
Hemoptysis as the presenting manifestation of bronchiectasis-associated hospitalization in Korea
Highlight box
Key findings
• Hemoptysis is associated with a lower risk of short-term mortality compared to infective exacerbation (IE) in bronchiectasis-associated hospitalization.
What is known and what is new?
• Although IE is a major cause of hospitalization in patients with bronchiectasis, hospitalization due to hemoptysis is not uncommon.
• Bronchiectasis patients hospitalized with hemoptysis had a lower bronchiectasis severity and short-term mortality than those with IE.
What is the implication, and what should change now?
• Since bronchiectasis patients hospitalized with hemoptysis had distinct clinical characteristics from those with IE, we should consider this in the management and prognosis prediction in patients with bronchiectasis.
Introduction
Bronchiectasis is a heterogeneous chronic lung disease, characterized by abnormal and permanent dilatation of the airway (1,2). Patients may present with a variety of etiologies, clinical features, and outcomes (3,4). Previous studies reported different characteristics of clinical phenotypes according to the symptoms, microbiological characteristics (3), and frequency of exacerbation (5). Patients with bronchiectasis have chronic respiratory symptoms such as cough and sputum production, and commonly experience exacerbations during the disease course (5,6). The British Thoracic Society (BTS) has defined exacerbation as deterioration with worsening local symptoms (cough; increased amount, purulence, or viscosity change in sputum with or without increasing dyspnea; wheezing; and hemoptysis) and/or systemic distress (2).
Exacerbations of bronchiectasis are associated with poor quality of life (5), deterioration of lung function (7), increased mortality (6), and increased healthcare costs (8), and often require emergency visits and hospitalization (6). Although infective exacerbation (IE) is a major cause of hospital admission in patients with bronchiectasis, hospitalization due to hemoptysis is not uncommon (9). Bronchiectasis, a frequent cause of hemoptysis, can cause varying amount of bleeding, ranging from mild to massive hemoptysis (10). In previous studies that examined data from bronchiectasis cohorts in the United States (11) and France (12), hemoptysis occurred in 22–25% of bronchiectasis patients.
We hypothesized that bronchiectasis patients hospitalized with hemoptysis would present a distinct phenotype, with differences in clinical characteristics compared to those admitted with IE. In this study, we compared the clinical features, radiological findings, and treatment outcomes between hospitalized patients having bronchiectasis with and without hemoptysis. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1541/rc).
Methods
Study design and population
This study was conducted on consecutive patients hospitalized with bronchiectasis-associated conditions at Kyungpook National University Hospital (KNUH), a tertiary referral center located in Daegu, South Korea, between June 2017 and December 2021. Data on baseline characteristics and clinical outcomes were extracted from the electronic medical records. The inclusion criteria were: (I) patients with bronchiectasis diagnosed by computed tomography (CT) scan; and (II) hospitalization due to hemoptysis or clinical symptoms suggestive of respiratory tract infection, including cough, increased sputum volume or change of consistency, increased sputum purulence with or without increasing wheezing, dyspnea, and systemic upset (2). The exclusion criteria were as follows: cystic fibrosis, pulmonary fibrosis with secondary traction bronchiectasis, active tuberculosis (TB), active nontuberculous mycobacterial pulmonary disease, and active thoracic malignancy.
The patients were categorized into two groups: (I) bronchiectasis patients with hemoptysis more than 10 mL of hemoptysis per 24 hours (hemoptysis group), and (II) those with clinical symptoms of respiratory tract infection, with or without less than 10 mL of hemoptysis per 24 hours (IE group). The group of patients who were hospitalized more than once during the study period was determined based on the first episode. Clinical variables, including baseline characteristics, treatment outcomes, radiological findings, and blood laboratory findings, were compared between the hemoptysis and IE groups. Subsequently, we examined the clinical and radiological characteristics associated with hemoptysis in patients who experienced bronchiectasis-associated hospitalization through multiple logistic regression analysis. Next, the enrolled patients were reclassified into survivors and non-survivors according to 30-day mortality; we examined the predictors affecting 30-day mortality in these patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Kyungpook National University Hospital (No. 2022-09-033). Written informed patient consent was not required due to the retrospective nature of the study.
Clinical data
Patient baseline characteristics, including age, sex, smoking history, alcohol consumption, body mass index (BMI), and use of antithrombotic drugs, were reviewed. Data regarding the Eastern Cooperative Oncology Group performance status (ECOG-PS) (13), Charlson Comorbidity Index (CCI) (14), and colonization of Pseudomonas aeruginosa (P. aeruginosa) or other pathogens were also collected. Chronic colonization was defined as the isolation of potential pathogens in sputum culture on two or more occasions in a 1-year period, at least 3 months apart (6). We collected data regarding vital signs, symptoms at presentation, use of mechanical ventilation and vasopressors, and outcome variables, including 30-day mortality, in-hospital mortality, and length of hospital stay. Massive hemoptysis was defined as expectoration of at least 100 mL of blood within a 24-h period (15). The etiology of bronchiectasis was determined in accordance with the BTS guidelines (2). In patients with results of pulmonary function test, which were performed within 12 months before or after hospitalization, forced expiratory volume in 1 second (FEV1) and bronchiectasis severity scores, including Bronchiectasis Severity Index (BSI) (6) and FACED score (16), were measured.
Radiologic and laboratory data
Two chest radiologists (J.P. and J.K.L.) evaluated the number of lobes affected by bronchiectasis (with the lingular segment considered as a separated lobe) and the type of bronchiectasis (tubular, varicose, and cystic) on chest CT scans. Subsequently, the modified Reiff scores indicating radiological severity of bronchiectasis were calculated (6). Furthermore, we evaluated the presence of mycetoma (17), TB-destroyed lung, emphysema, bronchial anthracofibrosis, and bronchial artery hypertrophy (18). Bronchial arteries were deemed hypertrophied when they were measured larger than 2 mm proximally.
Blood laboratory findings included complete blood cell counts, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin, N-terminal of prohormone brain natriuretic peptide (NT-proBNP), albumin, total protein, blood urea nitrogen, creatinine, and sodium.
Statistical analysis
SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) for Windows (Microsoft Corporation, Redmond, WA, USA) was used for statistical analyses. Chi-squared test was used to compare categorical variables, and data were presented as numbers with percentage. Mann-Whitney U test was used to compare continuous variables, and data were shown as medians with interquartile ranges. Statistics were deemed to be significant at P values <0.05. Multiple logistic regression analyses were performed to identify factors associated with hemoptysis and to predict 30-day mortality in patients who experienced bronchiectasis-associated hospitalization. Hosmer-Lemeshow test was used to evaluate the goodness-of-fit for logistic regression models. When performing multiple logistic regression analyses, some continuous variables were changed to categorical variables, and their cut-off values were determined through analysis of the receiver operating characteristic curves with MedCalc version 20.110 (MedCalc Software, Ostend, Belgium).
Results
Baseline and clinical characteristics
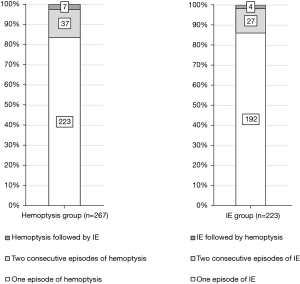
Overall, 490 patients were enrolled in this study. The patients were classified into the hemoptysis [267 (54.5%)] and IE [223 (45.5%)] groups (Table 1). Among the 44 (16.5%) patients in the hemoptysis group who experienced recurrent bronchiectasis-associated hospitalization, 37 (84.1%) experienced hemoptysis and seven (15.9%) experienced IE (Figure 1). Of the 31 IE patients (13.9%) who had recurrent bronchiectasis-associated hospitalization, 27 (87.1%) had IE and 4 (12.9%) had hemoptysis.
Table 1
| Characteristics | Hemoptysis (n=267) | Infective exacerbation (n=223) | P value |
|---|---|---|---|
| Age, years | 66 [59–73] | 72 [64–79] | <0.001 |
| Male sex | 125 (46.8) | 141 (63.2) | <0.001 |
| Smoking | |||
| Ever-smoker | 97 (36.7) | 106 (47.7) | 0.014 |
| Pack-years | 0 [0–20] | 0 [0–30] | 0.010 |
| BMI (kg/m2) | 21.4 [19.1–23.9] | 20.9 [18.7–23.7] | 0.522 |
| Excessive alcohol use | 20 (7.6) | 17 (8.0) | 0.879 |
| ECOG | 1 [1–1] | 2 [1–3] | <0.001 |
| CCI | 0 [0–1] | 1 [0–1] | <0.001 |
| Antithrombotic drugs | 60 (22.5) | 49 (22.0) | 0.895 |
| Colonization | |||
| Pseudomonas aeruginosa | 10 (3.7) | 23 (10.3) | 0.004 |
| Other pathogens | 6 (2.2) | 7 (3.1) | 0.541 |
| Systolic blood pressure, mmHg | 150 [134–167] | 130 [116–151] | <0.001 |
| Pulse rate, /min | 90 [80–102] | 100 [87–111] | <0.001 |
| Respiratory rate, /min | 20 [18–21] | 22 [20–28] | <0.001 |
| Symptoms at presentation | |||
| Duration of symptom, days | 1 [1–3] | 5 [3–7] | <0.001 |
| Cough | 198 (74.2) | 197 (88.3) | <0.001 |
| Sputum production | 144 (53.9) | 194 (87.0) | <0.001 |
| Dyspnea | 101 (37.8) | 171 (76.7) | <0.001 |
| Fever | 22 (8.2) | 120 (53.8) | <0.001 |
| Altered mental status | 3 (1.1) | 12 (5.4) | 0.006 |
| Chest pain | 7 (2.6) | 38 (17.0) | <0.001 |
| Mechanical ventilation | 3 (1.1) | 15 (6.7) | 0.001 |
| Vasopressor infusion | 3 (1.1) | 26 (11.7) | <0.001 |
| Systemic corticosteroids | 75 (28.1) | 85 (38.1) | 0.018 |
| Etiology | |||
| Idiopathic | 90 (33.7) | 84 (37.7) | 0.362 |
| Previous pulmonary TB | 125 (46.8) | 77 (34.5) | 0.006 |
| Previous NTM pulmonary disease | 13 (4.9) | 6 (2.7) | 0.214 |
| Post-infectious | 33 (12.4) | 35 (15.7) | 0.288 |
| Measles | 20 (7.5) | 23 (10.3) | 0.271 |
| Pertussis | 9 (3.4) | 10 (4.5) | 0.525 |
| Unclassified RTI | 4 (1.5) | 2 (0.9) | 0.693 |
| COPD | 5 (1.9) | 11 (4.9) | 0.058 |
| Others† | 1 (0.4) | 10 (4.5) | 0.002 |
| FEV1 (%) (n=195)‡ | 80 [63–97] | 70 [52–87] | 0.015 |
| FACED score (n=195)‡ | 1 [0–3] | 3 [1–3] | <0.001 |
| BSI score (n=195)‡ | 5 [3–8] | 7 [5–11] | <0.001 |
| 30-day mortality | 4 (1.5) | 20 (9.0) | <0.001 |
| In-hospital mortality | 3 (1.1) | 16 (7.2) | 0.001 |
| Length of hospital stay, days | 6 [4–8] | 10 [7–13] | <0.001 |
Data are presented as median [interquartile range] or n (%). †, others include rheumatoid arthritis [0 (0.0%) vs. 6 (2.7%)], Mounier-Kuhn syndrome [0 (0.0%) vs. 1 (0.4%)], diffuse panbronchiolitis [1 (0.4%) vs. 2 (0.9%)], and Kartagener’s syndrome [0 (0.0%) vs. 1 (0.4%)]. ‡, FEV1, FACED score, and BSI score were obtained from 195 patients (74 patients in the hemoptysis group and 121 patients in the infective exacerbation group). BMI, body mass index; CCI, Charlson Comorbidity Index; mMRC, modified Medical Research Council; BAE, bronchial artery embolization; FACED score comprises forced expiratory volume in one second, age, Pseudomonas aeruginosa colonization, radiological extension and dyspnea; BSI, bronchiectasis severity index; FEV1, forced expiratory volume in 1 second; TB, tuberculosis; NTM, nontuberculous mycobacterial; RTI, respiratory tract infection; COPD, chronic obstructive pulmonary disease.
In the hemoptysis group, 89 (33.3%) patients experienced massive hemoptysis and 79 (29.6%) patients underwent bronchial artery embolization. Compared with the IE group, the hemoptysis group was significantly younger and had lower proportions of males and ever-smokers, lower ECOG-PS and CCI, and a lower frequency of P. aeruginosa colonization (Table 1). Regarding etiology, previous pulmonary TB was significantly more common in the hemoptysis group than in the IE group. The hemoptysis group had a significantly higher percent predicted FEV1 and significantly lower disease severity scores, such as FACED and BSI than the IE group. In terms of the treatment outcome, the hemoptysis group had a significantly lower 30-day mortality [4 (1.5%) vs. 20 (9.0%), P<0.001] and in-hospital mortality [3 (1.1%) vs. 16 (7.2%), P=0.001], as well as a significantly shorter length of stay [6 days (4–8 days) vs. 10 days (7–13 days), P<0.001] than the IE group (Table 1).
Radiological and laboratory findings
In the hemoptysis group, the proportion of patients with cystic bronchiectasis, the number of involved lobes, and the modified Reiff score were significantly lower than in the IE group (Table 2). In comparison to the IE group, the hemoptysis group was more likely to have mycetomas and bronchial artery hypertrophy, and less likely to have emphysema. Among the blood laboratory findings, inflammatory markers, including white blood cell count, ESR, CRP, and procalcitonin, and NT-proBNP were significantly lower in the hemoptysis group than in the IE group (Table S1).
Table 2
| CT findings | Hemoptysis (n=267) | Infective exacerbation (n=223) | P value |
|---|---|---|---|
| Cystic bronchiectasis | 94 (35.2) | 124 (55.6) | <0.001 |
| Number of involved lobes | 2 [1–4] | 4 [3–5] | <0.001 |
| Involved lobes ≥3 | 133 (49.8) | 170 (76.2) | <0.001 |
| Modified Reiff score | 3 [2–6] | 6 [3–10] | <0.001 |
| Mycetoma | 39 (14.6) | 10 (4.5) | <0.001 |
| Tuberculosis-destroyed lung | 26 (9.7) | 14 (6.3) | 0.164 |
| Emphysema | 54 (20.2) | 78 (35.0) | <0.001 |
| Bronchial anthracofibrosis | 31 (11.6) | 38 (17.0) | 0.085 |
| Bronchial artery hypertrophy on enhanced CT | 118/257 (45.9) | 64/184 (34.8) | 0.019 |
Data are presented as n (%) or median [interquartile range]. CT, computed tomography.
Risk factors for hemoptysis in patients with bronchiectasis-associated hospitalization
Multiple logistic regression analysis was performed to determine the risk factors for hemoptysis in patients with bronchiectasis-associated hospitalization (Table 3). Clinical and radiological variables with P values <0.05 in the univariate analysis were selected as candidate factors for the multivariable analysis. Previous pulmonary TB [odds ratio (OR) =1.66, 95% confidence interval (CI): 1.03–2.69, P=0.038), mycetoma (OR =3.80, 95% CI: 1.55–9.31, P=0.004), and bronchial artery hypertrophy (OR =3.37, 95% CI: 2.00–5.68, P<0.001) were independently associated with the hemoptysis group. In contrast, male sex, ECOG-PS ≥2, P. aeruginosa colonization, ≥3 involved lobes, cystic bronchiectasis, and emphysema were negatively associated with the hemoptysis group (Hosmer-Lemeshow test, P=0.841).
Table 3
| Variables | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | P value | Odds ratio | 95% confidence interval | P value | ||
| Age | 0.947 | 0.929–0.965 | <0.001 | 0.987 | 0.964–1.011 | 0.294 | |
| Male | 0.512 | 0.356–0.736 | <0.001 | 0.436 | 0.221–0.858 | 0.016 | |
| Ever-smoker | 0.636 | 0.442–0.914 | 0.014 | 1.888 | 0.945–3.772 | 0.072 | |
| ECOG ≥2 | 0.198 | 0.134–0.292 | <0.001 | 0.248 | 0.153–0.401 | <0.001 | |
| CCI ≥1 | 0.511 | 0.355–0.735 | 0.001 | 0.754 | 0.463–1.227 | 0.255 | |
| Pseudomonas aeruginosa colonization | 0.338 | 0.157–0.727 | 0.006 | 0.308 | 0.119–0.795 | 0.015 | |
| Previous pulmonary tuberculosis | 1.669 | 1.157–2.407 | 0.006 | 1.663 | 1.030–2.686 | 0.038 | |
| Involved lobes ≥3 | 0.309 | 0.209–0.457 | <0.001 | 0.328 | 0.195–0.550 | <0.001 | |
| Cystic bronchiectasis | 0.434 | 0.301–0.625 | <0.001 | 0.414 | 0.249–0.689 | 0.001 | |
| Mycetoma | 3.643 | 1.775–7.481 | <0.001 | 3.796 | 1.548–9.312 | 0.004 | |
| Emphysema | 0.471 | 0.314–0.707 | <0.001 | 0.531 | 0.297–0.950 | 0.033 | |
| Bronchial artery hypertrophy | 1.592 | 1.078–2.351 | 0.020 | 3.373 | 2.002–5.684 | <0.001 | |
ECOG, Eastern Cooperative Oncology Group performance status; CCI, Charlson Comorbidity Index.
Predictors of 30-day mortality in patients with bronchiectasis-associated hospitalization
The patients were reclassified as survivors [466 (95.1%)] and non-survivors [24 (4.9%)], according to the results of the 30-day mortality (Table S2). Multiple logistic regression analysis was performed to determine the predictors affecting 30-day mortality in patients with bronchiectasis-associated hospitalization (Table 4). Clinical and radiological variables with P values <0.05 in the univariate analysis were chosen as candidate factors for the multivariable analysis. Along with BMI <18.4 kg/m2, ECOG-PS ≥2, altered mental status, and TB-destroyed lung, the absence of hemoptysis (OR =5.32, 95% CI: 1.27–22.37, P=0.023) was an independent predictor of 30-day mortality in patients with bronchiectasis-associated hospitalization (Hosmer-Lemeshow test, P=0.505).
Table 4
| Variables | Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | P value | Odds ratio | 95% confidence interval | P value | ||
| Age | 1.076 | 1.027–1.127 | 0.002 | 1.023 | 0.964–1.085 | 0.452 | |
| Male | 3.369 | 1.237–9.175 | 0.017 | 1.975 | 0.542–7.191 | 0.302 | |
| BMI <18.4 kg/m2 | 6.062 | 2.428–15.139 | <0.001 | 3.667 | 1.293–10.399 | 0.015 | |
| ECOG ≥2 | 17.637 | 4.098–75.907 | <0.001 | 4.858 | 1.019–23.171 | 0.047 | |
| Sputum production | 11.025 | 1.475–82.409 | 0.019 | 2.852 | 0.331–24.603 | 0.340 | |
| Dyspnea | 9.504 | 2.210–40.880 | 0.002 | 1.291 | 0.232–7.192 | 0.771 | |
| Altered mental status | 12.000 | 3.734–38.567 | <0.001 | 6.221 | 1.440–26.869 | 0.014 | |
| No or minimal hemoptysis | 6.478 | 2.180–19.248 | 0.001 | 5.321 | 1.266–22.369 | 0.023 | |
| Cystic bronchiectasis | 3.202 | 1.303–7.868 | 0.011 | 3.142 | 0.963–10.248 | 0.058 | |
| Tuberculosis-destroyed lung | 6.781 | 2.698–17.042 | <0.001 | 5.766 | 1.642–20.249 | 0.006 | |
| Emphysema | 3.446 | 1.503–7.899 | 0.003 | 1.510 | 0.481–4.739 | 0.481 | |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group performance status.
Discussion
In the present study conducted in Korea, >50% of the patients with bronchiectasis-associated hospitalization were categorized to the hemoptysis group. Patients who initially presented with hemoptysis tended to present with hemoptysis rather than IE at the second hospitalization. The hemoptysis group had a better short-term prognosis and a less severe disease compared with the IE group. The characteristics of the hemoptysis group included a previous pulmonary TB, mycetoma, and bronchial artery hypertrophy. Male sex, ECOG-PS ≥2, P. aeruginosa colonization, ≥3 involved lobes, cystic bronchiectasis, and emphysema were more likely to be associated with the IE group. No or minimal hemoptysis was an independent predictor of 30-day mortality in patients with bronchiectasis-associated hospitalization.
In a previous population-based study using diagnosis codes conducted in the United States, hemoptysis was the most common secondary diagnosis (21%) among hospitalizations with bronchiectasis as a primary diagnosis (19). In a similar study performed in Germany, hemoptysis was a secondary diagnosis in 15% of hospitalizations where bronchiectasis was as the primary diagnosis (20). Compared to the previous studies, the present study showed that a higher percentage of hospitalized patients with bronchiectasis presented with hemoptysis. Since the previous studies were conducted using diagnosis codes, the incidence of hemoptysis may have been underestimated due to the omission or misclassification of diagnosis codes. Furthermore, bronchiectasis is a disease that exhibits geographic variation in etiology, epidemiology, and microbiology (21). In Koreans, TB is a common cause of bronchiectasis (22), which may explain the higher frequency of hemoptysis in our study. Lastly, because the Regional Emergency Medical Center was located at our institution, the possibility that more patients with hemoptysis were transferred for bronchial artery embolization should be considered.
Our findings suggest that hospitalized bronchiectasis patients with hemoptysis have a comparatively lower short-term mortality than the IE group. A previous study reported that in-hospital mortality of hospitalized patients with exacerbation of bronchiectasis was 9% (23). Conversely, in a study of hospitalized patients with bronchiectasis-related hemoptysis, the in-hospital mortality was 4.5% (9). These findings are somewhat consistent with the results of our study, wherein the in-hospital mortality of the hemoptysis group was 1.1%, which was significantly lower than that of the IE group (7.2%). Similarly, the hemoptysis group exhibited a lower 30-day mortality than the IE group.
This study demonstrated that in bronchiectasis-associated hospitalization, patients with hemoptysis had less severe bronchiectasis than those with IE. Our study suggests that hemoptysis phenotype is not associated with severe bronchiectasis, but with specific etiology and radiological findings. In the present study, previous pulmonary TB, mycetoma, and bronchial artery hypertrophy were factors independently associated with the hemoptysis group. In a previous study, patients with post-TB bronchiectasis had more frequent hemoptysis compared to those with other etiologies (24), which is in line with the findings of our study. Mycetoma develops in patients with underlying conditions resulting in chronic lung destruction, such as bronchiectasis (17). Previous studies have reported that bronchiectasis was present in 60–70% of patients with mycetoma, and that hemoptysis is a common complication of mycetoma (25,26). In patients with bronchiectasis and mycetoma, more careful management is needed because inflammation of the tissues surrounding the mycetoma may lead to severe hemoptysis (25). Bronchial artery hypertrophy is one of the various mechanisms of hemoptysis, and is a major cause of hemoptysis in bronchiectasis (18). One previous study showed that in patients with bronchiectasis, hemoptysis occurs more frequently when the diameters of the bronchial arteries increase (27). These characteristics support the findings that patients presenting initially with hemoptysis were more likely to experience recurrent hemoptysis rather than IE.
The present study suggests that patients with IE have a higher risk of 30-day mortality than those with hemoptysis in bronchiectasis-associated hospitalization. Although the characteristics of the IE group, such as poor performance status, may be related to the higher risk of short-term mortality, ‘no or minimal hemoptysis’ in multiple logistic regression analysis was an independent predictor of 30-day mortality. Several previous studies have reported that older age, male sex, lower FEV1, P. aeruginosa colonization, and emphysema were associated with poor long-term prognosis in patients with bronchiectasis (6,23,28,29). In this study, these factors were highly associated with the IE group than with the hemoptysis group. In another study which aimed to determine the risk factors for 5-year mortality in patients with bronchiectasis, the occurrence of hemoptysis was higher in the survivors than the non-survivors (29). Based on these findings, it can be assumed that the long-term mortality of the hemoptysis group might be lower than that of the IE group (29). In previous studies, underweight, poor function status, and altered mental status were found to be associated with an increased risk of short-term mortality in patients with community-acquired pneumonia (30-33). Similarly, these factors may be independently associated with an increased risk of short-term mortality in patients with bronchiectasis exacerbation in the present study. TB-destroyed lung is associated with lung function decline, recurrent exacerbation, respiratory failure, and poor prognosis (34,35). Our study demonstrated that TB-destroyed lung was an independent factor predicting 30-day mortality in patients with bronchiectasis-associated hospitalization.
This study has several limitations which should be mentioned. First, there may be selection bias due to the retrospective, single-center design. In addition, bronchiectasis exhibits geographical differences. The findings may very well not be generalizable to other countries with different causes of bronchiectasis. Thus, a large, multicenter, multinational study is needed to reach a definite conclusion. Second, the possibility that patients were hospitalized at other institutions due to bronchiectasis-associated hospitalization could not be excluded. Third, as the amount of expectorated blood was determined from the medical records, there may be some limitations in assessing hemoptysis severity. Lastly, not all patients underwent spirometry; in particular, smaller proportion of patients in the hemoptysis group underwent spirometry than those in the IE group. For this reason, there may be limitations in comparing the disease severity and lung function between the two groups.
Conclusions
Bronchiectasis patients hospitalized with hemoptysis had a lower bronchiectasis severity and short-term mortality than those with IE. Those with the hemoptysis phenotype are more likely to have previous pulmonary TB, mycetoma, and bronchial artery hypertrophy on CT. Hemoptysis is associated with a lower risk of short-term mortality compared to IE in bronchiectasis-associated hospitalization. Our results demonstrated that the hemoptysis phenotype had distinct clinical characteristics from those with IE, which may be helpful in the management and prognosis prediction in patients with bronchiectasis.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1541/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1541/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1541/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1541/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Kyungpook National University Hospital (No. 2022-09-033). Written informed patient consent was not required due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J 2015;45:1446-62. [Crossref] [PubMed]
- Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019;74:1-69. [Crossref] [PubMed]
- Aliberti S, Lonni S, Dore S, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016;47:1113-22. [Crossref] [PubMed]
- Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018;392:880-90. [Crossref] [PubMed]
- Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the "Frequent Exacerbator Phenotype" in Bronchiectasis. Am J Respir Crit Care Med 2018;197:1410-20. [Crossref] [PubMed]
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. [Crossref] [PubMed]
- Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007;132:1565-72. [Crossref] [PubMed]
- de la Rosa D, Martínez-Garcia MA, Olveira C, et al. Annual direct medical costs of bronchiectasis treatment: Impact of severity, exacerbations, chronic bronchial colonization and chronic obstructive pulmonary disease coexistence. Chron Respir Dis 2016;13:361-71. [Crossref] [PubMed]
- Lim RK, Tremblay A, Lu S, et al. Evaluating hemoptysis hospitalizations among patients with bronchiectasis in the United States: a population-based cohort study. BMC Pulm Med 2021;21:392. [Crossref] [PubMed]
- Mondoni M, Carlucci P, Job S, et al. Observational, multicentre study on the epidemiology of haemoptysis. Eur Respir J 2018;51:1701813. [Crossref] [PubMed]
- Aksamit TR, O'Donnell AE, Barker A, et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017;151:982-92. [Crossref] [PubMed]
- Buscot M, Pottier H, Marquette CH, et al. Phenotyping Adults with Non-Cystic Fibrosis Bronchiectasis: A 10-Year Cohort Study in a French Regional University Hospital Center. Respiration 2016;92:1-8. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J 2008;32:1131-2. [Crossref] [PubMed]
- Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014;43:1357-67. [Crossref] [PubMed]
- Chabi ML, Goracci A, Roche N, et al. Pulmonary aspergillosis. Diagn Interv Imaging 2015;96:435-42. [Crossref] [PubMed]
- Marquis KM, Raptis CA, Rajput MZ, et al. CT for Evaluation of Hemoptysis. Radiographics 2021;41:742-61. [Crossref] [PubMed]
- Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest 2010;138:944-9. [Crossref] [PubMed]
- Ringshausen FC, de Roux A, Pletz MW, et al. Bronchiectasis-associated hospitalizations in Germany, 2005-2011: a population-based study of disease burden and trends. PLoS One 2013;8:e71109. [Crossref] [PubMed]
- Chandrasekaran R, Mac Aogáin M, Chalmers JD, et al. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med 2018;18:83. [Crossref] [PubMed]
- Lee H, Choi H, Chalmers JD, et al. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology 2021;26:619-21. [Crossref] [PubMed]
- Finklea JD, Khan G, Thomas S, et al. Predictors of mortality in hospitalized patients with acute exacerbation of bronchiectasis. Respir Med 2010;104:816-21. [Crossref] [PubMed]
- Fong I, Low TB, Yii A. Characterisation of the post-tuberculous phenotype of bronchiectasis: A real-world observational study. Chron Respir Dis 2022;19:14799731221098714. [Crossref] [PubMed]
- Lee JK, Lee YJ, Park SS, et al. Clinical course and prognostic factors of pulmonary aspergilloma. Respirology 2014;19:1066-72. [Crossref] [PubMed]
- Kim TH, Koo HJ, Lim CM, et al. Risk factors of severe hemoptysis in patients with fungus ball. J Thorac Dis 2019;11:4249-57. [Crossref] [PubMed]
- Kosar M, Kurt A, Keskin S, et al. Evaluation of effects of bronchiectasis on bronchial artery diameter with multidetector computed tomography. Acta Radiol 2014;55:171-8. [Crossref] [PubMed]
- Goeminne PC, Nawrot TS, Ruttens D, et al. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014;108:287-96. [Crossref] [PubMed]
- Tang X, Bi J, Yang D, et al. Emphysema is an independent risk factor for 5-year mortality in patients with bronchiectasis. Clin Respir J 2017;11:887-94. [Crossref] [PubMed]
- Lee J, Kim K, Jo YH, et al. Severe thinness is associated with mortality in patients with community-acquired pneumonia: a prospective observational study. Am J Emerg Med 2015;33:209-13. [Crossref] [PubMed]
- Corrales-Medina VF, Valayam J, Serpa JA, et al. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis 2011;15:e54-7. [Crossref] [PubMed]
- Jeon K, Yoo H, Jeong BH, et al. Functional status and mortality prediction in community-acquired pneumonia. Respirology 2017;22:1400-6. [Crossref] [PubMed]
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377-82. [Crossref] [PubMed]
- Ryu YJ, Lee JH, Chun EM, et al. Clinical outcomes and prognostic factors in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis 2011;15:246-50. i. [PubMed]
- Rhee CK, Yoo KH, Lee JH, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis 2013;17:67-75. [Crossref] [PubMed]


