
Effects of dexmedetomidine on inflammation and pulmonary function after thoracoscopic surgery for lung cancer: a systematic review and meta-analysis
Highlight box
Key findings
• The use of Dex in lung cancer patients after radical surgery can reduce serum inflammatory factors, which may play a key role in diminishing postoperative inflammatory reaction and improving lung function.
What is known and what is new?
• To minimize lung function damage caused by one-lung ventilation and inflammatory reactions caused by surgery are new challenges in the field of anesthesiology.
• Dex can ameliorate the postoperative lung function of lung cancer patients after radical resection.
What is the implication, and what should change now?
• Dex can reduce the inflammatory response after radical resection for lung cancer. The conclusions we obtained need to be further validated by larger sample, high-quality, multi-center clinical studies.
Introduction
Lung cancer is a common malignant tumor which poses an immense threat to the health and life of those affected. Though decreasing in the United States (US), over the last few years, the incidence rate and mortality of lung cancer worldwide have been on the rise (1,2). The latest statistics show that lung cancer has become the tumor with the highest incidence rate and mortality in China (3). Patients with lung cancer may have clinical symptoms, such as cough, chest pain, chest tightness and shortness of breath, or hemoptysis (4,5). Coupled with smoking related lung injury pulmonary reserve may be compromised. Malnutrition, frailty and metabolic disorders can further increase surgical risk (6). These factors together diminish patient tolerance to surgery and anesthesia (7). Currently, surgical resection remains the most effective method for treating early-stage lung cancer. Baseline lung function as well as the impact of surgery, positive pressure ventilation and single lung isolation affect perioperative lung function and outcomes (8-10). Hypoxemia occurs more frequently during one-lung ventilation, and the degree of ischemia and hypoxia increases as the duration of single lung ventilation exceeds 3 hours. Lung injuries caused by the single lung ventilation can bring secondary harm to patients who have mild-to-moderate abnormal lung function before surgery, and severe cases can eventually develop into acute lung injury (11,12). Moreover, surgical treatment may lead to adverse stress reactions such as activation of inflammatory reactions, limitation of immune functions, and an increase in the risks of tumor recurrence and metastasis. Minimizing damage caused by one-lung ventilation and inflammatory cascade of the surgical insult are ongoing challenges faced by the perioperative team (13,14).
Dexmedetomidine (Dex), a sedative frequently used in intensive care unit (ICU), can interact and combine with the presynaptic membrane of α2 adrenergic receptors, and inhibit the release of neurotransmitters in the presynaptic membrane, to produce exact analgesic and sedative effects (15). Dex can also inhibit cell apoptosis, oxidative stress reactions and inflammatory reactions, potentially protecting the heart, the brain, the lungs, and other important organs, improving perioperative pulmonary function. Other study has also reported that Dex can diminish the production of reactive oxygen species (ROS) and the release of cytochrome C in lung tissues, therefore it can reduce the apoptosis of alveolar epithelial cells (16). The influence of Dex on lung injury and inflammatory factors in elderly lung cancer patients who undergo thoracoscopic lobectomy remains unknown. With this meta-analysis we hope to examine the existing data on Dex in lung resection, and consider more aggressive use of Dex for anesthetic management in patients with lung cancer. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-651/rc).
Methods
This study has been registered in the PROSPERO platform (No. CRD42022359855).
Literature retrieval
We performed a computer search of the databases of PubMed, Embase, Cochrane Library, Web of Science for controlled trials (CTs) about the effect of Dex on inflammation and lung function after thoracoscopic surgery for lung cancer. The time period for retrieval was set from the inception of the database to 1 August 2022. The search was conducted through medical subject headings (MeSH) plus free text terms. The terms (keywords) used were: dexmedetomidine, precedex, thoracoscopy, and thoracoscopic surgery. The specific retrieval strategies are provided in Table S1. There was no restriction regarding region and/or publication status, and non-English studies were not included in this study.
Inclusion and exclusion criteria
We included the studies that met the following criteria: adults diagnosed with lung cancer; the experimental group was treated with Dex; the primary outcome measures were: interleukin-8 (IL-8), interleukin-6 (IL-6), interleukin-10 (IL-10), and forced expiratory volume in the first second (FEV1); the secondary outcome measures were: partial pressure of oxygen (PaO2) and adverse events.
The following studies were excluded: conference summaries, systematic reviews, duplicate publications, meta-analyses, animal experiments, case reports; unobtainable full-text.
Data extraction
The literature was screened independently by two researchers based on the inclusion and exclusion criteria. The titles and abstracts of the literature were checked to delete irrelevant studies. Dissents on whether a study should be included, if any, were resolved by a third researcher. Then, the full texts of the remaining articles were downloaded and reviewed to select eligible studies. Relevant data were extracted from the included studies by two independent researchers, who then cross checked their results. The extracted data encompassed the name of first author, experimental design, year of publication, age, country, intervention measures, sample size, and outcome indicators.
Quality evaluation
The quality of the studies included was independently assessed by two researchers utilizing the risk-of-bias assessment tool provided by Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (https://training.cochrane.org/handbook), which involves 7 domains: generation of random sequence (selection bias), allocation concealment (selection bias), blinding of the operators and participants (performance bias), blinding of the outcome evaluators (detection bias), integrity of data results (attrition bias), selective reporting of results (reporting bias), and other sources of bias. Each study was evaluated using the above criteria. If the original study fully met the criteria listed above, the overall risk of bias was low, indicating high quality. If the original study only partially met the above criteria, the risk of bias was unclear, indicating moderate quality. If the original study did not meet the above criteria at all, the risk of bias was high, and the study was of low quality.
Statistical analysis
Meta-analysis was carried out using Stata 15.0 software (StataCorp. L lung cancer, College Station, TX, USA). Continuous variables were presented as weighted mean difference (WMD) and 95% confidence interval (CI), and dichotomous variables were expressed by relative risk (RR) and 95% CI. The heterogeneity test was conducted. P≥0.1 and I2<50% suggested low heterogeneity, and the fixed-effects model was utilized. P<0.1 and I2>50% indicated the presence of heterogeneity, and subgroup analysis and sensitivity analysis were carried out to probe into the sources of heterogeneity. If it was impossible to identify the causes of heterogeneity, a random-effects model was employed for data analysis. The presence of publication bias in the meta-analysis results was determined by whether the funnel chart was symmetrical. A statistically significant difference was considered when P<0.05.
Results
Literature retrieval process results
The retrieval of the databases yielded 268 articles, of which 218 remained after removing duplicates. After reviewing the titles and abstracts, 20 were then retained. According to a full-text reading, 11 (14,17-26) RCTs were finally included. The literature screening process is illustrated in Figure 1.
Baseline and quality evaluation of the included literature
In total, 11 CTs were included, involving 1,026 patients. Of them, 512 were in the Dex group and 514 were in the control group. Most of the articles were from China. The amount of Dex used was from 0.2 to 0.5 µg/(kg·h). Table 1 displays the specific characteristics of the articles. The risk of bias assessment for the included studies is shown in Figures 2,3.
Table 1
| Study | Country | Sample size | Gender (M/F) | Mean age (years) | Intervention | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| EG | CG | EG | CG | EG | CG | |||||
| Ding J, 2022 | China | 60 | 60 | 71/49 | 68.26 | 68.52 | Dex 0.3 μg/(kg·h) | Saline | IL-6, IL-10, TNF-α, IL-1β, PaO2, PaCO2 | |
| Shi HX, 2020 | China | 40 | 40 | 80/0 | 66.4 | 67.6 | Dex 0.2 μg/(kg·h) | Saline | FVC, FEV1, adverse event | |
| Xie Y, 2020 | China | 58 | 58 | 76/40 | 67.4 | 68.1 | Dex 0.3 μg/(kg·h) | Saline | IL-6, TNF-α, adverse event, IL-8 | |
| Kong L, 2018 | China | 60 | 60 | 73/47 | 40.8 | 41 | Dex 0.5 μg/(kg·h) | Saline | IL-6, TNF-α, IL-8 | |
| Meng J, 2020 | China | 20 | 20 | 24/16 | 52 | 58 | Dex 0.5 μg/(kg·h) | Saline | Adverse event, IL-8 | |
| Jannu V, 2020 | India | 40 | 40 | 73/7 | 54 | 56 | Dex 0.5 μg/(kg·h) | Saline | PaO2, PaCO2, FEV1, adverse event | |
| Chen J, 2021 | China | 38 | 38 | 41/35 | 55.93 | 55.64 | Dex 0.6 μg/(kg·h) | Saline | FEV1, adverse event | |
| Liu GC, 2020 | China | 60 | 60 | 73/47 | 65 | 66 | Dex 0.5 μg/(kg·h) | Saline | IL-6, IL-10, TNF-α, IL-8 | |
| Lee SH, 2016 | Korea | 50 | 50 | 49/51 | 62 | 62 | Dex 1.0 μg/(kg·h) | Saline | PaO2, PaCO2, FEV1, adverse event | |
| Kim JA, 2019 | Korea | 60 | 60 | 58/62 | 63 | 59 | Dex 0.5 μg/(kg·h) | Saline | IL-6, IL-10, TNF-α, PaO2, PaCO2, adverse event, IL-8 | |
| Wen QP, 2020 | China | 26 | 28 | 33/21 | 54 | 58 | Dex 0.4 μg/(kg·h) | Saline | IL-6, adverse event | |
EG, experimental group; CG, control group; M, male; F, female; Dex, dexmedetomidine; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; IL-8, interleukin-8.
Meta-analysis results
IL-6
A total of 6 (17,19,20,22,24,25) studies mentioned the index of IL-6, involving a total of 646 patients. Heterogeneity testing indicated (I2=95.9%, P=0.000), so the random-effects model was employed to conduct data analysis. The results indicated that Dex can significantly reduce IL-6 after lung cancer surgery [standardized mean difference (SMD) =−2.09; 95% CI: −3.03, −1.14], with a statistically significant difference (P=0.003), as shown in Figure 4. Since the heterogeneity of the indicator was more than 50%, sensitivity analysis was carried out by excluding the included studies one by one. The analysis results showed that Ding et al. (17) may be the source of heterogeneity, as shown in Figure 5.

IL-8
A total of 5 (19,21,22,24,25) studies mentioned the index of IL-8, involving 512 patients overall. Heterogeneity testing indicated (I2=78.5%, P=0.001), so the random-effects model was utilized for data analysis. The results suggested that Dex was able to significantly reduce IL-8 after lung cancer surgery (SMD =−1.12; 95% CI: −1.54, −0.71), with a statistically significant difference (P=0.001), as shown in Figure 6. Since the heterogeneity of the indicator was more than 50%, sensitivity analysis was carried out by excluding the included studies one by one. The analysis results showed that Kong et al. (25) may be the source of heterogeneity, as shown in Figure 7.

Tumor necrosis factor-α (TNF-α)
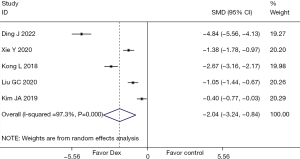
A total of 5 (17,19,22,24,25) studies mentioned TNF-α as an index, involving 592 cases in total. Heterogeneity testing indicated (I2=97.3%, P=0.000), so data were consolidated and analyzed using the random effects model. The results showed that Dex reduced TNF-α after lung cancer surgery (SMD =−2.04; 95% CI: −3.24, −0.84), and the difference was statistically significant (P=0.001), as shown in Figure 8. Since the heterogeneity of the indicator was more than 50%, sensitivity analysis was carried out by excluding the included studies one by one. The analysis results showed that the sensitivity of this indicator was small and that the analysis results were stable, as shown in Figure 9.
FEV1
A total of 4 (14,18,23,26) studies mentioned FEV1 as an index, involving 336 patients in total. Heterogeneity testing indicated (I2=30.1%, P=0.231), so the random-effects model was adopted to conduct data analysis. The results showed that Dex increased FEV1 after lung cancer surgery (SMD =0.50, 95% CI: 0.24, 0.76), with a statistically significant difference (P=0.003), as illustrated in Figure 10.

PaO2
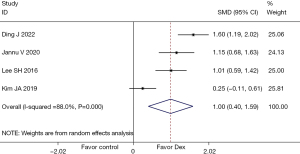
A total of 4 (17,23,24,26) studies mentioned the FEV1 index, involving 420 patients in total. Heterogeneity testing indicated (I2=88.0%, P=0.000), so the random-effects model was employed for data analysis. The results demonstrated that Dex increased PaO2 after lung cancer surgery (SMD =1.00, 95% CI: 0.40, 1.59), and the difference was statistically significant (P=0.001), as illustrated in Figure 11. Since the heterogeneity of the indicator was more than 50%, sensitivity analysis was carried out by excluding the included studies one by one. The analysis results showed that the sensitivity of this indicator was small and that the analysis results were stable, as shown in Figure 12.
Adverse reactions
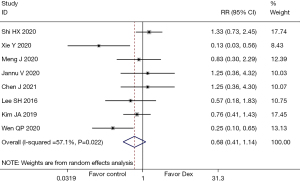
A total of 8 (14,18-21,23,24,26) studies mentioned the indicator of adverse reactions, involving 666 patients in all. Heterogeneity testing indicated (I2=57.1%, P=0.022), so the random-effects model was adopted for data analysis. The results revealed that regarding adverse reactions after lung cancer surgery, no significant difference was found between Dex treatment and the control group (RR =0.68; 95% CI: 0.41, 1.14; P=0.27), as illustrated in Figure 13. Since the heterogeneity of the indicator was more than 50%, sensitivity analysis was carried out by excluding the included studies one by one. The analysis results showed that the sensitivity of this indicator was small and that the analysis results were stable, as shown in Figure 14.
Publication bias
Funnel charts were drawn for IL-6 and adverse reactions, to conduct publication bias evaluation. The analysis results showed that the two sides of the funnel charts for IL-6 and adverse reactions were not completely symmetrical, suggesting that there was great possibility of publication bias for these two indicators (Figures S1,S2).
Discussion
In total, 11 RCTs were included in this study. The results showed that after Dex treatment, inflammatory factors IL-6 (SMD =−2.09; 95% CI: −3.03, −1.14; P=0.003), IL-8 (SMD =−1.12; 95% CI: −1.54, −0.71; P=0.001), and TNF-α (SMD =−2.04; 95% CI: −3.24, −0.84) were all decreased in lung cancer patients who received curative intent surgical resection. When lung cancer surgery is performed with one-lung ventilation, distribution of perfusion is altered, causing local hypoxia and increasing the number of alveolar macrophages and pulmonary neutrophils. This results in increased synthesis and release of proinflammatory factors and intercellular adhesion molecules (15,27). Taniguchi et al. (28) found in a rat model of endotoxin-induced shock that Dex can significantly reduce the level of serum inflammatory factors TNF-α and IL-6, in a dose- and time-dependent manner, and improve the survival rate of animals. Further studies confirmed that pretreatment with Dex significantly reduced the level of serum inflammatory factors TNF-α, IL-6, and IL-8 in endotoxemia rats. This suggests that Dex may, through increasing vagal efferent impulse, cause nerve endings to release acetylcholine (ACh) and bind to α7 ACh receptor (α7AChR) located in immune cells. In turn, this inhibits the release of the above inflammatory cytokines, thus playing an anti-inflammatory and protective role (29,30). In 2011, Zhang et al. (31) found that when the choice for the loading dose of Dex was 1.0 µg/kg before anesthesia induction, and 0.5 µg/kg for the maintenance of anesthesia, the concentration of serum IL-6 and IL-8 decreased during anesthesia. The above research also further confirmed our conclusion, that Dex can reduce the inflammatory response after resection for lung cancer.
Resection with curative intent for lung cancer entails the removal of all the tumor and systematic lymph node dissection. The standard of care and the most common surgical treatment is lobectomy, though sub-lobar resection has become increasingly utilized in very small tumors. Surgical manipulation coupled with one-lung ventilation can induce significant tissue inflammatory reaction. A multitude of inflammatory factors then enter the alveoli, which destroy the alveolar-capillary barrier, change the permeability, and cause interstitial edema, which then leads to reduced lung ventilation and diffusion function (32). In addition, during one-lung ventilation, the reduction of the collapsed lung’s ventilation will cause hypoxemia in the body, resulting in imbalance of lung ventilation (33). Our present study revealed that Dex can increase FEV1 (SMD =0.50; 95% CI: 0.24, 0.76; P=0.003) and PaO2 (SMD =1.00; 95% CI: 0.40, 1.59; P=0.001) in patients diagnosed with lung cancer who have undergone resection, indicating that Dex may ameliorate the postoperative lung function of lung cancer patients after radical resection, the likely mechanism is reduction of pulmonary inflammation. Our study found that there was no statistically significant difference in adverse reactions regarding the use of Dex in postoperative lung cancer patients (RR =0.68; 95% CI: 0.41, 1.14; P=0.27). A possible reason may be that the elimination half-life of Dex is relatively short, yet the time period for which we have documented adverse reactions was longer than the half-life. This study also has the following limitations. First, there was important heterogeneity among the included studies. However, in view of the limited data provided by the original studies, it was impossible to identify the source of heterogeneity through the method of different subgroup analyses. In addition, the type of surgery in each study was different, and the size of trauma caused by different surgeries was also different, which also leads to different degrees of inflammatory reaction. Second, the number of individuals included in the studies was small, and the dose of Dex varied, which can lead to potential heterogeneity. Third, most of the included literature presented multiple potential biases, such as unclear randomization, unclear blinding, and/or unclear allocation concealment.
Conclusions
In summary, the use of Dex in lung cancer patients with surgery for curative intent can reduce serum inflammatory factors, which may play a key role in diminishing postoperative inflammatory reaction and improving lung function. Limited by the quantity and quality of the studies that were included, the conclusions we have obtained need to be further validated by larger sample, high-quality, multi-center clinical studies.
Acknowledgments
We appreciate the work of the researchers and the study participants.
Funding: This work was supported by the Project “Effect of Cis-Atracurium on Postoperative Immune Function in Patients with Colorectal Tumors” (No. YK2021068).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-651/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-651/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-651/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bo JH, Wang JX, Wang XL, et al. Dexmedetomidine Attenuates Lipopolysaccharide-Induced Sympathetic Activation and Sepsis via Suppressing Superoxide Signaling in Paraventricular Nucleus. Antioxidants (Basel) 2022;11:2395. [Crossref] [PubMed]
- Han DW, Oh JE, Lim BJ, et al. Dexmedetomidine attenuates subarachnoid hemorrhage-induced acute lung injury through regulating autophagy and TLR/NFκB signaling pathway. Korean J Anesthesiol 2022;75:518-29. [PubMed]
- Hemsinli D, Tumkaya L, Ergene S, et al. Dexmedetomidine attenuates pneumocyte apoptosis and inflammation induced by aortic ischemia-reperfusion injury. Clin Exp Hypertens 2022;44:595-600. [Crossref] [PubMed]
- Li Y, Wu B, Hu C, et al. The role of the vagus nerve on dexmedetomidine promoting survival and lung protection in a sepsis model in rats. Eur J Pharmacol 2022;914:174668. [Crossref] [PubMed]
- Lin Y, Cai J, Huang D, et al. Effects of dexmedetomidine on the expression profile of tsRNAs in LPS-induced acute lung injury. J Clin Lab Anal 2022;36:e24115. [Crossref] [PubMed]
- Lu Y, Shimizu H, Nakamura R, et al. Dexmedetomidine improves acute lung injury by activating autophagy in a rat hemorrhagic shock and resuscitation model. Sci Rep 2023;13:4374. [Crossref] [PubMed]
- Ren B, Cheng M, Liu C, et al. Perioperative lidocaine and dexmedetomidine intravenous infusion reduce the serum levels of NETs and biomarkers of tumor metastasis in lung cancer patients: A prospective, single-center, double-blinded, randomized clinical trial. Front Oncol 2023;13:1101449. [Crossref] [PubMed]
- Xiao S, Wang Q, Gao H, et al. Dexmedetomidine alleviates airway hyperresponsiveness and allergic airway inflammation through the TLR4/NF-κB signaling pathway in mice. Mol Med Rep 2022;25:74. [Crossref] [PubMed]
- Xiao S, Zhou Y, Gao H, et al. Dexmedetomidine attenuates airway inflammation and oxidative stress in asthma via the Nrf2 signaling pathway. Mol Med Rep 2023;27:2. [Crossref] [PubMed]
- Zhang ZT, Xie K, Luo RJ, et al. Dexmedetomidine alleviates acute lung injury by promoting Tregs differentiation via activation of AMPK/SIRT1 pathway. Inflammopharmacology 2023;31:423-38. [Crossref] [PubMed]
- Zhou Y, Dong X, Zhang L. Dexmedetomidine Can Reduce the Level of Oxidative Stress and Serum miR-10a in Patients with Lung Cancer after Surgery. Thorac Cardiovasc Surg 2023;71:197-205. [Crossref] [PubMed]
- Zhou Y, Du X, Wang Q, et al. Dexmedetomidine Protects against Airway Inflammation and Airway Remodeling in a Murine Model of Chronic Asthma through TLR4/NF-κB Signaling Pathway. Mediators Inflamm 2023;2023:3695469. [Crossref] [PubMed]
- An G, Zhang Y, Chen N, et al. Opioid-free anesthesia compared to opioid anesthesia for lung cancer patients undergoing video-assisted thoracoscopic surgery: A randomized controlled study. PLoS One 2021;16:e0257279. [Crossref] [PubMed]
- Shi HX, Du XJ, Wu F, et al. Dexmedetomidine for early postoperative cognitive dysfunction after video-assisted thoracoscopic lobectomy in elderly male patients with lung cancer. Medicine (Baltimore) 2020;99:e21691. [Crossref] [PubMed]
- Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth 2015;115:171-82. [Crossref] [PubMed]
- Congregado M, Merchan RJ, Gallardo G, et al. Video-assisted thoracic surgery (VATS) lobectomy: 13 years' experience. Surg Endosc 2008;22:1852-7. [Crossref] [PubMed]
- Ding J, Zhu M, Lv H, et al. Application Effect of Dexmedetomidine and Dezocine in Patients Undergoing Lung Cancer Surgery under General Anesthesia and Analysis of Their Roles in Recovery Time and Cognitive Function. Comput Math Methods Med 2022;2022:9889534. [Crossref] [PubMed]
- Chen J, Chen B, Chen A. Effects of anesthesia induction using dexmedetomidine in thoracoscopic pulmonary segmentectomy on the safety and protection of lung function. Int J Clin Exp Med 2021;14:565-72.
- Xie Y, Jiang W, Zhao L, et al. Effect of dexmedetomidine on perioperative inflammation and lung protection in elderly patients undergoing radical resection of lung cancer. Int J Clin Exp Pathol 2020;13:2544-53. [PubMed]
- Miao Z, Wu P, Wang J, et al. Whole-Course Application of Dexmedetomidine Combined with Ketorolac in Nonnarcotic Postoperative Analgesia for Patients with Lung Cancer Undergoing Thoracoscopic Surgery: A Randomized Control Trial. Pain Physician 2020;23:E185-93. [PubMed]
- Meng J, Lv Q, Yao J, et al. Effect of Dexmedetomidine on Postoperative Lung Injury during One-Lung Ventilation in Thoracoscopic Surgery. Biomed Res Int 2020;2020:4976205. [Crossref] [PubMed]
- Liu GC, Sun K, Fu HG, et al. Effects of dexmedetomidine on injury of lungs and CHOP protein expression in elderly patients with lung cancer during one-lung ventilation. Zhonghua Yi Xue Za Zhi 2020;100:37-41. [PubMed]
- Jannu V, Dhorigol MG. Effect of Intraoperative Dexmedetomidine on Postoperative Pain and Pulmonary Function Following Video-assisted Thoracoscopic Surgery. Anesth Essays Res 2020;14:68-71. [Crossref] [PubMed]
- Kim JA, Ahn HJ, Yang M, et al. Intraoperative use of dexmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial. Can J Anaesth 2019;66:371-9. [Crossref] [PubMed]
- Kong L, Lu XH. Effect of dexmedetomidine on perioperative inflammatory response and cellular immune in patients undergoing radical operation of thoracoscopic lung cancer. Zhonghua Yi Xue Za Zhi 2018;98:2929-32. [PubMed]
- Lee SH, Lee CY, Lee JG, et al. Intraoperative Dexmedetomidine Improves the Quality of Recovery and Postoperative Pulmonary Function in Patients Undergoing Video-assisted Thoracoscopic Surgery: A CONSORT-Prospective, Randomized, Controlled Trial. Medicine (Baltimore) 2016;95:e2854. [Crossref] [PubMed]
- Li Q, Yao H, Xu M, et al. Dexmedetomidine combined with sufentanil and dezocine-based patient-controlled intravenous analgesia increases female patients' global satisfaction degree after thoracoscopic surgery. J Cardiothorac Surg 2021;16:102. [Crossref] [PubMed]
- Taniguchi T, Kurita A, Kobayashi K, et al. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth 2008;22:221-8. [Crossref] [PubMed]
- Wu CY, Lu YF, Wang ML, et al. Effects of Dexmedetomidine Infusion on Inflammatory Responses and Injury of Lung Tidal Volume Changes during One-Lung Ventilation in Thoracoscopic Surgery: A Randomized Controlled Trial. Mediators Inflamm 2018;2018:2575910. [Crossref] [PubMed]
- Xu B, Gao H, Li D, et al. Nebulized dexmedetomidine improves pulmonary shunt and lung mechanics during one-lung ventilation: a randomized clinical controlled trial. PeerJ 2020;8:e9247. [Crossref] [PubMed]
- Zhang RZ, Shi YS, Zhang YM, et al. Effects of different doses of dexmedetomidine on perioperative inflammatory response in patients undergoing one-lung ventilation. Chinese Journal of Anesthesiology 2011;31:1443-5.
- Ding Y, Su SY, Lin YL, et al. Effect of transcutaneous acupoint electrical stimulation at Neiguan (PC 6) on general anesthesia under preserving spontaneous breathing in thoracoscopic lobectomy. Zhongguo Zhen Jiu 2023;43:282-6. [PubMed]
- Zhou ZG, Liu R, Tan HL, et al. The application of dexmedetomidine combined with dezocine in thoracoscopic radical resection of lung cancer and its effect on awakening quality of patients. Eur Rev Med Pharmacol Sci 2019;23:7694-702. [PubMed]
(English Language Editor: J. Jones)










