2007;67:1531-9. [Crossref] [PubMed]
- Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236-9. [Crossref] [PubMed]
- Schumacher L, Leino AD, Park JM. Tacrolimus intrapatient variability in solid organ transplantation: A multiorgan perspective. Pharmacotherapy 2021;41:103-18. [Crossref] [PubMed]
- Kuypers DRJ. Intrapatient Variability of Tacrolimus Exposure in Solid Organ Transplantation: A Novel Marker for Clinical Outcome. Clin Pharmacol Ther 2020;107:347-58. [Crossref] [PubMed]
- Section 2: AKI Definition. Kidney Int Suppl (2011) 2012;2:19-36. [Crossref] [PubMed]
- Braithwaite HE, Darley DR, Brett J, et al. Identifying the association between tacrolimus exposure and toxicity in heart and lung transplant recipients: A systematic review. Transplant Rev (Orlando) 2021;35:100610. [Crossref] [PubMed]


