Comparison of treatment outcomes between single-port video-assisted thoracoscopic anatomic segmentectomy and lobectomy for non-small cell lung cancer of early-stage: a retrospective observational study
Introduction
There is an increasing trend toward performing minimally invasive surgery, and video-assisted thoracoscopic technology is a typical representative of new technology for minimally invasive thoracic surgery (1).
Compared with those who undergo traditional lobectomy, patients who undergo anatomic segmentectomy have less lung tissue resected and more lung function preserved, contributing to a subsequent higher quality of life (2-4). There are reportedly no significant differences in local tumor recurrence rate and total 5-year survival rate between these two surgical options (5-7). Anatomic segmentectomy is clearly the less invasive. The Non-small Cell Lung Cancer Clinical Practice Guidelines issued by the National Comprehensive Cancer Network in 2010 recommend anatomic segmentectomy as a suitable operative procedure for treating early-stage non-small cell lung cancer (NSCLC) (8). The size and number of operative incisions can be used as an index of the severity of operative wounds (9,10). In accordance with the above data, surgeons have gradually begun to attempt single-port video-assisted thoracoscopic surgery (S-VATS), which is less invasive than previously performed procedures. Although Gonzalez et al. have reported outcomes of S-VATS lobectomy (11) and anatomic segmentectomy (12) and Zhu et al. have reported the comparison of the feasibility and safety between single-port and triple-port complete thoracoscopic lobectomy for NSCLC (13), there are few reports comparing S-VATS anatomic segmentectomy and lobectomy for early stage NSCLC.
In this study we retrospectively analyzed data on 32 patients who had undergone S-VATS anatomic segmentectomy and 47 patients who had undergone S-VATS lobectomy for early stage NSCLC in our institution and explored the safety, efficacy, advantages, and disadvantages of these two operative procedures.
Methods
In this study, data for 79 patients who had undergone S-VATS procedures [32 anatomic segmentectomies (segmentectomy group) and 47 lobectomies (lobectomy group)] for early-stage NSCLC from April 2014 to July 2015 in the Department of Thoracic Surgery, Union Hospital Affiliated to Fujian Medical University were examined.
All patients had undergone a preoperative work-up including pulmonary function tests, computed tomography (CT) chest scans with or without positron emission tomography (PET)-CT scans, flexible bronchoscopy, and brain magnetic resonance imaging. Their preoperative images showed nodules or ground glass opacities in the lung without evidence of enlarged mediastinal lymph nodes or extensive adhesion to the chest wall. None had clinical, laboratory, or imaging evidence of contraindications to surgery or tumor distant metastases. There were no statistically significance differences between the two groups in general data, such as age and sex (P>0.05); thus, the groups were comparable (Table 1).
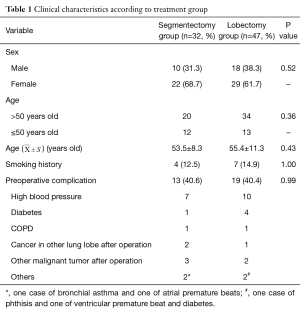
Full table
Surgical techniques
Patients in both groups were placed in a lateral position on the non-affected side and subjected to intravenous anesthesia with double-lumen intubation. During the procedure, one-lung ventilation was performed on the healthy side and the lung collapsed on the affected side. A 3.5–4.5 cm incision was made between the fourth and fifth ribs in the anterior axillary line, silica gel incision protective casing introduced, and a 30° video-assisted thoracoscope inserted to check for any extensive compact adhesions to the chest as well and identify the diseased region and its relationship with surrounding organs and the situation regarding dissection of the lung hilum. Rib retractors were not used during the procedures. The laparoscopic apparatus was guided by a monitor and video-assisted thoracoscope and other instruments were inserted and removed through this single port. In patients with deep lesions according to preoperative imaging, segmentectomy and lobectomy were performed immediately; otherwise, pulmonary wedge resection was performed first. The results of examination of a fast frozen section of the excised lung tissues and of a sampled lymph node from the lobar node and segmental node stations were assessed to determine whether to then perform anatomic segmentectomy or lobectomy. On completion of the procedure, bleeding was completely stopped and the lung inflated to check for leakage. When the surgeon had confirmed there was no air leakage under an airway pressure of 30 cmH2O (2.94 kPa), the wound surfaces at the lymph node and tracheal end were covered with hemostatic gauze and sprayed with fibrin glue. Next, a 28# or 32# chest tube was placed though the incision and an Abel drainage tube (Central Venous Catheter, 8Fr-20, Baihe Medical, China) connected to a drainage pack placed between the seventh and eighth ribs in the posterior axillary line. Thus the upper tube discharged air and the lower tube drained liquid.
Segment dissociation
We identified the vein and artery by CT photographs preoperatively and by the surrounding lung tissue and source and branch of the segmental vascular intraoperatively. The way to identify the segmental bronchus was similar to the segmental vascular. If the segmental bronchus was variational and different to identify, fiber bronchoscope could be applied to recognize the target bronchus intraoperatively. The surgeon would use vessel smooth forceps to clamp the target segmental bronchus and then the lungs were dilated to confirm the border of the target segmental lung issues. Divided the bronchus using the linear staple. At last, removed the target segment using the linear stapler along its border. The order to dissociate the vein, artery and bronchus of the target segment were unfixed (13).
Postoperative care
On the first postoperative day, the drainage volume of closed thoracic drainage tube was <150 mL/day, there was no air leakage occurred, and posteroanterior and lateral films of the chest showed satisfactory placement of tubes on the affected side in both groups. When the drainage volume of the chest drainage pack was <100 mL/day and posteroanterior and lateral films of the chest showed no obvious pleural effusion after the closed thoracic drainage tube had been removed, the Abel drainage tube was removed. When the patients were able to get out of bed without supplementary oxygen and had no postoperative complications (or any complications had resolved or improved markedly), they were discharged from hospital.
Variables assessed
The operation time, intraoperative blood loss, numbers and stations of mediastinal lymph nodes dissected, numbers of staples used, postoperative drainage volume and duration, duration of hospital stay, costs, incidence and nature of postoperative complications, and mortality rate were compared between two groups.
Statistical analysis
All data are expressed as mean ± standard deviation  . The χ2 and Student’s t-tests were performed with SPSS 19.0 statistical software. P<0.05 indicates a statistically significant difference.
. The χ2 and Student’s t-tests were performed with SPSS 19.0 statistical software. P<0.05 indicates a statistically significant difference.
The study was approved by the ethics committee of the Union Hospital Affiliated to Fujian Medical University.
Results
All 79 patients underwent S-VATS operative treatment as planned; the operations proceeded smoothly with no need for thoracotomy or second operative procedures.
Distribution of pulmonary tissue dissected
Lobectomy group: 11 cases of the left upper lobectomy; 6 cases of the left lower lobectomy; 15 cases of the right upper lobectomy; 4 cases of the right middle lobectomy; 10 cases of the right lower lobectomy; 1 case of the right middle and lower lobectomy.
Segmentectomy group: 3 cases of the left upper lobe posterior segmentectomy (S2); 1 case of the left upper lobe apicoposterior segmentectomy (S1 + S2); 4 cases of the left upper lober trisegmentectomy (S1 + S2 + S3); 3 cases of the left upper lobe lingular segmentectomy (S4 + S5); 1 case of the left upper lobe anterior lingular segmentectomy (S3 + S4 + S5); 2 cases of the left lower lobe dorsal segmentectomy (S6); 1 case of the left lower lobe anterior medial basal segmentectomy (S7 + S8); 1 case of the left lower lobe basal segmentectomy (S7 + S8 + S9 + S10); 4 cases of the right upper lobe apical segmentectomy (S1); 5 cases of the right upper lobe posterior segmentectomy (S2); 2 cases of the right upper lobe anterior segmentectomy (S3); 2 cases of the right upper lobe apicoposterior segmentectomy (S1 + S2); 3 cases of the right lower lobe dorsal segmentectomy (S6).
Postoperative pathological results
According to postoperative pathological examination of the operative specimens, the maximum diameters of pulmonary tumours resected were 7.3±2.4 and 16.2±9.0 mm in the segmentectomy and lobectomy groups, respectively; this difference is statistically significant (P<0.01) (Table 2).
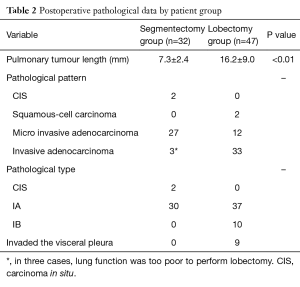
Full table
Differences between the two groups in operation time, intraoperative blood loss, postoperative hospital stay, costs, numbers of staples used, and rate of postoperative complications were all not statistically significant (all P>0.05). However, differences between the segmentectomy and lobectomy groups in the numbers of lymph nodes dissected and postoperative drainage volume and duration were all statistically significant (all P<0.05) (Table 3).
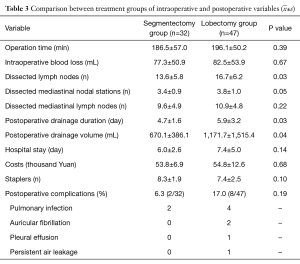
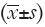
Full table
Follow-up
Patients in the two groups were followed up for 105–577 days (mean 313.9 days) clinically and via telephone. There were no tumor recurrences or tumor-related deaths.
Discussion
Gonzalez-Rivas et al. reported 17 patients who underwent S-VATS anatomic segmentectomy (12). The mean surgical time was 94.5±35.0 minutes (range, 40–150 minutes). The mean number of nodal stations explored was 4.1±1.0 (range, 0–5) with a mean of 9.6±1.8 (range, 7–12) lymph nodes resected. The mean maximum tumor diameter was 2.3±1.0 cm (range, 1–4 cm). In our study, S-VATS anatomic segmentectomy was performed in 32 patients. Their mean surgical time was 186.5±57.0 minutes (range, 100–320 minutes). The mean number of nodal stations explored was 3.6±0.8 (range, 4–7) with a mean of (13.6±5.8) (range, 5–29) lymph nodes resected. The mean maximum tumor diameter was 0.7±0.2 cm (range, 0.4–1 cm). Because S-VATS segmentectomy has not been performed for very long in our institution, the durations of our procedures were longer than those reported by others; however, the durations of these procedures at our institution are progressively decreasing. The other findings listed above are similar to those reported by others.
In this study, the tumors of the patients in the segmentectomy group had maximum diameters of 0.7±0.2 cm (range, 0.4–1.0 cm) according to measurement of operative specimens; all met the criteria for eligibility for anatomic segmentectomy (8). To guarantee acceptable margins, resection of adjoining pulmonary segments was performed for lesions that were determined by intraoperative examination to be located in or close to the boundary of a pulmonary segment. Additionally, fast pathologic examination detected no metastases in lymph nodes from the lobar node and segmental node stations in all cases in the segmentectomy group; postoperative pathological examination confirmed no metastases in their mediastinal lymph nodes. Therefore, anatomic segmentectomy in these patients with NSCLC was considered safe and effective.
It is currently recommended that mediastinal lymph node dissection should cover three or more mediastinal nodal stations (including subcarinal lymph nodes) and the number of mediastinal lymph nodes dissected should not be less than 10 (14-17). In this study, S-VATS was successfully used to perform lymph node dissection in 79 patients with primary bronchogenic carcinoma. The number of groups of mediastinal lymph nodes dissected was 3–7 (mean 3.7±1.0) and of mediastinal lymph nodes dissected 4–31 (mean 10.4±4.9), which is in accordance with the requirements for mediastinal lymph node dissection. These results are similar to those reported by Gonzalez-Rivas et al., who dissected 16±8 lymph nodes (18). Significantly fewer total lymph nodes and groups of lymph nodes were dissected in the segmentectomy than lobectomy group (P<0.05). In the segmentectomy group, only conventional lymph node sampling was performed for the hilar node, interlobar node and lobar node stations (134 lymph nodes in total; mean per case, 4.2), whereas conventional lymph node dissection was performed in the lobectomy group (271 lymph nodes in total; mean per case, 5.8).
In this study, according to postoperative measurement, the tumors resected in the segmentectomy group had smaller diameters than those in the lobectomy group; similar findings have been reported by others both in China and other countries (18,19). Because this study was retrospective and there were no standardized guidelines for selecting the procedure, individual surgeons decided which patients would undergo anatomic segmentectomy, which may have skewed the results. In any case, anatomic segmentectomy results in a larger pulmonary surface and greater trauma than lobectomy. However, in our study, the groups did not differ significantly in terms of intraoperative blood loss and incidence of postoperative complications. In terms of the absolute data, the segmentectomy group (6.3%) had fewer postoperative complications than the lobectomy group (17.0%). Therefore, we consider that the influence of tumor size was minimal and did not determine the outcomes. Prospective studies are required to verify this contention.
Two patients in the lobectomy group required thoracocentesis and catheterization because of postoperative pleural effusion; the drainage volume and duration in these two cases is included in the total drainage duration and volume. Drainage duration was shorter and the volume less in the segmentectomy than lobectomy group (both significant at P<0.05). Because less lung tissue is removed when anatomic segmentectomy is performed, the residual cavity is smaller. Additionally, only few inter-lobar fissures need to be incised to provide an adequate field of vision, the lung lobe structure and position do not change markedly, more of the tissue in the affected lung lobe can be left in situ, and the recruitment maneuver is unnecessary: unlike lobectomy, anatomic segmentectomy does not require a maneuver to recruit other lung lobes on the affected side to fill the residual cavity. Other studies (19,20) have reported that S-VATS anatomic segmentectomy is associated with a more rapid postoperative recovery.
More staples were used in the segmentectomy than the lobectomy group; however, this difference was not statistically significant. This finding is not in agreement with previous reports concerning non-S-VATS (21). We believe the following points are important regarding number of staples required. (I) In some patients in the lobectomy group, an adequate inter-lobar fissure did not develop and a linear stapling device was required to seal the deficiency, increasing the number of staples used; (II) because the arteries and veins of segments are thinner than those of lobes, silk ligation could be substituted for linear stapling in some patients in the segmentectomy group, decreasing the number of staples required; (III) in some patients in the segmentectomy group who had peripheral pulmonary nodules, wedge resection for lesions that were not close to the visceral pleura would have resulted in a similar functional loss to that caused by direct pulmonary segment dissection; the latter was therefore performed.
Previous studies (19,20) comparing the treatment outcomes of anatomic segmentectomy and lobectomy have reported that anatomic segmentectomy involves a longer operation time, fewer lymph node groups and mediastinal lymph nodes dissected, smaller postoperative drainage volume, shorter duration of postoperative drainage, and use of more staples than lobectomy. However, in our study, we found no statistically significant differences between S-VATS anatomic segmentectomy and S-VATS lobectomy in operation time, intraoperative blood loss, or number of staples used (P>0.05); S-VATS anatomic segmentectomy did not increase the number of staples used or the cost. Additionally, we found significantly fewer groups of lymph nodes and number of mediastinal lymph nodes dissected, a smaller volume of postoperative drainage, and shorter duration postoperative drainage than in the lobectomy group (all P<0.05). Therefore, segmentectomy does facilitate rapid postoperative recovery. Because S-VATS requires fewer ports for instruments, provides a smaller operation space, and is a more difficult procedure requiring greater technical competence than lobectomy, S-VATS anatomic segmentectomy should be performed only by surgeons who have mastered S-VATS lobectomy. S-VATS conforms with the modern concept that surgical procedures should guarantee safety and be minimally invasive. Because we did not have data about comparison with non-single-port segmentectomy, considering S-VATS segmentectomy superior to non- S-VATS segmentectomy would be not enough evidence and needed more related research in the future.
Chest wall pain after S-VATS is clearly milder than that after traditional multi-port VATS (22). Patients are therefore generally less fearful and stressed about undergoing the former procedure. Because the incision is located posterior to the breast, it can readily be covered and putting on clothes is easier. In conclusion, S-VATS lobectomy and segmentectomy are safe and have similar effect. Segmentectomy better facilitates rapid postoperative recovery and can be performed in patients with early-stage NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- He J. Mini-invasive thoracic surgery: History, current status, and future. Int J Pathol Clin Med 2013;33:1-7.
- Takizawa T, Haga M, Yagi N, et al. Pulmonary function after segmentectomy for small peripheral carcinoma of the lung. J Thorac Cardiovasc Surg 1999;118:536-41. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Computed tomography-defined functional lung volume after segmentectomy versus lobectomy. Eur J Cardiothorac Surg 2010;37:1433-7. [Crossref] [PubMed]
- Yoshimoto K, Nomori H, Mori T, et al. Quantification of the impact of segmentectomy on pulmonary function by perfusion single-photon-emission computed tomography and multidetector computed tomography. J Thorac Cardiovasc Surg 2009;137:1200-5. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11. [Crossref] [PubMed]
- Okada M, Mimae T, Tsutani Y, et al. Segmentectomy versus lobectomy for clinical stage IA lung adenocarcinoma. Ann Cardiothorac Surg 2014;3:153-9. [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [PubMed]
- Yang HC, Noh D. Single incision thoracoscopic lobectomy through a 2.5 cm skin incision. J Thorac Dis 2015;7:E122-5. [PubMed]
- Liu CY, Lin CS, Shih CH, et al. Single-port video-assisted thoracoscopic surgery for lung cancer. J Thorac Dis 2014;6:14-21. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Mendez L, Delgado M, et al. Uniportal video-assisted thoracoscopic anatomic segmentectomy. J Thorac Dis 2013;5 Suppl 3:S226-33. [PubMed]
- Zhu Y, Xu G, Zheng B, et al. Single-port video-assisted thoracoscopic surgery lung resection: experiences in Fujian Medical University Union Hospital. J Thorac Dis 2015;7:1241-51. [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Shapiro M, Kadakia S, Lim J, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013;144:1615-21. [Crossref] [PubMed]
- Watanabe S. Lymph node dissection for lung cancer: past, present, and future. Gen Thorac Cardiovasc Surg 2014;62:407-14. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Ohtsuka T, Kamiyama I, Asakura K, et al. Thirty-day outcomes after lobectomy or segmentectomy for lung cancer surgery. Asian Cardiovasc Thorac Ann 2015;23:828-31. [Crossref] [PubMed]
- Liu H, Chen L, Zhu Q, et al. The clinical treatment comparison on completely anatomic thoracoscopic segmentectomy and lobectomy for small pulmonary nodes. Chinese Journal of Clinicians 2012;6:103-5. (Electronic Edition).
- Wang GS, Wang Z, Wang J, et al. Uniportal complete video-assisted thoracoscopic surgery: a retrospective analysis of 106 cases of single-institution. China Journal of Endoscopy 2014;20:118-23.


