
Retrospective on global pulmonary hypertension clinical trials: 1999–2021
Highlight box
Key findings
• The participation of developing countries in global PH clinical trials was low. There are large gaps between developed and developing countries in children’s representation, and type of clinical trial, interventions, study phase and sponsorship.
What is known and what is new?
• The distinct differences in clinical trials on PH between developed and developing countries have not been elucidated.
• Developed countries play a leading role in initiating PH clinical trials, while participation in developing countries is progressively increasing.
What are the implications, and what should change now?
• There are large gaps between developed and developing countries in children’s representation, and types of clinical trial, interventions, study phase and sponsorship. Women with pulmonary arterial hypertension (PAH) were relatively better represented in Group 1 PH trials, drug intervention trials, phase III studies, and industry-sponsored studies. More importantly, effective strategies to improve the representation of developing countries in PH clinical trials are needed.
Introduction
Pulmonary arterial hypertension (PAH) is a rare but life-threatening cardiopulmonary disease (1). Although pulmonary hypertension (PH) was first described in 1891, it was not until 1973 that the World Health Organization (WHO) formally established a clinical classification and management guidelines. More in-depth research has led to consistent improvements in our understanding of PH, and the prognosis of patients has been significantly improved. The 1- and 3-year survival rates of PAH patients included in the U.S. National Institutes of Health (NIH) registry in the 1980s were only 68% and 48%, respectively (1). In 1990, phase III premarketing clinical trials of intravenous epoprostenol infusion demonstrated remarkably improved survival in patients with idiopathic PAH (IPAH) (conventional therapy vs. epoprostenol, P=0.003) (2), inaugurating the era of targeted PAH drug therapy (3). In fact, in contemporary registries, the 1-, 3-, and 5-year survival rates of heritable PAH or IPAH were 85%, 68%, and 57%, respectively, in the US REVEAL Registry (4), consistent with the survival rate reported in the French Registry (85.7%, 69.6%, and 54.9%, respectively) (5).
However, the PAH prognosis seems to be unbalanced worldwide. In fact, the survival rate in developing countries is far lower than that in developed countries. In 2007, the 5-year survival rate of the untreated PAH cohort in China was only 20.8%, the largest developing country in Asia (6), which is similar to the US survival rate before the availability of PH therapies (1). Meanwhile, in the modern management era, when patients with PAH had access to targeted therapies, the survival rate of IPAH patients in China improved remarkably, reaching a level similar to that in developed countries, with 1- and 3-year survival rates of 92.1% and 75.1%, respectively (7). It can be inferred that successful clinical trials of targeted PH therapies may be responsible for the improvement in survival during this period. The availability of “study sponsored” drugs might have alleviated the economic burden of PH treatment in developing countries. Taking China as an example, most patients have no access to targeted therapy except through enrollment in clinical trials; in this way, they have the opportunity to receive adequate early combination therapy (8).
However, a detailed analysis of global PH clinical trials is still lacking. Based on this, we collected ClinicalTrials.gov data before December 2021. All registered clinical trials on PH were analyzed and summarized to investigate the differences in PH characteristics between developed and developing countries.
Men and adults account for the majority of patients with common cardiovascular diseases; however in PH, there is a higher disease prevalence in women and similar prevalence rate in children compared to adults (9,10). Therefore, we summarized PH clinical trials enrolling women and children. The current study aimed to (I) provide a comprehensive analysis of the PH clinical trials in developed and developing countries and (II) assess the participation of women and children.
Methods
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data source and study selection
We searched for clinical trials registered at ClinicalTrials.gov using the search terms “pulmonary hypertension” as the disease condition, “all studies” as the recruitment status, and “with results” as the result status. We further restricted “completed” to recruitment status and excluded “children only” for the age group. The search period was from January 1, 1999, and January 31, 2021. Trials were excluded if (I) the disease type was not PH and (II) the result status was “without results”.
Data extraction
Only trials that met the aforementioned inclusion criteria were selected. The following information was extracted: (I) National Clinical Trial number; (II) primary completion date; (III) trial locations; (IV) intervention types (drugs, devices, lifestyle changes, procedures, or multiple interventions); (V) clinical classification of PH (Groups 1–5 PH); (VI) sponsor types [research institute (hospital/medical center and university), industry, government, industry and research institute and multiple sponsors]; (VII) sponsor name; (VIII) trial size; (IX) proportion of women; (X) mean age; (XI) funding sources; (XII) interventional model (parallel assignment, single group assignment, and crossover assignment); (XIII) masking [single (outcome assessor), double (participant, investigator), triple (participant, care provider, investigator), quadruple (participant, care provider, investigator, outcome assessor), none (open label)]; (XIV) allocation (non-randomized, randomized); (XV) clinical trial type (observation and intervention); (XVI) study phase (I, II, III, and IV) and completion status (completed, terminated, active, not recruiting). Trials conducted both in developed and developing countries were categorized as “developed and developing countries”, and those only in developed countries as “developed countries”. Countries were assigned to income levels according to the WHO categorization, which is based on a country’s gross national income per capita. The high-income level group included a total of 53 countries (income per capita of US $10,066 or more), the middle-income level group included 93 countries (income per capita of US $826 to $10,065), and the low-income level group included 59 countries (income per capita of US $825 or less) (11). Developed countries, included sovereign states with highly advanced economies and technological infrastructure. Developing countries, including underdeveloped and less developed countries, have a low Human Development Index and a less developed industrial base.
The participants were divided into four groups based on the following age ranges: ≤47, 48–53, 54–59, and ≥60 years according to the interquartile range. Trial size was divided into four groups by quartiles (quartile 1, ≤19; quartile 2, 20–36; quartile 3, 37–148; quartile 4, ≥149 study participants). The clinical classification of PH was categorized according to the 6th World Symposium on PH (12,13).
Statistical analysis
We tested the differences between developed and developing countries depending on trial size, intervention type, study phase, sponsor type, clinical classification of PH, clinical trial type, allocation, completion status, and intervention model. The comparisons of adults and children were calculated and stratified by age, intervention type, trial size, clinical classification of PH, study phase, sponsor type, country category, allocation, clinical trial type, intervention model, and masking. The participation to prevalence ratio (PPR) in the PH population, which is a measure that describes the representation of women in trials based on their proportion in the PH population, was computed using the formula below, as previously described (14,15).
The proportion of females in the PH population was 57.2%, which was obtained from the most recent or large epidemiological population-based data available in the literature (9,16,17). The influence of PH prevalence on actual female participation rates could be adjusted by the PPR. A PPR of 1 suggested that the sex distribution in the respective trial was comparable to that of the disease population. Women were considered to be underrepresented or overrepresented when the PPR was <0.8 or >1.2, respectively, relative to women in the disease population (14). We also compared the PPR differences between developed and developing countries, as well as between children and adults.
The differences between groups were assessed using the t-test, Mann-Whitney U test, Wilcoxon rank sum test, Kruskal-Wallis test, or one-way Analysis of Variance (ANOVA). The Bonferroni method for correcting the significance level for multiple comparisons was applied. Pairwise comparisons of continuous variables were performed using the Mann-Whitney U test. All analyses were conducted using R (version 4.1.0, Camp Pontanezen, TX) and GraphPad Prism software (version 7.0.1, San Diego, California, USA).
Results
General characteristics of trials
Initially, 1,181 clinical trials were found by the condition or disease of “pulmonary hypertension” from January 1, 1999, to January 31, 2021. We excluded studies on healthy volunteers (n=4), trials without updated study results (n=973), and trials sponsored by developing countries (n=1). Ultimately, 203 trials were included for further analysis (Figure 1), including 23,402 participants with a mean age of 50±15 years, among whom 67.8% were women (Table 1). Almost all countries on all continents have participated in PH clinical trials; however, most trials were carried out by European and American countries (nearly 90%) (Figure S1).
Table 1
| Characteristics | Developed countries (N=171) | Developed and developing countries (N=32) | Total (N=203) | P value* |
|---|---|---|---|---|
| Female (%) | N=167 | N=32 | 0.140 | |
| Mean (SD) | 68.3 (23.2) | 64.8 (16.3) | 67.8 (22.3) | |
| Age (years) | N=136 | N=25 | ||
| Mean (SD) | 50.8 (15.2) | 46.9 (16.0) | 50.2 (15.3) | 0.246 |
| Sample size (n) | ||||
| Median (min, max) | 27 (1, 3,337) | 165 (3, 1,192) | 32 (1, 3,337) | <0.001 |
| Sponsor type, n (%) | <0.001 | |||
| Government | 2 (1.2) | 0 | 2 (1.0) | |
| Industry | 91 (53.2) | 30 (93.8) | 121 (59.6) | |
| Industry and research institute | 10 (5.8) | 0 | 10 (4.9) | |
| Multiple sponsors | 1 (0.6) | 0 | 1 (0.5) | |
| Research institute | 67 (39.2) | 2 (6.2) | 69 (34.0) | |
| Phase, n (%) | <0.001 | |||
| Phase 1 | 5 (2.9) | 2 (6.2) | 7 (3.4) | |
| Phase 2 | 53 (31.0) | 4 (12.5) | 57 (28.1) | |
| Phase 3 | 49 (28.7) | 21 (65.6) | 70 (34.5) | |
| Phase 4 | 31 (18.1) | 5 (15.6) | 36 (17.7) | |
| Phase 1 & Phase 2 | 5 (2.9) | 0 (0.0) | 5 (2.5) | |
| Phase 2 & Phase 3 | 4 (2.3) | 0 (0.0) | 4 (2.0) | |
| Not applicable | 24 (14.0) | 0 (0.0) | 24 (11.8) | |
| Adults or children, n (%) | 0.194 | |||
| Children | 15 (8.8) | 4 (12.5) | 19 (9.4) | |
| Adults | 144 (84.2) | 23 (71.9) | 167 (82.3) | |
| Children and adults | 12 (7.0) | 5 (15.6) | 17 (8.4) | |
| Completion status, n (%) | 0.686 | |||
| Completed | 119 (69.6) | 20 (62.5) | 139 (68.5) | |
| Terminated | 49 (28.7) | 11 (34.4) | 60 (29.6) | |
| Active, not recruiting | 3 (1.8) | 1 (3.1) | 4 (2.0) | |
| Clinical trial type, n (%) | 0.223 | |||
| Observation | 13 (7.6) | 0 (0.0) | 13 (6.4) | |
| Intervention | 158 (92.4) | 32 (100.0) | 190 (93.6) | |
| Allocation, n (%) | N=158 | N=32 | 0.168 | |
| Randomized | 71 (41.5) | 20 (62.5) | 91 (44.8) | 0.183 |
| Non-randomized | 86 (50.3) | 12 (37.5) | 98 (48.3) | |
| N/A | 1 (0.6) | 0 (0.0) | 1 (0.5) | |
| Intervention model, n (%) | N=157 | N=32 | 0.056 | |
| Parallel assignment | 69 (40.4) | 22 (68.8) | 91 (44.8) | |
| Single group assignment | 76 (44.4) | 10 (31.2) | 86 (42.4) | |
| Crossover assignment | 11 (6.4) | 0 (0.0) | 11 (5.4) | |
| Factorial assignment | 1 (0.6) | 0 (0.0) | 1 (0.5) | |
| Masking, n (%) | N=158 | N=32 | 0.378 | |
| Double | 19 (11.1) | 4 (12.5) | 23 (11.3) | |
| None (open label) | 91 (53.2) | 15 (46.9) | 106 (52.2) | |
| Quadruple | 28 (16.4) | 10 (31.2) | 38 (18.7) | |
| Triple | 14 (8.2) | 3 (9.4) | 17 (8.4) | |
| Single | 6 (3.5) | 0 (0.0) | 6 (3.0) | |
| Intervention type, n (%) | N=170 | N=32 | 0.457 | |
| Drug | 162 (94.7) | 32 (100.0) | 194 (95.6) | |
| Devices | 7 (4.1) | 0 (0.0) | 7 (3.4) | |
| Multiple interventions | 1 (0.6) | 0 (0.0) | 1 (0.5) | |
| PH group, n (%)† | N=35 | N=184 | N=219 | 0.353 |
| Group 1 PH | 26 (74.3) | 141 (76.6) | 167 (76.3) | |
| Group 2 PH | 2 (5.7) | 13 (7.1) | 15 (6.8) | |
| Group 3 PH | 2 (5.7) | 16 (8.7) | 18 (8.2) | |
| Group 4 PH | 4 (11.4) | 6 (3.3) | 10 (4.6) | |
| Group 5 PH | 1 (2.9) | 8 (4.3) | 9 (4.1) |
*, when the Bonferroni method was employed for correcting the significance level of 43 comparisons made in this study, the adjusted significance level was 0.001; †, numbers may add up to >100% as some clinical trials enrolled more than one Group PH. SD, standard deviation; N/A, not applicable; PH, pulmonary hypertension.
The sample sizes of trials involving developing countries were significantly larger than those of trials involving developed countries (P<0.01). From a global perspective, clinical research on PH has surged in recent years; with higher number of developing countries participating in global PH clinical research, although still far less than that of developed countries (Figure S2). Of the 203 trials included, 95.6% evaluated drug interventions, 59.6% were fully sponsored by pharmaceutical companies, 76.3% included Group I PH (PAH), and 84.2% were conducted only in developed countries (n=171) (Table 1).
PH clinical trials between developed and developing countries
The sponsors of clinical trials in developed countries were mainly the pharmaceutical industry (53.2%) and research institutions (39.2%), while the pharmaceutical industry (93.8%) was the main sponsor in developing countries. As for the study phase, early phases, such as phase I or II, were mainly conducted in developed countries (Table 1), while phase III PH clinical trials were mainly conducted in developing countries (65.6%).
Regardless of whether studies were conducted in developed or in both developed and developing countries, intervention drugs were dominant. In terms of the intervention type, developing countries only participated in the clinical trials of drug interventions, while developed countries participated in more diversified clinical studies, including drug- or device-related studies and studies with multiple interventions, even though the drug intervention type was predominant (Figure 2A). In terms of the study phase, patients in developing countries mainly participated in phase III clinical trials, accounting for more than 50% of trials (Figure 2B). Among the clinical studies conducted only in developed countries, pharmaceutical companies were the major sponsor type, followed by research institutions (Figure 2C). As shown in Figure 2D, Group 1 PH was the predominant type for all PH clinical studies. In addition, non-Group 1 PH pediatric patients were only recruited in developed countries. All participants from developing countries were recruited only for intervention trials, while observational clinical studies were only conducted in developed countries (Figure 3A). Notably, there was no significant difference in the allocation model between developed and developing countries, which indicates that the research design has a certain homogeneity and reflects the high quality of the clinical trials (Figure 3B). Of the 23,402 study participants, 557 pediatric patients (<17 years old) were involved in clinical trials without any adult participation. No significant differences existed in the completion rate of clinical studies between adults and children regardless of the developmental status of the countries (Figure 3C). Single group and parallel assignment were the main intervention patterns of PH clinical studies regardless of the developmental status of the countries (Figure 3D).

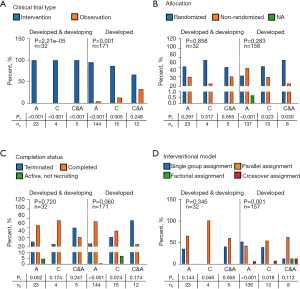
Children in PH clinical trials
Regarding the sample size of PH clinical trials, there were significant differences in adult and pediatric participants in trials including only developed countries, which were abrogated if developing countries were included (Figure S4). In developed and developing countries, PPR was highest in clinical trials involving both adults and children, while PPR of clinical trials showed homogeneity in developed countries independent of age (Figure S5). All PH clinical trials including children tested drug interventions (Figure 2A). Children in developed countries were enrolled in more diverse PH clinical trials with various phase stages than those in developing countries (P<0.001) (Figure 2B). Trials involving children in developing countries were funded only by drug companies. Similar to the results for adults, those for children showed that Group 1 PH was the predominant type for all PH clinical studies (Figure 2D). The clinical trial type (observation and intervention), allocation model, completion status, intervention model, and masking in children are also shown (Figure 3A-3D).
Women in PH clinical trials
A total of 129 PH clinical trials were included to analyze the participation of women in PH clinical trials (Figure 1). Among the whole clinical trial population, the PPR of elderly PH patients (>60 years old) was lower than that of younger PH patients (Figure 4A). No differences in the female PPR existed between developed and developing countries (Figure 4B). The PPR of Group 1 PH was much higher than those of the other PH groups (1.259, 1.058, 0.720, 1.053, and 0.982 for Groups 1–5, respectively) (Figure 4C). In trials involving only developed countries, the female PPR of Group 3 PH was much lower than those of Group 1 PH and Group 2 PH (0.69 vs. 1.29, P<0.001; 0.69 vs. 1.06, P=0.02, respectively). In trials involving developing countries, women were overrepresented in Group 1 and Group 4 PH, and the PPRs of Group 1 PH were higher than those of WHO Group 4 (1.28 vs. 1.22, P=0.004) (Figure 4D). Moreover, the PPR was also higher in phase III clinical trials (P=0.03) (Figure 4E). There were no differences in the female PPR in terms of phase staging (Figure 4F). The PPR was also higher in trials sponsored by research institutions (P=0.004) (Figure 4G). There were no differences in terms of sponsors (Figure 4H). Compared with those in device intervention-related clinical studies, female patients participating in clinical studies of drug interventions tended to have a higher PPR (1.229 vs. 0.9533, respectively, P=0.05) (Figure 4I). However, there were no differences in the female PPR in terms of intervention type (Figure 4J), trial size (Figure 4K) or clinical trial design, including allocation, clinical trial type, and masking (Figure S3). Similar results were observed between developed and developing countries in terms of trial sizes (Figure 4L).

Discussion
General characteristics of trials
From the world map distribution of clinical trials and the increasing trend of the number of trials, PH, a rare disease, has been receiving increasing attention from countries, regardless of their economic status. The multinational design is very popular in clinical drug research worldwide (8,18). Developed countries play a leading role in the promotion of PH trials, which also drives the participation of developing countries. Globally, clinical research on PH has surged in recent years. In fact, the number of enrolled patients, the duration of drug clinical trials, and the participation of developing countries have all increased. However, there is a difference between developed and developing countries in the acceleration time/mode to reach the peak number of PH trials, where developed countries peaked in an earlier period compared to developing countries. Such is the scenario in the introduction of endothelin receptor antagonists and related targeted drugs in developing countries (7). In other words, the results of these clinical trials have led to a different era of PH treatment.
In initial PH treatment studies, the average study duration was 3–4 months, and the primary outcome was a change in the 6-minute walking distance. Today, the design of clinical trials was more complex; with the main goal of reducing the incidence rate of composite poor outcome. There are many reasons for this change, including insufficient early recognition, high mortality, and unclear drug efficacy. The length and endpoint of the clinical trials were considered only after the survival rate was improved in the later stage. This also reflects the continuous improvement of disease awareness and the gradual exploration of drugs. In the same way, the different degrees of drug acceptance and compliance among patients affect the setting and implementation of test results. These changes also indicate that the understanding and development of this key disease have entered a new stage.
PH clinical trials between developed and developing countries
We found that almost all PH clinical trials were initiated by developed countries. Developed countries have actively engaged in all global clinical trials, with a participation rate that is significantly higher than that of developing countries. Developed countries may recognize and add importance to the treatment of rare diseases earlier that developing countries that may have other more pressing concerns. In developed countries, scientific research institutions, universities, government institutions, and other institutions support PH clinical trials, while in developing countries, only companies support PH clinical trials. Therefore, clinical trials in developing countries are mainly phase III trials, and the participation rate in early clinical trials is much lower. However, due to the differences in drug safety and tolerance between different nationalities and races, we strongly suggest that phase I and II clinical trials also be carried out in developing countries in the future.
Despite participating in fewer PH clinical trials than developed countries, developing countries were involved in clinical trials with larger sample sizes. From a global perspective, transnational designs are very popular in the clinical design of PH trials (8,17). On the one hand, the population of patients with PAH in the developed world is now relatively small for meeting demands for the simultaneous enrollment of patients and rapid progress. On the other hand, it certain types of PH are more prevalent in developing countries than in developed countries (8). The age, sex, and etiological subgroups of Group 1 PH have different epidemiological distributions between developed and developing countries. In the former, the patients are older, and the most common etiological group is the IPAH group; in the latter, the patients are younger, without comorbidities, and the most common etiological group is the group with PAH associated with congenital heart diseases. This further verified our conjecture that the etiology type is inconsistent between developing and developed countries and highlights a strategic significance for global collaboration in clinical trials.
Several factors that are intrinsic to developing countries add to the complexity of managing patients with PH (19-22). These factors include the late presentation of diseases, multiple comorbidities (e.g., infections, malnutrition), the lack of expertise, the unreliable availability of medications, the cost of care, ‘out-of-pocket’ payment models, limited follow-up, and the absence of multidisciplinary patient units where all services necessary to treat PH patients are available. However, the completion status, clinical trial types, allocations, intervention models, and masking models in developing countries participating in trials are comparable to those in developed countries participating in trials, demonstrating that the quality, homogeneity, reliability, and data authenticity of multinational clinical studies are good. The increase in global clinical trials is conducive to economic relief, regular follow-up, and better treatments for some patients with PH in developing countries and ultimately improving the prognosis.
Children and women in PH clinical trials
Congenital heart disease is a global health issue, especially in children, and 5–10% of patients with congenital heart disease develop PAH (23-25), which is an important cause of morbidity and mortality in children. Approved targeted therapies for the treatment of adults with PAH have been adopted to treat children; however, evidence-based therapeutic strategies for children are lacking and warrant further investigation, as children are affected by many complex factors, including drug dosage, disease tolerance, and parental concern. The enrollment of children is more difficult than that of adults (18-25). Comparatively speaking, more children are enrolled in trials in developed countries with strong economic strength. However, the population base in developing countries is larger, and more pediatric clinical trials in developing countries in the future will also help advance science.
The representation of women in trials relative to the overall disease population reportedly varies from disease to disease, with a higher rate of representation in PH trials (9) because females have a higher prevalence of PH than males (6,26). Our findings were generally consistent with previous publications (9). We observed that the >60 years age group had a lower PPR than all the other age groups, which might have several explanations. Firstly, PAH (especially IPAH) is more common in young women of childbearing age (27,28). Secondly, young patients have much more awareness of opportunities to participate in clinical trials (8). The cultural background, investigators’ communication approach, and written trial materials are all crucial elements that could affect patients’ comfort with enrollment (29). For cardiovascular disease studies worldwide, multi-sponsor trials were more likely to recruit women (9). Globally, the PPR of female patients was highest in Group 1 PH, which is also related to sex skew in this subtype of PH, while that of patients in Group 3 PH (PH due to lung disease and/or hypoxia) was the lowest, which might be due to the higher proportion of smokers among men leading to chronic lung diseases.
Area of uncertainty
Randomized clinical trials have demonstrated that PAH is a pathological condition with clinical manifestations not confined to the lungs. Substantial evidence has documented that coronary artery disease (CAD) occurs 4 times more frequently in PH than in the worldwide population unaffected by this disease, providing a plausible reason to assert a correlation between these two clinical pathological entities (30,31). Several non-randomized studies have observed that many signaling pathways described in PH also play a crucial role in other diseases in which vascular remodeling occurs. For example, both PH and CAD patients develop sustained inflammation and deranged smooth muscle cell proliferation/apoptosis. Therefore, randomized trials aimed at a better understanding between the manifestation of PH and the development of CAD are required both in developed and in developing country.
Limitations
There were some limitations in our study that should be noted. Firstly, our findings were only based on the analyzed results from clinical trials registered at ClinicalTrials.gov instead of all clinical trial platforms. However, ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. Therefore, our findings are general and representative. Secondly, the funding sources were clearly different, which may lead to expanded participation or might be associated with specific legal requirements for participation. However, it is very important to focus on both academic and industry-sponsored studies and provide a detailed description of PH clinical trials. Thirdly, pediatric studies are well known to be challenging (irrespective of where patients and investigators live) and are mostly driven by drug market authorization. Therefore, bias is created due to the small number of patients recruited worldwide. Hence, more studies on the representation of children in PH clinical trials on a larger scale are warranted in the future.
Conclusions
We found that the participation of developing countries in global PH clinical trials was low despite the growing number of trials. There are large gaps between developed and developing countries in children’s representation, diversification of clinical trial types, intervention types, study phases, and sponsor types. Women with PAH were relatively better represented in trials of Group 1 PH, drug intervention trials, phase III studies, and industry-sponsored studies. Importantly, effective strategies to improve the representation of developing countries in PH clinical trials are needed.
Acknowledgments
Funding: The work was supported by the Program of National Natural Science Foundation of China (Nos. 81870042 and 82200065), the Program of Shanghai Pulmonary Hospital (No. FKLY20011), and the Shanghai Pujiang Program (No. 22PJ1410100).
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-701/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-701/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343-9. [Crossref] [PubMed]
- Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996;334:296-301. [Crossref] [PubMed]
- Rubin LJ, Mendoza J, Hood M, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med 1990;112:485-91. [Crossref] [PubMed]
- Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142:448-56. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156-63. [Crossref] [PubMed]
- Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007;132:373-9. [Crossref] [PubMed]
- Zhang R, Dai LZ, Xie WP, et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest 2011;140:301-9. [Crossref] [PubMed]
- Sastry BK, McGoon MD, Gibbs JS. Clinical trials for pulmonary hypertension in the developing world: pulmonary vascular disease: the global perspective. Chest 2010;137:62S-8S. [Crossref] [PubMed]
- Jin X, Chandramouli C, Allocco B, et al. Women's Participation in Cardiovascular Clinical Trials From 2010 to 2017. Circulation 2020;141:540-8. [Crossref] [PubMed]
- U.S. Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. Fed Regist 1993;58:39406-16. [PubMed]
- Organization. WH. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008. Available online: www.who.int/evidence/bod, accessed April 9, 2011.
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Scott PE, Unger EF, Jenkins MR, et al. Participation of Women in Clinical Trials Supporting FDA Approval of Cardiovascular Drugs. J Am Coll Cardiol 2018;71:1960-9. [Crossref] [PubMed]
- Eshera N, Itana H, Zhang L, et al. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA From 2010 to 2012. Am J Ther 2015;22:435-55. [Crossref] [PubMed]
- Kajimoto K, Sato N, Keida T, et al. Association between length of stay, frequency of in-hospital death, and causes of death in Japanese patients with acute heart failure syndromes. Int J Cardiol 2013;168:554-6. [Crossref] [PubMed]
- George MG, Schieb LJ, Ayala C, et al. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest 2014;146:476-95. [Crossref] [PubMed]
- Badesch DB. Clinical trials in pulmonary hypertension. Annu Rev Med 1997;48:399-408. [Crossref] [PubMed]
- Kovacs G, Dumitrescu D, Barner A, et al. Definition, clinical classification and initial diagnosis of pulmonary hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018;272S:11-9. [Crossref] [PubMed]
- Thienemann F, Dzudie A, Mocumbi AO, et al. The causes, treatment, and outcome of pulmonary hypertension in Africa: Insights from the Pan African Pulmonary Hypertension Cohort (PAPUCO) Registry. Int J Cardiol 2016;221:205-11. [Crossref] [PubMed]
- Bigna JJ, Noubiap JJ, Nansseu JR, et al. Prevalence and etiologies of pulmonary hypertension in Africa: a systematic review and meta-analysis. BMC Pulm Med 2017;17:183. [Crossref] [PubMed]
- Stewart S, Wilkinson D, Hansen C, et al. Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation 2008;118:2360-7. [Crossref] [PubMed]
- Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation 2007;115:1039-50. [Crossref] [PubMed]
- Ramjug S, Hussain N, Hurdman J, et al. Pulmonary arterial hypertension associated with congenital heart disease: Comparison of clinical and anatomic-pathophysiologic classification. J Heart Lung Transplant 2016;35:610-8. [Crossref] [PubMed]
- Li L, Zhu X, Chen X, et al. Advances in targeted therapy for pulmonary arterial hypertension in children. Eur J Pediatr 2023;182:2067-76. [Crossref] [PubMed]
- Humbert M, Sitbon O, Yaïci A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010;36:549-55. [Crossref] [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- Athanasiou KA, Sahni S, Rana A, et al. Diagnosing and managing scleroderma-related pulmonary arterial hypertension. JAAPA 2017;30:11-8. [Crossref] [PubMed]
- Rothbard N, Agrawal A, Fischer C, et al. Pulmonary arterial hypertension in the elderly: Clinical perspectives. Cardiol J 2020;27:184-93. [Crossref] [PubMed]
- Meloche J, Lampron MC, Nadeau V, et al. Implication of Inflammation and Epigenetic Readers in Coronary Artery Remodeling in Patients With Pulmonary Arterial Hypertension. Arterioscler Thromb Vasc Biol 2017;37:1513-23. [Crossref] [PubMed]
- Nappi F, Avtaar Singh SS. Distinctive Signs of Disease as Deterrents for the Endothelial Function: A Systematic Review. Metabolites 2023;13:430. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



