Successful extracorporeal membrane oxygenation (ECMO) support in two pediatric heart transplant patients with extreme donor/recipient size mismatch
Introduction
Heart transplantation remains the gold standard therapy for patients with end-stage heart failure (1). With increasing gap between organ demand and supply, there is growing interest in utilizing organs from high-risk and marginal donors for pediatric heart transplantation. The use of marginal donor hearts may lead to post-transplant graft dysfunction and graft failure, necessitating the use of extracorporeal membrane oxygenation (ECMO) support.
From September 2008 to March 2015, 20 cases of pediatric heart transplantation were performed in Wuhan Union Hospital. Two (10%) patients required ECMO support due to low cardiac output syndrome. Here we report on these two cases of ECMO support in pediatric heart transplant patients using marginal donors with extreme donor/recipient size mismatch, exceeding our institution’s usual criteria of less than 20%.
Case presentation
Case 1
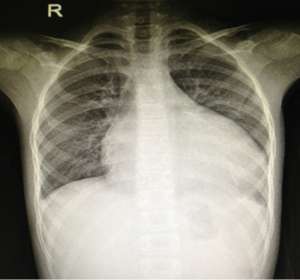
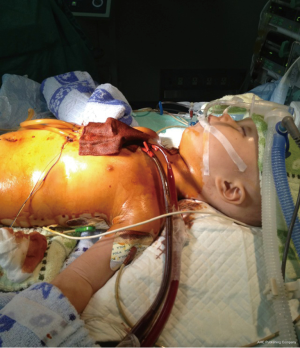
An 8-year-old female was admitted to our hospital with failure to thrive and oliguria. On admission, the chest X-ray revealed cardiomegaly (Figure 1). Transthoracic echocardiogram (TTE) revealed dilated cardiomyopathy with left ventricular ejection fraction of 25% with a measured left ventricular end diastolic dimension of 46 mm. Due to her deterioration, a donor was identified for her: 4-year-old male weighing 15 kg; the donor/recipient weight ratio was 0.71. The donor heart had a cold ischemic time of 452 min. Her heart transplant required cardiopulmonary bypass (CPB) time of 217 min. Veno-arterial ECMO (VA-ECMO) was initiated in operation room due to graft dysfunction and inability to wean off CPB. The patient was able to be weaned off ECMO after 63 hours of support. Her left ventricular ejection fraction recovered 68% on the TTE performed on post-operative day #21. She was discharged home on post-operative day #32 and continues to do well now (Figure 2).
Case 2
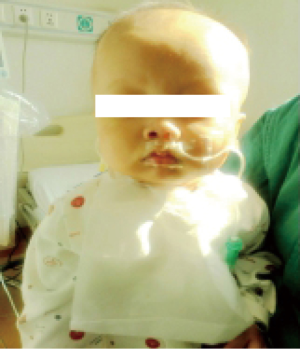
A 9-month-old boy weighing 7.5 kg was admitted to our hospital with dyspnea and poor oral intake. On admission, TTE echocardiogram revealed left-ventricular non-compaction with left ventricular ejection fraction of 33% and left ventricular end diastolic dimension of 41 mm. Due to continued deterioration despite optimal medical therapy, he underwent urgent heart transplantation. His donor was a 3-year-old female weighing 13 kg. The donor/recipient weight ratio was 1.73. Total cold ischemic time was 379 min and CPB time was 185 min. VA-ECMO was initiated in operation room due to inadequate cardiac output on maximal inotropic support. The patient was weaned off ECMO after 115 hours of support and his left ventricular ejection fraction was measured at 67% on post-operative day #21 (Figures 3,4). He was discharged home on post-operative day #28 and continues to do well.
Discussion
Today, development of pediatric orthotopic heart transplantation in china is still in its infancy. There are many challenges to face, such as shortage of pediatric heart donors, complex anatomy of congenital heart disease, and difficult perioperative management (2). With the number of potential recipients far exceeds the donor pool, more and more hearts from marginal donors are being considered and utilized for pediatric heart transplantation. In our institution, we performed 20 cases of pediatric orthotopic heart transplantation from September 2008 to March 2015. A total of nine cases used marginal heart donors (45%, 9/20=45%). Two (10%) of these patients required ECMO after heart transplantation. Both of these patients had significant donor/recipient weight mismatch exceeding our usual 20% limit. It has been reported that weight difference between donor and recipient of greater than 20% can directly affect the cardiac graft function (3,4). Secondary hypertension and reactive pulmonary vascular spasm may occur more commonly when large donor hearts transplanted to smaller recipients. In these transplant patients, they often require a week or longer post-transplant to adapt to the extreme size discrepancy. Difficulty to wean off CPB occurs more commonly when small donor hearts transplanted to larger recipients. Often faster heart rate is required to provide adequate cardiac output (5). If necessary, ECMO support may be needed during the postoperative period.
Of the mechanical circulatory support available for pediatric population, ECMO is the most feasible because of its flexibility to accommodate anatomy as well as rapid initiation of support. With ECMO support, the donor heart may recover its function and adapt to hemodynamic support of the recipient. For the weight difference between donor and recipient more than 20%, ECMO support is helpful to assist with recovery of cardiac function, particularly if recipients have severe pulmonary hypertension and donor ischemia time of greater than 4 hours (6,7).
These two pediatric patients supported by ECMO have chronic pulmonary congestion and high pulmonary vascular resistance; cold ischemic times of more than 6 hours, and donor/recipient weight mismatch of more than 20%. All these conditions predispose the heart to significant ischemic-reperfusion injury, primary graft dysfunction, and inability to wean off CPB. VA-ECMO was applied in these two cases to allow rapid stabilization of cardiopulmonary function. Improved hemodynamics allows for improved coronary and systemic perfusion. In alternative cannulation configurations, central VA-ECMO can be quickly converted to ventricular assist device and other circulation support, avoiding potential complications such as limb ischemia and peripheral catheter embolization (8-10).
In our experience, ECMO can be effectively implemented in pediatric patients who are unable to wean from CPB after heart transplantation, thus allowing hemodynamic support of the patient while awaiting graft recovery. A large prospective cohort study will be needed to define criteria for the use of ECMO after pediatric heart transplantation and perhaps allow great donor organ ulitization.
Acknowledgements
Funding: This study was supported by a grant from National Natural Science Foundation of China (No. 81570090).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Stendahl G, Bobay K, Berger S, et al. Organizational structure and processes in pediatric heart transplantation: a survey of practices. Pediatr Transplant 2012;16:257-64. [Crossref] [PubMed]
- Li F, Cai J, Sun YF, et al. Pediatric Heart Transplantation: Report from a Single Center in China. Chin Med J (Engl) 2015;128:2290-4. [Crossref] [PubMed]
- Delmo Walter EM, Huebler M, Schubert S, et al. Influence of size disparity of transplanted hearts on cardiac growth in infants and children. J Thorac Cardiovasc Surg 2012;143:168-77. [Crossref] [PubMed]
- Amarelli C, De Santo LS, Marra C, et al. Early graft failure after heart transplant: risk factors and implications for improved donor-recipient matching. Interact Cardiovasc Thorac Surg 2012;15:57-62. [Crossref] [PubMed]
- Mather PJ, Jeevanandam V, Eisen HJ, et al. Functional and morphologic adaptation of undersized donor hearts after heart transplantation. J Am Coll Cardiol 1995;26:737-42. [Crossref] [PubMed]
- Patel ND, Weiss ES, Nwakanma LU, et al. Impact of donor-to-recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation 2008;118:S83-8. [Crossref] [PubMed]
- Kertesz NJ, Gajarski RJ, Towbin JA, et al. Effect of donor-recipient size mismatch on left ventricular remodeling after pediatric orthotopic heart transplantation. Am J Cardiol 1995;76:1167-72. [Crossref] [PubMed]
- Undar A, Wang S. Current devices for pediatric extracorporeal life support and mechanical circulatory support systems in the United States. Biomed Mater Eng 2013;23:57-62. [PubMed]
- Crews KA, Kaiser SL, Walczak RJ, et al. Bridge to transplant with extracorporeal membrane oxygenation followed by HeartWare ventricular assist device in a child. Ann Thorac Surg 2013;95:1780-2. [Crossref] [PubMed]
- Jefferies JL, Morales DL. Mechanical circulatory support in children: bridge to transplant versus recovery. Curr Heart Fail Rep 2012;9:236-43. [Crossref] [PubMed]