
Evidence-based opioid prescribing guidelines after lung resection: a prospective, multicenter analysis
Highlight box
Key findings
• In this statewide, quality improvement study, patients reported using significantly fewer opioids than they were prescribed at discharge including nearly half of patients who required none.
What is known and what is new?
• Implementation of opioid guidelines have significantly decreased overprescribing and post-discharge use; however, limited recommendations exist for general thoracic surgery.
• Patient-reported outcomes and in-hospital data prior to discharge were used to develop opioid prescribing recommendations and then distributed to participating institutions.
What is the implication, and what should change now?
• Evidence-based guidelines should be used to inform opioid prescribing after lung cancer resection.
Introduction
Opioid prescribing is a cornerstone of pain management after surgery. Yet, overprescribing remains a common occurrence with larger prescription sizes associated with higher opioid use as well as an increased risk of new persistent opioid use after surgery (1-4). Moreover, patients undergoing cardiac or general thoracic surgery are among the highest risk populations for new persistent opioid use (2,5-9) despite being among the least likely to be preoperative opioid users (10,11).
In an effort to reduce opioid use and improve patient safety, evidence-based guidelines have been developed to help mitigate overprescribing after surgery (12-17). Such guidelines have been particularly effective for general surgery procedures, many of which are performed in ambulatory or short-stay settings (13,15,17). In contrast, Brescia et al. (12) recently proposed recommendations for opioid prescribing after cardiac surgery where median hospital stay can be over a week (18). The authors showed that a stratified prescribing strategy guided by inpatient opioid use significantly decreased post-discharge opioid use without changes in pain levels and refill rate, an approach which may be similarly beneficial for general thoracic surgery patients. At present, the relationship between in-hospital opioid use and post-discharge use after lung resection is not well understood. Variation in operative approach, including minimally invasive (MIS) versus open thoracotomy, yields a range of post-operative pathways, whereas cardiac surgery patients typically undergo median sternotomy for most operations performed. Recent efforts have thus aimed to better characterize opioid use and prescribing after general thoracic surgery (19-21); however, there is no consensus on appropriate opioid prescription quantities for patients undergoing lung resection.
In this prospective, multicenter study, we utilized a statewide quality collaborative to (I) characterize opioid prescribing and post-discharge use after thoracic surgery, (II) assess patient-reported pain scores after surgery, and (III) evaluate inpatient opioid use and operative approach as predictors of post-discharge opioid use. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1621/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was deemed exempt by the University of Michigan Institutional Review Board (No. HUM00156194), and individual consent for this retrospective analysis was waived.
Data source
Clinical data were collected through the Michigan Society of Thoracic and Cardiovascular Surgeons (MSTCVS) Quality Collaborative. Initially developed in 2001 as a cardiac surgeon-led quality improvement effort embedded within the MSTCVS, it has grown to become a statewide, multidisciplinary, large-scale collaborative that has expanded to now include general thoracic surgery since 2014. The Quality Collaborative data warehouse is composed of standardized harvest files sent from each of the participating sites to the Society of Thoracic Surgeons (STS) national database.
Patient-reported outcomes (PROs) were captured via questionnaires administered at 30-day clinic follow-up. Questionnaire items included (I) post-operative pain scores, (II) quantity and duration of post-discharge opioid use, and (III) pain medication storage and disposal. Data managers at each site also collated information including in-hospital opioid use before discharge, prescription size at discharge, and prescription refills after discharge. All patients receiving an opioid prescription were provided an Opioid Start Talking form and reviewed with a provider prior to discharge. Current state law allows prescription sizes up to 7-days for treatment of acute post-operative pain, where a Michigan Automated Prescription System (MAPS) report is required for prescriptions exceeding a 3-day supply.
Questionnaire responses and opioid prescription and use data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at the MSTCVS Coordinating Center (22). Patient responses and opioid-specific information were then linked to STS data from the Quality Collaborative by unique record identification numbers as well as dates of surgery and discharge.
Study population
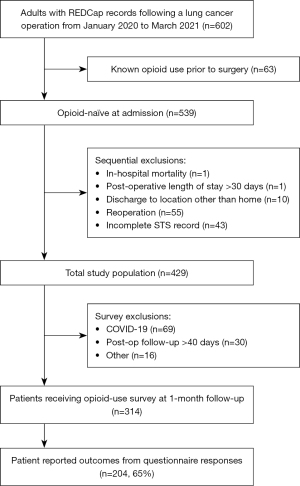
Patients undergoing lung cancer resection between January 2020 to March 2021 were identified across 11 participant centers. Patients taking opioids at the time of admission (i.e., not opioid-naïve) were excluded from this analysis. Additional sequential exclusions included patients undergoing reoperation, postoperative length of stay (LOS) greater than 30 days, discharge to location other than home, in-hospital mortality during the index admission, and patients with an incomplete STS record (Figure 1). Former cigarette smokers were defined as those patients who quit smoking cigarettes greater than 30 days prior to surgery. Major psychiatric disorder refers to a formal psychiatric diagnosis requiring regular behavioral therapy, counseling, and/or pharmaceutical treatment (e.g., depression, anxiety, bipolar disorder).
Statistical analysis
Clinical characteristics and opioid prescription and use data were collected for all patients as described above. Primary outcomes for this study were opioid prescription size and patient-reported opioid use after discharge. Secondary outcomes included in-hospital opioid use day before discharge, prescription refills before follow-up, duration of opioid use after discharge, and patient-reported pain scores. Pain assessment included pain in the first week after surgery (e.g., none, minimal, moderate, or severe) as well as incisional pain and overall body pain for the week immediately prior to follow up (e.g., 0 denoting no pain and 10 denoting worst pain).
Opioid prescription and use quantities were converted to oral morphine equivalents to standardize across individual sites, with the data quantified as the number of 5-mg oxycodone pills and presented as mean ± standard deviation unless otherwise stated. Two-tailed Student’s t-test, nonparametric Wilcoxon rank sum test, and chi-square testing for categorical data were used for comparisons as appropriate with P<0.05 considered to be statistically significant. Cochran-Armitage test was used to detect trends in prescription refill rate. Data analysis was performed using MATLAB (R2021a; MathWorks, Natick, MA).
Results
From the study cohort of 602 patients, there were 539 patients considered to be opioid naïve. Following additional sequential exclusions, the resulting population included 429 patients. Due to staff limitations and follow-up imposed by the COVID-19 pandemic, there were 314 patients who received surveys with a questionnaire response rate of 65%.
Patient demographics
Among the survey responders (n=204), mean age was 68.2±8.7 years, 57.8% female, body mass index (BMI) 29.3±6.6 kg/m2, and predominately Caucasian, 87.7% (Table 1). Hypertension (65.2%), major psychiatric disorder (29.9%), and diabetes (21.6%) were the most common comorbidities. The majority of patients were former cigarette smokers (60.8%) compared to current (22.5%) or never smokers (16.7%). Most patients underwent MIS operations (n=171) versus open thoracotomy (n=20). None of the patients meeting inclusion criteria were discharged with chest tubes remaining in place.
Table 1
| Variable | Overall (n=429) | Responders (n=204) | Non-responders (n=225) | P value |
|---|---|---|---|---|
| Age, years | 67.0±9.1 | 68.2±8.7 | 66.0±9.3 | 0.600 |
| Female | 226 (52.7) | 118 (57.8) | 108 (48.0) | 0.030 |
| BMI, kg/m2 | 28.0±6.1 | 29.3±6.6 | 29.8±5.5 | 0.470 |
| Race | 0.100 | |||
| Caucasian | 382 (89.0) | 190 (87.7) | 192 (86.2) | |
| Black | 36 (8.4) | 16 (7.8) | 20 (8.9) | |
| Race other than Black or Caucasian | 18 (4.2) | 6 (2.9) | 12 (5.3) | |
| Not stated | 4 (0.9) | 3 (1.5) | 1 (0.02) | |
| Diabetes | 81(18.9) | 44 (21.6) | 37 (16.4) | 0.180 |
| Hypertension | 275 (64.1) | 133 (65.2) | 142 (63.1) | 0.650 |
| Cerebrovascular disease | 54 (12.6) | 34 (16.7) | 20 (8.9) | 0.010* |
| Aortic or peripheral vascular disease | 48 (11.2) | 22 (10.8) | 26 (11.6) | 0.810 |
| Congestive heart failure | 22 (5.1) | 15 (7.4) | 7 (3.1) | 0.047* |
| Major psychiatric disorder | 96 (22.4) | 61 (29.9) | 35 (15.6) | <0.001* |
| Smoking status | 0.850 | |||
| Never | 70 (16.3) | 34 (16.7) | 36 (16.0) | |
| Former | 257 (60.0) | 124 (60.8) | 133 (59.1) | |
| Current | 102 (23.8) | 46 (22.5) | 56 (24.9) | |
| Procedure | 0.85 | |||
| Minimally invasive surgery | 356 (83.0) | 171 (83.8) | 185 (82.2) | |
| Open thoracotomy | 44 (10.3) | 20 (9.8) | 24 (10.7) | |
| Not reported | 30 (7.0) | 13 (6.4) | 17 (7.6) | |
| Post-operative length of stay, days | 4.2±3.3 | 3.7±2.8 | 4.7±3.6 | 0.10 |
Data expressed as mean ± standard deviation, or total number (percentage). *, P<0.05. BMI, body mass index.
Among the survey non-responders (n=225), there were fewer female respondents (48.0% vs. 57.8%, P=0.030) and a lower proportion with a history of cerebrovascular disease (8.9% vs. 16.7%, P=0.010), congestive heart failure (3.1% vs. 7.4%, P=0.047), and major psychiatric disorder (15.6% vs. 29.9%, P<0.001). Average post-operative LOS was not significantly different between groups (4.7±3.6 vs. 3.7±2.8 days, P=0.10).
Opioid prescribing and refills
At discharge, 83.4% of patients were provided a prescription for opioids with mean size of 20.5±13.1 pills (Figure S1). Of these patients, 90.0% reported filling their prescription. Patients who were provided a prescription for opioids at discharge were more likely to need a refill before follow up compared with patients who were not prescribed any opioids at discharge (21.5% vs. 12.7%). For patients prescribed opioids at discharge, refill rate was unrelated to the initial prescription size (P=0.748).
Pain scores and duration of opioid use
The majority of patients described their pain as moderate (51.2%) during the first week after surgery compared to none (3.0%), minimal (21.2%), and severe (24.6%). Pain scores for incisional pain and overall body pain during the week immediately prior to follow up were 2.4±2.5 and 3.0±2.8 (scale 0 to 10), respectively. Most patients reported using opioids for less than 2 weeks after discharge with 33.8% reporting <1 week, 36.0% for 1–2 weeks, 12.9% for 2–3 weeks, 6.5% for 3–4 weeks, and 10.8% for >4 weeks. Post-operative, non-narcotic pain adjuncts included PO acetaminophen (91.6%), IV acetaminophen (29.1%), PO nonsteroidal anti-inflammatory drugs (NSAIDs, 17.2%), IV ketorolac (24.2%), and PO gabapentin (48.1%).
Opioid use after discharge
Patient-reported opioid use was 8.2±13.0 pills after discharge (Figure 2), significantly fewer than the 20.5±13.1 pills prescribed at discharge (P<0.001). This included 43.7% of patients who reported using no opioids after discharge. There was no difference in the number of pills used after discharge between those undergoing MIS and open thoracotomy (8.2±13.2 vs. 8.0±11.1 pills, respectively; P=0.84); however, stratification of opioid use into categories of 10 pill increments revealed that a higher proportion of open thoracotomy patients used 11 to 20 pills compared to 1 to 10 pills (P=0.007; Figure S2).
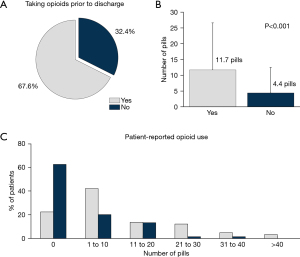
Approximately one-third of patients (32.4%) were not taking opioids in-hospital on the day prior to discharge (Figure 3A). After discharge, these patients used significantly fewer number of pills (4.4±8.1 vs. 11.7±14.9 pills; P<0.001; Figure 3B) and had a higher proportion requiring no opioids after discharge (62.7% vs. 22.6%; Figure 3C), representing a nearly 3-fold increase over those who were taking opioids on the day prior to discharge.
Additional sub-analyses (Appendix 1) were performed to compare the effects of patient demographics and clinical factors on opioid prescribing and post-discharge use (Table S1). Findings included (I) patients undergoing robot-assisted operations used the fewest opioids (5.2±9.7 pills, P=0.004); (II) more than 80% of patients with LOS >7 days required no opioids after discharge, with mean 1.5±4.0 pills; and (III) the type of resection performed was not associated with difference in post-discharge opioid usage (P=0.835).
Discussion
In this assessment of opioid-prescribing practices in our general thoracic surgery statewide quality collaborative, patient-reported post-discharge opioid use was significantly less than discharge opioid prescribing, including nearly half of patients who used no opioids. We found that opioid refill rate was unrelated to prescription size at discharge, while few patients discharged without opioids required a subsequent prescription. In addition, opioid use on the day prior to discharge may be predictive of significantly less opioid use after discharge. Together these findings were used to develop guidelines (Figure 4), as presented here, which can be used to standardize opioid prescribing after lung cancer resection.

Opioid overprescribing after surgery is common and despite therapeutic intent, can serve as the sentinel event for new persistent opioid use (3,4). Thoracic surgery patients are among the highest risk population for new persistent opioid use (5), although among the least likely to be receiving opioids prior to surgery (10). Recent studies (8,9) have suggested rates of new persistent opioid use may even be as high as 14% to 17% after lung resection, highlighting the urgent need for a more targeted approach to opioid prescribing. Opioid prescription sizes in our cohort were nearly 2.5-fold higher than actual patient-reported use, similar to Holst et al. (19) who reported median prescription sizes ranging from 1.5- to 3.5-fold higher than actual opioid consumption. Reducing prescription size at discharge, therefore, represents a high-impact area for improvement.
General surgery has been at the forefront of implementing standardized prescribing guidelines, reducing opioid prescribing by 40–60% across multiple studies (13,15-17). Their effectiveness has provided a template for the development of prescribing recommendations across other surgical subspecialties. In cardiac surgery, Brescia et al. (12) recently reported median prescription size decreased from 20 to 12 pills and median opioid use decreased from 3 to 0 pills with no change in refill rate or pain scores after the implementation of similar prescribing recommendations. These findings demonstrated the feasibility of implementing prescribing guidelines after more invasive procedures (i.e., via sternotomy) compared to the ambulatory or short-stay procedures typical of general surgery (13,16). In comparison, thoracic surgery spans both MIS and open approaches with variable LOS, such that prescribing guidelines must be inclusive of all types of lung resection procedures performed.
In this study, we estimate that a threshold of 15 pills would be sufficient for nearly 80% of the patients in this cohort. Surprisingly, there was no difference in the mean number of pills used after discharge between MIS and open approaches, although a higher proportion of patients undergoing open thoracotomy used 11–20 pills. In addition, we found that patients not requiring any opioids prior to discharge used significantly fewer pills after discharge (4.4±8.1 vs. 11.7±14.9) and were nearly three times more likely to not use any opioids after discharge. Moreover, nearly 70% of all patients used opioids for two weeks or less after discharge, suggesting a relatively short course for the majority of patients after surgery. Based on these data, we recommend a prescription size of 0–5 pills for patients not using opioids on the calendar day prior to discharge, and 0–15 pills for patients after MIS or 0–20 pills for patients after thoracotomy. As not all patient may be discharged with oxycodone, 15 pills correspond to approximately 5 mg of tramadol, 50 mg of hydrocodone, and 7.5 mg of codeine.
Stratifying recommendations based on in-patient use further helps tailor prescribing to promote patient-centered care (12,17). Similarly, providing a range for prescription sizes (e.g., 0–15 pills) discourages one-size-fits-all guidelines that may discount experiences of the individual patient (11), promotes a more holistic approach to post-operative pain control, and allows for additional flexibility in clinical judgement. For example, although type of lung resection was not associated with post-discharge opioid use, patients undergoing robot-assisted procedures may actually use the fewest number of opioids after discharge while more than 80% of patients with LOS greater than 1 week used none. Surgical centers already familiar with stratified prescribing guidelines, as recently developed for cardiac surgery (12), may have already implemented and more readily adopt such recommendations. Nevertheless, in the setting of any clinical practice changes, future work should evaluate for changes in respiratory infection, emergency room visits, and readmissions secondary to inadequate pain control.
These recommendations are consistent with prior findings. Skelhorne-Gross et al. (23) recently described a standardized limited opioid prescription of 15 tablets of 2-mg hydromorphone after general thoracic surgery. Although smaller in size (n=122) with more elective procedures excluding open surgery, they demonstrated adequate pain control and a refill rate of 17% with 54% of patients using no opioids after discharge, comparable to our findings here. In another report, Thiels et al. (14) proposed tiered guidelines with a standard dose of 20 tabs of oxycodone after MIS; however, there was considerable variability among low (0 tabs), standard, and high dose (40 tabs) tiers, with even larger prescription sizes after thoracotomy (low 5 tabs, standard 50 tabs, high 60 tabs). A follow up to this study (19) demonstrated average opioid use after discharge was only 28% to 67% of prescription size using the proposed guidelines. Others have also investigated ERAS (enhanced recovery after surgery) programs that do not routinely prescribe opioids at discharge for minimally-invasive foregut and lung resection procedures (21), with up to 72% of patients not taking opioids at home.
This study has several limitations. First, risk of recall bias is inherent with all studies involving PROs; however, surveys were collected at a fixed interval in the post-operative clinical course to standardize response times. Second, given the challenges presented by the COVID-19 pandemic, not all patients were provided surveys (314 out of 429 patients who met inclusion criteria); however, of those patients, 65% completed the questionnaires, a response rate which is identical to or better than previous studies using similar methodology (12,15). Moreover, the measured characteristics of the responder and non-responder groups were similar, with slightly fewer comorbidities, including major psychiatric disorders, observed for the latter group suggesting the PROs may even over-estimate opioid usage. Third, peri-operative use of pain adjuncts, including intercostal nerve blocks, may vary among different institutions, while benzodiazepine use at the time of admission, as well as prior but not current opioid or non-narcotic treatment of pain syndromes were not tracked as part of this study. Fourth, we do not account for socioeconomic difficulties and geographic populations, which could be associated with differences in obtaining opioid prescriptions, perioperative care, and even genetic variance. Finally, the proposed guidelines do not address pain recommendations for patients taking opioids prior to surgery, those with prolonged LOS, or discharge to location other than home. Nevertheless, these guidelines represent one of the largest, most robust datasets integrating data across 11 geographically diverse surgical centers with multiple surgeons, teams, and approaches, and thus, make recommendations widely generalizable to a variety of practice types.
Conclusions
In summary, this multi-center, quality collaborative study proposes evidence-based opioid prescribing guidelines for patients undergoing lung cancer resection. These stratified recommendations aim to promote patient-centered care and improve safety by decreasing opioid overprescribing after general thoracic surgery.
Acknowledgments
The authors wish to acknowledge essential members of the surgical healthcare team who helped to make this quality improvement effort possible, including Lisa Reiff (Beaumont Royal Oak and Troy), Tara Richter (Ascension Borgess), Christi Bartlett (Henry Ford Allegiance), Anna Gurgul (Henry Ford Detroit), Donna Bonaldi-Swan (Henry Ford Macomb), Alina Vermurlen (McLaren Lansing), Amy Storey (McLaren Port Huron), Elise Hollenbeck (Munson Medical Center), Ruth Raleigh (St. Joe’s Ann Arbor), and Shari Barnett, Judy Miller, and Angela Cloutier (University of Michigan). Support for the MSTCVS Quality Collaborative is provided by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of the BCBSM Value Partnerships program. Although Blue Cross Blue Shield of Michigan and the MSTCVS Quality Collaborative work collaboratively, the opinions, beliefs and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1621/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1621/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1621/coif). RMA reports grants and travel expenses when proctoring for Edwards Lifesciences, Intuitive Surgical, and Ethicon – Johnson & Johnson. JR Martin reports consulting fees and webinars for Terumo. CMB reports consulting fees from Heron Therapeutics, Vertex Pharmaceuticals, Benter Foundation, and Alosa Health, as well as receiving payment for providing expert testimony. ACC previously served as the President of the Michigan Society of Thoracic & Cardiovascular Surgeons. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was deemed exempt by the University of Michigan Institutional Review Board (No. HUM00156194), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howard R, Fry B, Gunaseelan V, et al. Association of Opioid Prescribing With Opioid Consumption After Surgery in Michigan. JAMA Surg 2019;154:e184234. [Crossref] [PubMed]
- Brescia AA, Waljee JF, Hu HM, et al. Impact of Prescribing on New Persistent Opioid Use After Cardiothoracic Surgery. Ann Thorac Surg 2019;108:1107-13. [Crossref] [PubMed]
- Hill MV, McMahon ML, Stucke RS, et al. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg 2017;265:709-14. [Crossref] [PubMed]
- Bartels K, Mayes LM, Dingmann C, et al. Opioid Use and Storage Patterns by Patients after Hospital Discharge following Surgery. PLoS One 2016;11:e0147972. [Crossref] [PubMed]
- Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014;348:g1251. [Crossref] [PubMed]
- Clement KC, Canner JK, Lawton JS, et al. Predictors of new persistent opioid use after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2020;160:954-963.e4. [Crossref] [PubMed]
- Clement KC, Canner JK, Whitman GJR, et al. New Persistent Opioid Use After Aortic and Mitral Valve Surgery in Commercially Insured Patients. Ann Thorac Surg 2020;110:829-35. [Crossref] [PubMed]
- Brescia AA, Harrington CA, Mazurek AA, et al. Factors Associated With New Persistent Opioid Usage After Lung Resection. Ann Thorac Surg 2019;107:363-8. [Crossref] [PubMed]
- Nelson DB, Niu J, Mitchell KG, et al. Persistent Opioid Use Among the Elderly After Lung Resection: A SEER-Medicare Study. Ann Thorac Surg 2020;109:194-202. [Crossref] [PubMed]
- Hilliard PE, Waljee J, Moser S, et al. Prevalence of Preoperative Opioid Use and Characteristics Associated With Opioid Use Among Patients Presenting for Surgery. JAMA Surg 2018;153:929-37. [Crossref] [PubMed]
- Brown LM, Kratz A, Verba S, et al. Pain and Opioid Use After Thoracic Surgery: Where We Are and Where We Need To Go. Ann Thorac Surg 2020;109:1638-45. [Crossref] [PubMed]
- Brescia AA, Clark MJ, Theurer PF, et al. Establishment and Implementation of Evidence-Based Opioid Prescribing Guidelines in Cardiac Surgery. Ann Thorac Surg 2021;112:1176-85. [Crossref] [PubMed]
- Howard R, Waljee J, Brummett C, et al. Reduction in Opioid Prescribing Through Evidence-Based Prescribing Guidelines. JAMA Surg 2018;153:285-7. [Crossref] [PubMed]
- Thiels CA, Ubl DS, Yost KJ, et al. Results of a Prospective, Multicenter Initiative Aimed at Developing Opioid-prescribing Guidelines After Surgery. Ann Surg 2018;268:457-68. [Crossref] [PubMed]
- Vu JV, Howard RA, Gunaseelan V, et al. Statewide Implementation of Postoperative Opioid Prescribing Guidelines. N Engl J Med 2019;381:680-2. [Crossref] [PubMed]
- Lee JS, Howard RA, Klueh MP, et al. The Impact of Education and Prescribing Guidelines on Opioid Prescribing for Breast and Melanoma Procedures. Ann Surg Oncol 2019;26:17-24. [Crossref] [PubMed]
- Hill MV, Stucke RS, Billmeier SE, et al. Guideline for Discharge Opioid Prescriptions after Inpatient General Surgical Procedures. J Am Coll Surg 2018;226:996-1003. [Crossref] [PubMed]
- Fernandez FG, Shahian DM, Kormos R, et al. The Society of Thoracic Surgeons National Database 2019 Annual Report. Ann Thorac Surg 2019;108:1625-32. [Crossref] [PubMed]
- Holst KA, Thiels CA, Ubl DS, et al. Postoperative Opioid Consumption in Thoracic Surgery Patients: How Much Is Actually Used? Ann Thorac Surg 2020;109:1033-9. [Crossref] [PubMed]
- Pommerening MJ, Landau A, Hrebinko K, et al. An analysis of analgesia and opioid prescribing for veterans after thoracic surgery. Sci Rep 2020;10:11348. [Crossref] [PubMed]
- Hodges JD, Nguyen DT, Doan J, et al. Factors associated with home opioid use after thoracic surgery. JTCVS Open 2021;5:173-86. [Crossref] [PubMed]
- Harris PA, Taylor R, Minor BLThe REDCap consortium, et al. Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [Crossref] [PubMed]
- Skelhorne-Gross G, Simone C, Gazala S, et al. A Standardized Minimal Opioid Prescription Post-Thoracic Surgery Provides Adequate Pain Control. Ann Thorac Surg 2022;113:1901-10. [Crossref] [PubMed]



