
The determination of pleurodesis using sonography after surgical pleurectomy for pneumothorax: a pilot study
Introduction
The hospital length of stay after surgical pleurodesis (SP) for pneumothorax is generally determined by the need for chest tube drainage (1). The optimal drainage duration is unclear and obviously differs between patients. Our local hospital protocol uses a chest tube drainage duration of at least 72 hours, theoretically allowing pleural adhesions to develop and reducing the recurrence of pneumothorax.
Animal studies have shown that ultrasonographic pleurodesis, i.e., absence of lung sliding, correlates with pleurodesis scores measured during autopsy (r=0.55–0.81) (2,3). The average duration till measurable ultrasonographic pleurodesis was 3 days in rabbits, with ultrasonographic evaluation performed on days 0, 1, 3, 14, 21 (3). It is unclear if this is comparable with human ultrasonographic pleurodesis.
Monitoring the formation of adhesions indirectly by assessing pleural movement with thoracic ultrasound (TUS) could provide useful information on the extent and success of pleurodesis.
The aim of this study is to evaluate the development of ultrasonographic pleurodesis after SP. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-116/rc).
Methods
Study design
We conducted an exploratory prospective single-center cohort study in Isala Hospital, Zwolle, the Netherlands. Patient aged ≥18 years undergoing complete SP after pneumothorax were eligible. Excluded were patients with concomitant pneumonia or pleural empyema, with previous ipsilateral pleurodesis, either chemical or surgical, and those with persistent (partial) pneumothorax after surgery (as evaluated with standard post-operative imaging). All patients were treated according to the local treatment-protocol with chest tube drainage for 72 hours digital drainage system (Thopaz Chest Drain System, Medela, Switzerland), with a standard drainage pressure of −8 cmH2O. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Isala Hospital (No. 200127). Written informed consent was obtained for all patients.
Primary outcome: pleural assessment
Ultrasonographic pleurodesis was assessed with bedside TUS examination of the affected hemi-thorax and pleura each consecutive day after surgery, until discharge or complete ultrasonographic pleurodesis. TUS was performed using the Sparq Ultrasound System (Philips Healthcare, Andover, MA, USA) with a 12 MHz linear array transducer. The pleura was assessed in six locations by using the anterior and posterior axillary lines as anatomical landmarks, three areas per hemithorax (anterior, lateral, and posterior) were identified, and each area was divided in two, superior and inferior (Figure 1). This methodology was previously applied in ventilated critically ill patients (4).
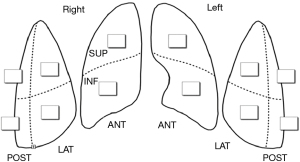
The primary outcome parameters were lung sliding and pleural thickening. Both were graded according to a modified system obtained from two previous studies (Table 1 and Video 1) (2,5). The sum of the scores of each examined thoracic region (Figure 1), was divided by the number of examined sites. Ideally, all six regions were examined. TUS was performed by one of two investigators with training and experience in ultrasound of the pleura. All images were labeled and stored for later scoring by the operator, and a second observer blinded to the scoring of the operator.
Table 1
| Grade | Lung sliding | Pleural thickening |
|---|---|---|
| 0 | Lung sliding present | Thickness conform baseline |
| 1 | Lung sliding questionable | Pleural thickening questionable |
| 2 | Lung sliding absent | Pleural thickening present |
Secondary outcomes
Secondary outcomes were number of recurrent ipsilateral pneumothoraces, duration of chest tube drainage, hospital length of stay, and interrater reliability of ultrasonographic pleurodesis grading.
Statistical analysis
Statistical analyses were performed by using SPSS statistics 27.0 software. Categorical data were presented as n (%), and continuous data were presented as mean ± standard deviation (SD) or median (range), depending on the distribution. For analyses of the primary endpoint, ultrasonographic pleurodesis, descriptive statistics and 95% confidence intervals are supplied. Other data were analyzed using paired samples t-test, Wilcoxon signed rank test or Mann-Whitney U test depending on data distribution and paired or unpaired data. P values less than 0.05 were considered significant.
Interrater reliability was analyzed by using the intraclass correlation coefficient (ICC) with a two-way mixed effects model and an absolute agreement definition for single measurements: values less than 0.5, between 0.5–0.75, between 0.75–0.9 and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively, all based on 95% confidence interval of the ICC estimate (6).
Results
Between February 2020 and September 2021, 14 patients were included shortly after admission or decision for intended surgical pleurodesis. Baseline characteristics are depicted in Table 2 (see Table S1 for individual patient characteristics).
Table 2
| Characteristic | Values (n=14) |
|---|---|
| Median age in years (range) | 29 (18 to 66) |
| Female, n [%] | 4 [29] |
| Smoking, n [%] | |
| Never | 7 [50] |
| Current | 4 [29] |
| Former | 3 [21] |
| Comorbidities, n [%] | |
| Diabetes mellitus | 1 [7] |
| Hypertension | 2 [14] |
| Chronic lung disease (asthma/COPD) | 3 [21] |
| Median number of ipsilateral pneumothoraxes (range) | 2 (1 to 3) |
| Median number of days from surgical pleurodesis till discharge (range) | 4 (3 to 11) |
COPD, chronic obstructive pulmonary disease.
Primary outcome
Mean lung sliding was 0.25±0.26 on day 1, 0.39±0.48 on day 2, 0.84±0.49 on day 3, and 1.12±0.56 on day 4. Mean pleural thickening was 1.0±0.56 on day 1, 1.17±0.48 on day 2, 1.44±0.44 on day 3, and 1.54±0.34 on day 4. Only two patients could be assessed on day 5, with a median lung sliding and pleural thickening of 1.63 (1.50–1.75) and 1.63 (1.50–1.75), respectively (Figure 2). Lung sliding decreased and pleural thickening increased significantly between day 1 and day 4 (P=0.002 and P=0.023, respectively). Before discharge, 1 (7%) patient reached the maximum achievable grade for lung sliding, and 3 (21%) patients for pleural thickening. Three (21%) patients reached ≥80% of the maximum achievable grade for lung sliding, and 9 (64%) patients for pleural thickening.

Secondary outcomes
Three patients had a recurrent ipsilateral pneumothorax, with a mean maximum observed lung sliding of 0.6±0.5, and a mean maximum observed pleural thickening of 1.3±0.6. This was not significantly lower than the rest of the group, 1.2±0.6 and 1.7±0.2, respectively (P=0.149 and P=0.103, respectively). One recurrent pneumothorax was found during TUS examination for this study (participant number 6, Table S1).
Median duration of chest tube drainage after surgery was 4 days (range, 3–11). Median duration of hospital stay after surgery was 3.5 days (range, 3–15), with one patient being discharged with ambulant chest tube drainage.
Interrater reliability was considered excellent for lung sliding and good for pleural thickening, with an ICC for single measures of 0.866 (lower bound 0.777, upper bound 0.921) and 0.784 (lower bound 0.651, upper bound 0.871), respectively.
Discussion
The results of this study show a significant increase in TUS grading for lung sliding and pleural thickening during the first postoperative days after SP, probably attributable to progressing pleurodesis. However, in general, complete pleurodesis on all examination sites was not reached before discharge, despite complete pleurectomy. Interestingly, lung sliding and pleural thickening grades tended to be lower in patients with recurrent pneumothorax during follow-up, although this was not statistically significant due to the small groups.
Previously, the evaluation of whether a pleurodesis was realized was indirectly concluded when there was a lack of recurrence of pleural fluid or pneumothorax (7). Other imaging modalities such as chest radiographs and computed tomography may identify pleural thickening and pleural calcification, although a fusion of visceral and parietal pleura, the hallmark of pleurodesis, remains uncertain (8). Animal studies have demonstrated the accuracy of ultrasound as a non-invasive modality for the evaluation of pleurodesis. The absence of lung sliding on ultrasound correlates well with the presence of an anatomical pleurodesis (2,3).
A recent study showed that ultrasound guided care for talc pleurodesis in patients with malignant pleural effusion resulted in shorter hospital length of stay, without reducing success rate (9). This study confirms the usefulness of sonographic evaluation of pleurodesis after surgery with less ultrasound assessment locations. The results of this study might be a starting point for further research on the clinical implementation of TUS in the treatment of pneumothorax. Pneumothorax is a common clinical problem and treatment has barely changed in the last decades, however academic efforts are growing to ameliorate pneumothorax management. The advancing integration of TUS in pulmonologic practice results in search for wider applications. Monitoring pleurodesis might be one of them and is an easy performable and broadly accessible diagnostic modality. Generally, preventative treatment options for recurrent pneumothorax attempt to create pleurodesis, and, if feasible, resection of blebs and bullae. This study shows that TUS is feasible for evaluating pleurodesis. TUS guidance raises the opportunity for patient tailored approaches, although it is unclear if TUS monitoring decreases hospital length of stay, or drainage time, or whether it influences recurrence rate.
In this study, sonographic pleurodesis grades tended to be lower in patients with recurrent pneumothorax. If confirmed in larger studies, longitudinal post-operative TUS assessment may identify patients at risk for ineffective pleurodesis and may even allow for additional methods of inducing pleurodesis while chest tubes are still in place, potentially reducing the incidence of recurrent pneumothorax. Furthermore, TUS assessment may prove a means to evaluate effectiveness of various sclerosing agents or techniques for inducing pleurodesis.
This study has several limitations. The number of patients studied was small and follow-up was short due to the discharge of participants. Furthermore, ultrasound is a technique known for interobserver variability although the interrater reliability was high in this study (6).
Conclusions
This study supports the feasibility to monitor pleurodesis after surgical pleurectomy, by performing repetitive TUS for pleural sliding and thickening trends. Future research could focus on TUS implementation and its effects on timing of chest tube removal and recurrence rate.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-116/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-116/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-116/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dearden AS, Sammon PM, Matthew EF. In patients undergoing video-assisted thoracic surgery for pleurodesis in primary spontaneous pneumothorax, how long should chest drains remain in place prior to safe removal and subsequent discharge from hospital? Interact Cardiovasc Thorac Surg 2013;16:686-91. [Crossref] [PubMed]
- Zhu Z, Donnelly E, Dikensoy O, et al. Efficacy of ultrasound in the diagnosis of pleurodesis in rabbits. Chest 2005;128:934-9. [Crossref] [PubMed]
- Tazi-Mezalek R, Frankel D, Fortin M, et al. Chest ultrasonography to assess the kinetics and efficacy of talc pleurodesis in a model of pneumothorax: an experimental animal study. ERJ Open Res 2018;4:00158-2017. [Crossref] [PubMed]
- Bouhemad B, Mongodi S, Via G, et al. Ultrasound for "lung monitoring" of ventilated patients. Anesthesiology 2015;122:437-47. [Crossref] [PubMed]
- Corcoran JP, Hallifax RJ, Mercer RM, et al. Thoracic Ultrasound as an Early Predictor of Pleurodesis Success in Malignant Pleural Effusion. Chest 2018;154:1115-20. [Crossref] [PubMed]
- Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016;15:155-63. [Crossref] [PubMed]
- Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Webb WR, Higgins CB. Thoracic imaging: pulmonary and cardiovascular radiology. Philadelphia, PA: Lippincott, Williams and Wilkins; 2004.
- Psallidas I, Hassan M, Yousuf A, et al. Role of thoracic ultrasonography in pleurodesis pathways for malignant pleural effusions (SIMPLE): an open-label, randomised controlled trial. Lancet Respir Med 2022;10:139-48. [Crossref] [PubMed]


