
Safety and feasibility of oesophageal ultrasound for the work-up of thoracic malignancy in patients with respiratory impairment
Introduction
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) are recommended for accessing mediastinal lymph nodes and centrally located lung tumours in patients suspected of lung cancer (1). These structures can also be biopsied with the use of FNA with the EBUS-endoscope via the oesophagus (EUS-B-FNA) (1-4).
EUS-B-FNA gives access to several locations both in thorax and below the diaphragm (5-8).
The safety of EBUS-TBNA/EUS-(B)-FNA is generally high with adverse events (AEs) reported in less than 1% (9), and no serious complications have been described (10). Obviously, some complications may occur (9,11,12) for example respiratory failure in relation to EBUS-TBNA (13). Thus, pre-existing respiratory failure in patients needing endobronchial biopsy may be a clinical challenge (14). The benefit of EUS-B-FNA in these patients has been described in case reports and series (15-18) and as a subgroup of 18 patients in a recent large retrospective study (19).
Furthermore, EUS-B-FNA was associated with less frequent events of oxygen desaturation compared with EBUS-TBNA in a randomized study of patients without pre-existing respiratory impairment (20) with similar results in a retrospective study (5). However, there is a paucity of prospective data with systematic reporting of how patients with respiratory impairment tolerate EUS-B-FNA. Therefore, we assessed the safety and feasibility and of the EUS-B-FNA procedure in these patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1705/rc).
Methods
Study design and patients
The study was a prospective observational feasibility study, following the STROBE checklist, performed in three Danish centres (Zealand University Hospitals, Roskilde and Naestved, and Odense University Hospital).
Consecutive patients admitted for diagnostic work-up of tumours (lung lesions or thoracic lymph nodes) suspected of malignancy were considered eligible if: (I) lesion located adjacent to the oesophagus; (II) EUS-B-FNA of the tumour was considered (by the operator) the least and only invasive procedure to provide diagnostic clarification; (III) respiratory impairment at referral [defined as either peripheral oxygen saturation (SpO2) level of ≤90%, requirement of continuous oxygen supply to maintain SpO2 >90%, or modified Medical Research Council (mMRC) dyspnoea scale score of ≥3]; and (IV) local tumour board advised a biopsy to diagnose potentially treatable conditions.
Outcomes
Primary outcome: proportion of patients with procedure-related AEs either during the procedure, recovery (1-hour post-procedure), or 30-day follow-up.
Secondary outcomes: diagnostic yield.
The EUS-B procedure
We used a flexible ultrasound bronchoscope in combination with a linear ultrasound scanning transducer (Olympus BF-UC 190-F or UC 180-F) 19-, 21- or 22-G needles were used for aspirations (21- or 22-G Olympus ViziShot or 19-G ViziShot Flex; all; Olympus Medical Systems Europe, Ltd., Hamburg, Germany).
The patient was in a supine position and in conscious sedation with midazolam and if indicated additional fentanyl/sufentanil. An oral approach was chosen if a nasal approach was not possible. Topical xylocaine 2% was applied in nostrils or on the mouthpiece and on the endoscope tip. At a few centimetres above the vocal cords the endoscope was slightly retracted, turned left and posteriorly before advancing carefully without pressure into the oesophagus while encouraging the patient to swallow. Either a structured (4,21) or targeted approach was used depending on the clinical situation. At least two FNA passes were made from each tumour in order to produce both smears and a coagulum. Rapid on-site evaluation was not available. All aspirates were processed for both cytological smears and cellblock analysis.
The procedures were performed by eight different operators all with several years’ experience both in the EUS-B and EBUS procedure. No prophylactic antibiotics were used.
Patient safety monitoring
Procedure time, medications used, oxygen supply and vital signs were recorded prospectively (22):
- Pre-procedure (5–10 min before): respiratory rate (RR), oxygen saturation (SaO2; pulse-oximetry), oxygen supply (flow in L/min), blood pressure (BP), heart rate (HR);
- During the procedure: SaO2, oxygen supply (L/min), HR (3-lead electrocardiogram) were monitored continuously and recorded every 5 min, whereas RR and BP were measured at least every 5 min;
- Post-procedure (at 5, 10, and 30 min): RR, SaO2, oxygen supply (L/min), BP, HR;
- Recovery period (at 30–60 min post-procedure): vital parameters only recorded if an intervention was needed.
Nasal oxygen supply was not administered routinely but added/increased if SaO2 decreased below basis level, see above.
We recorded the following from the electronic patient medical charts at day 30: post-procedure related events, 30-day mortality, final cytopathological diagnosis and follow-up.
Definitions of events
AEs during the procedure were defined as any of the following: decline in SpO2 to <90% (or further declines below baseline if baseline was below 90%) during >30 s or increase in oxygen supply (hypoxemia); change >20% from baseline in RR, HR, or BP requiring intervention (23,24); and any other episode requiring intervention such as, but not limited to, pain or bleeding.
Severe AEs (SAEs): escalation of care level, need for backup from anaesthesiologist on call, or respiratory or cardiac arrest.
Transient changes of >20% in vital parameters (RR, HR, or BP) from baseline not requiring intervention were recorded but not considered as AE/SAE.
AEs during recovery (1-hour post-procedure): any AE or SAE, as mentioned above, including pain with need of analgesics assessed to be related to the procedure.
Late AEs (after recovery to 30 days after procedure) were retrieved from electronic patient medical charts: any episode requiring treatment, re-admission or prolonged admission judged to be procedure-related by treating doctor or investigators such as, but not limited to, pneumothorax, respiratory infection, or mediastinitis.
Definitions of adequacy and diagnostic yield
Sample adequacy: sufficient material for cytopathological evaluation, and for lymph node samples either presence of lymphocytes or malignant cells.
Samples with a non-malignant diagnosis without confirmation or follow-up were considered false-negative.
Diagnostic yield: (number of true positive samples)/(total number of samples).
Ethics
The study did not include any interventions or randomization. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the regional scientific ethics system and the Danish Data Protection Agency (ID numbers: SJ-803 and REG-105-2019). All patients gave their verbal and written consent to participate.
Statistics
A sample size of 16 patients was considered sufficient to assess feasibility and most data is presented at a descriptive level (23,25) as mean with standard deviation for normally distributed continuous variables, or as median and interquartile range (IQR) for variables with a skewed distribution. For comparison of related variables, a Friedman’s test was performed. Significant differences were further analysed pairwise using Wilcoxon signed ranks test. A P value of ≤0.05 was considered significant. Analyses were performed using SPSS software version 27 (IBM®, Armonk, NY, USA).
Results
Patients
We included 16 patients between 1 April 2020 and 30 January 2021. Eight hospitalized patients continued hospitalization after the procedure, and eight out-patients were discharged after the procedure. Table 1 shows that respiratory impairment was completely or partially caused by tumour-compression in the majority (n=9, 56%). Severe dyspnoea (mMRC ≥3) was common (n=14, 88%) and supplementary oxygen was needed prior to EUS-B by 9 (56%) increasing to 14 (88%) patients during the procedure. Fourteen patients (88%) were diagnosed with malignancy, and twelve with primary lung cancer. Lung cancer stage varied between IIB and IV with seven patients having stage IV disease.
Table 1
| Variables | Value |
|---|---|
| Patients, n | 16 |
| Age (years), mean ± SD | 73.1±8.4 |
| Sex (female) (%) | 56.3 |
| mMRC dyspnoea scale score, n (%) | |
| 0 | 0 (0.0) |
| 1 | 1 (6.3) |
| 2 | 1 (6.3) |
| 3 | 8 (50.0) |
| 4 | 6 (37.5) |
| ECOG performance status, n (%) | |
| 0 | 2 (12.5) |
| 1 | 4 (25.0) |
| 2 | 2 (12.5) |
| 3 | 8 (50.0) |
| FEV1 (% of expected), mean ± SD | 37.9±12.5 |
| NA, n | 4 |
| Cardiopulmonary comorbidities, n (%) | |
| COPD | 10 (62.5) |
| Interstitial lung disease | 1 (6.3) |
| Cardiac insufficiency | 2 (12.5) |
| Main reason for respiratory impairment, n (%) | |
| Subacute tumour related | 5 (31.3) |
| Chronic respiratory failure | 7 (43.8) |
| Both | 4 (25.0) |
| Final diagnosis, n (%) | |
| Malignant—primary pulmonary | |
| Adenocarcinoma | 4 (25.0) |
| Squamous cell carcinoma | 3 (18.8) |
| Small cell lung cancer | 3 (18.8) |
| Non-small cell lung cancer NOS | 2 (12.5) |
| Malignant—other | |
| Lymphoma | 1 (6.2) |
| Metastasis, anal cancer | 1 (6.2) |
| Non-malignant | |
| Sarcoidosis | 1 (6.2) |
| Non-specific | 1 (6.2) |
SD, standard deviation; mMRC, modified Medical Research Council; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; NA, not available; COPD, chronic obstructive pulmonary disease; NOS, not otherwise specified.
The biopsied lesions included mediastinal lymph nodes, mediastinal tumour and intrapulmonary tumours with mediastinal invasion or pleural contact (Table 2).
Table 2
| Procedure related information | Value |
|---|---|
| Completed procedure, n (%) | 16 (100.0) |
| Procedure time† (min), median [IQR] | 15 [10–20] |
| Time to discharge (min), median [IQR] | 76 [57–83] |
| Structures biopsied‡, n | |
| Tumour | |
| Mediastinal | 3 |
| Intrapulmonary (LLL and RUL) | 3 |
| Mediastinal lymph nodes§ | 11 |
| Structures biopsied pr procedure, median [range] | 1 [1–3] |
| Medications during procedure | |
| Midazolam, n (%) | 15 (93.8) |
| Median dose (range) (mg) | 2.0 (1.1–2.9) |
| Fentanyl, n (%) | 3 (18.8) |
| Median dose (range) (μg) | 25.0 (12.5–25.0) |
| Sufentanil, n (%) | 5 (31.3) |
| Median dose (range) (μg) | 5.0 (2.5–5.0) |
| Other medications during procedure, n (%) | 0 (0.0) |
| Medications after procedure, n (%) | |
| Vaporized short-acting bronchodilators | 1 (6.3) |
| Flumazinil | 2 (12.5) |
†, time from insertion to removal of the endoscope; ‡, number exceeds total number of patients, as one patient had biopsied from both; §, lymph node stations: 4L, 4R, 5, 7 and 8. IQR, interquartile range; LLL, left lower lobe; RUL, right upper lobe.
Safety during procedure and recovery
Table 3 shows median oxygen supply and SaO2 during procedure and recovery. We observed significant changes in oxygen supply—but not in median vital parameters—with higher oxygen supply during EUS-B compared with before or during recovery (Wilcoxon signed ranks test, post-hoc pairwise comparison, P values <0.05) (Table S1). Episodes with vital parameter changes >20% were common: observed in 88% (n=14) during and in 63% (n=10) after the procedure (Table S2).
Table 3
| Observations | Oxygen supply† (L/min), median [IQR] | SaO2 (%), median [IQR] | Number of measurements |
|---|---|---|---|
| Baseline | 1.5 [0.0–3.0] | 94 [89–94] | 16 |
| During procedure | |||
| 5 min | 3.0 [2.0–4.0] | 94 [91–96] | 16 |
| 10 min | 3.0 [2.0–4.0] | 94 [90–98] | 16 |
| 15 min | 2.0 [2.0–3.5] | 96 [94–100] | 9 |
| 20 min | 3.0 [2.0–4.0] | 97 [94–99] | 5 |
| 25 min | 4.0 | 96 | 1 |
| Post-procedure | |||
| 5 min | 2.0 [0.0–3.8] | 94 [91–98] | 16 |
| 10 min | 2.0 [0.0–3.0] | 96 [92–98] | 16 |
| 30 min | 2.0 [0.0–3.0] | 94 [92–97] | 16 |
†, nasal cannula. IQR, interquartile range; SaO2, oxygen saturation.
Primary endpoints
AEs
No SAEs were observed. During the procedure, 12 AEs occurred in 8 (50%) patients, predominantly hypoxemic events (Table 4). All AEs happened within the first 15 min of the procedures, and all were managed satisfactorily by the endoscopy team. Eight cases of hypoxemia were treated with increased oxygen supply alone. Two cases of hypoxemia in one patient, secondarily to breath holding triggered by nausea and gag reflex, were treated with oxygen supply and continuous breathing instructions by the endoscopist. The procedure was successfully completed as the patient did not vomit or aspirate. One patient with hypoxemia and decreased systolic BP was treated with oxygen supply and intravenous flumazenil. One patient with decreasing RR during the procedure and post-procedural somnolence was treated with intravenous flumazenil alone.
Table 4
| AEs | During procedure | Post-procedure† |
|---|---|---|
| Total number of AEs, n | 12 | 3 |
| Hypoxemia | 10 | 3 |
| Decreased systolic BP | 1 | 0 |
| Decreased RR and somnolence | 1 | 0 |
| Procedures with AEs, n (%) | 8 (50.0) | 2 (12.5) |
| SAEs, n | 0 | 0 |
†, within 1-hour post-procedure. AE, adverse events; BP, blood pressure; RR, respiratory rate; SAE, severe AE.
Of the eight patients with AEs, three were hospitalized prior to, and after the procedure.
During the 1-hour post-procedure recovery, three AEs were observed in 2 (13%) patients—all were hypoxemic events that were treated by the endoscopy team (Table 4). Two cases (onset within 30 min) were treated with increased oxygen supply. One case (onset after 30 min) was treated with oxygen supply and inhalation of vaporized short-acting bronchodilators. All but one patient had returned to pre-procedure condition 60 min after ended EUS-B.
30-day follow-up
A total of five events were identified (one prolonged need of oxygen supply, one infection, one intensive care unit (ICU) admission, two hospitalizations) however only the first two were considered as likely procedure-related by treating physicians and research group. Thus, proportion of procedure-related AEs was 13% (2/16).
Likely EUS-B-related events
Prolonged oxygen treatment: one in-patient treated with flumazenil (see above) needed supplementary oxygen for 3 hours after ended EUS-B without any impact on length of hospitalization or other consequences.
Infection/pneumonia: one in-patient experienced fever and increased blood C-reactive protein (CRP) (78 mg/L) on day 5 post-procedure and was treated with oral antibiotics without prolonging admission. There was no radiological or microbiological confirmation of infection.
Unlikely EUS-B-related events
ICU: one patient was admitted a week earlier due to rapidly progressive breathlessness and clinical deterioration without pre-existing cardiopulmonary disease. Supplementary oxygen (3 L/min; nasal cannula) was needed to keep saturation >90%, and clinicians considered lymphoma likely and that EUS-B was the only tolerated procedure to diagnose the massive mediastinal masses (Figure 1). A few hours after an uncomplicated EUS-B procedure, progressive tumour pressure symptoms (partial compression of airways and the left pulmonary artery) were treated in the ICU with non-invasive ventilation. Acute analysis of the EUS-B-FNA sample was consistent with high-grade malignant lymphoma. High-dose glucocorticoid and chemotherapy was consequently commenced resulting in marked clinical response i.e., discharge from the hospital and cessation of supplemental oxygen therapy.
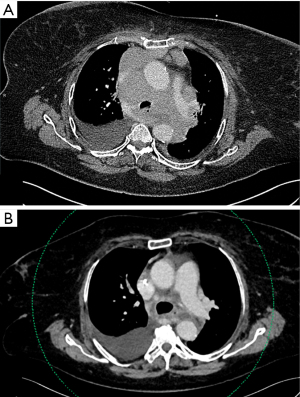
Hospitalization: two out-patient cases were hospitalized within 30 days due to their advanced cancer without relation to the endoscopic procedure.
Secondary endpoints
All planned procedures were completed. Table 2 shows additional procedure information.
All 16 EUS-B-FNA samples were adequate. Diagnostic yield was 81% with a malignant diagnosis in 13 patients, who were referred for cancer therapy without further invasive procedures.
In three patients EUS-B-FNA did not yield a specific diagnosis. Two needed further biopsying after clinical improvement; one patient had lung cancer diagnosed in a lesion not reachable by EUS-B-FNA and another had non-caseating granulomas without malignancy found by mediastinoscopy (final diagnosis: sarcoidosis). The last patient had suspected post-reactive pulmonary changes with clinical improvement. Thus, EUS-B-FNA detected malignancy in 93% (13/14) of cases with a final diagnosis of malignancy.
Of the six patients diagnosed with either adenocarcinoma or non-small cell lung cancer not otherwise specified, 5 (83%) patients had analyses for oncodriver markers and programmed death-ligand 1 (PD-L1) performed.
Discussion
In this prospective cohort we assessed the safety of EUS-B-FNA for centrally located tumours in a selected group of patients with significant respiratory challenges.
Though based on a limited number of patients, our results indicate high overall safety of EUS-B-FNA in patients with respiratory impairment examined in the bronchoscopy suite without anaesthesiologist present. Almost all patients experienced self-limiting changes >20% in vital parameters not needing interventions, but continuous monitoring of vital signs allowed early recognition of persistent changes and thus early correction of AEs with simple measures. All AEs were handled by the regular bronchoscopy staff with standard care interventions: oxygen supply, and benzodiazepine reversal.
Several systematic reviews on lung cancer work-up report an overall AE incidence of <2% during EBUS, EUS or EUS-B (2,9,10,26) with similar event rates (1–4%) in prospective studies with systematic AE registration including hypoxemia (12,20,24). Data on respiratory challenged patients are scarce but suggest that hypoxemia during endoscopic procedures occurs more frequently in populations with chronic obstructive pulmonary disease or other pre-disposing diseases in both bronchoscopy (14,22,27,28) and gastrointestinal endoscopy (29).
We found an AE incidence of 50% during procedure and 13% during recovery with the majority being hypoxemia handled with increased oxygen supply. In order to investigate what kind of events to expect in this group of patients, we chose very conservative definitions of AEs to capture even minor signals concerning safety that probably is below the detection level of reporting of AEs in retrospective studies (23,24). Indeed, our patients were at risk of developing AEs: mean forced expiratory volume in 1 s (FEV1) 38%, poor performance, hospitalization, and chronic respiratory failure with need for oxygen supply.
Several studies describe hypotension and bradycardia as sedation-related events (23,30,31). We used low-dose benzodiazepine and synthetic morphine to diminish the risk of further respiratory impairment (30,32,33). We observed 2 patients (13%) with probable sedation-related AEs since they were efficiently reversed with antidote. Furthermore, one patient experienced nausea and gag-reflexes, which are well-known AEs in gastroenterological endoscopy and possibly caused by sedatives, especially opioids (34). However, our design does not allow us to assess to what extend our AEs were related to either the procedure itself, the sedation or both.
During the 30-day follow-up, only minor procedure-related AEs not affecting treatment were seen in 13%. Though, we cannot know if EUS-B affected admission time in hospitalized patients, the diagnostic clarity provided by the procedure, contributed significantly by enabling targeted treatment and care.
We found a diagnostic yield of 81%, which is comparable with that of EUS-B-FNA in patients without respiratory insufficiency (3,20), and in the same proportion of patients, EUS-B-FNA as a single procedure offered diagnostic clarity sufficient for treatment decision without need of further invasive procedures.
Strengths to our study include that this, to our knowledge, is the first prospective cohort study that systematically reports how patients with respiratory impairment tolerate EUS-B-FNA with detailed procedure related events. Furthermore, we investigated EUS-B-FNA as a single procedure, thus no other procedures influenced the results. Additionally, it was a three-centre study with eight EUS-B operators, suggesting a high level of external validity.
There are several limitations. Firstly, considering the general low complication rate of endoscopic procedures, the sample size is too small to conclude safety statistically. However, patients fulfilling the inclusion criteria are luckily quite rare. It would be interesting to reproduce our findings in larger series and in other centres. We did not randomize the patients to more burdensome procedures as for example EBUS-TBNA or surgery, since it seemed unethical as EUS-B-FNA in each patient was chosen as a “back-against-the-wall-option”.
In conclusion, EUS-B-FNA of centrally located tumours was feasible and safe in this cohort of patients with respiratory impairment, when examined in the bronchoscopy suite. A variety of mostly mild and manageable complications occurred, a few even up to 30 days post-procedure.
Acknowledgments
Funding: ISC received unrestricted research grants from the following funds: the Danish Respiratory Society, Neye Fonden, Dagmar Marshalls Fond, and Else og Mogens Wedell Wedellsborgs Fond.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1705/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1705/coif). ISC received unrestricted research grants from the following funds: the Danish Respiratory Society, Neye Fonden, Dagmar Marshalls Fond and Else og Mogens Wedell Wedellsborgs Fond. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the regional scientific ethics system and the Danish Data Protection Agency (ID Nos: SJ-803 and REG-105-2019). All patients gave their verbal and written consent to participate.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015;46:40-60. [Crossref] [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Skovgaard Christiansen I, Kuijvenhoven JC, Bodtger U, et al. Endoscopic Ultrasound with Bronchoscope-Guided Fine Needle Aspiration for the Diagnosis of Paraesophageally Located Lung Lesions. Respiration 2019;97:277-83. [Crossref] [PubMed]
- Crombag LMM, Dooms C, Stigt JA, et al. Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J 2019;53:1800800. [Crossref] [PubMed]
- Meena N, Bartter T. Endosonography for mediastinal disease: esophageal ultrasound vs. endobronchial ultrasound. Endosc Int Open 2015;3:E302-6. [Crossref] [PubMed]
- Christiansen IS, Bodtger U, Naur TMH, et al. EUS-B-FNA for Diagnosing Liver and Celiac Metastases in Lung Cancer Patients. Respiration 2019;98:428-33. [Crossref] [PubMed]
- Christiansen IS, Ahmad K, Bodtger U, et al. EUS-B for suspected left adrenal metastasis in lung cancer. J Thorac Dis 2020;12:258-63. [Crossref] [PubMed]
- Crombag LMMJ, Szlubowski A, Stigt JA, et al. EUS-B-FNA vs conventional EUS-FNA for left adrenal gland analysis in lung cancer patients. Lung Cancer 2017;108:38-44. [Crossref] [PubMed]
- von Bartheld MB, van Breda A, Annema JT. Complication rate of endosonography (endobronchial and endoscopic ultrasound): a systematic review. Respiration 2014;87:343-51. [Crossref] [PubMed]
- Dhooria S, Aggarwal AN, Gupta D, et al. Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Respir Care 2015;60:1040-50. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
- Kang HJ, Hwangbo B, Lee GK, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 2014;69:261-8. [Crossref] [PubMed]
- Hashemi SM, Dahele M, Daniels JM, et al. Complications of endoscopic ultrasound-guided needle aspiration. Acta Oncol 2014;53:1265-8. [Crossref] [PubMed]
- Magalhães A. Evaluation of the patient undergoing respiratory endoscopic procedures. Rev Port Pneumol 2012;18:48-53. [Crossref] [PubMed]
- Prasad KT, Sehgal IS, Gupta N, et al. Endoscopic ultrasound (with an echobronchoscope)-guided fine-needle aspiration for diagnosis of a mediastinal lesion in a mechanically ventilated patient: A case report and systematic review of the literature. Indian J Crit Care Med 2016;20:608-12. [Crossref] [PubMed]
- Ito T, Oki M, Saka H, et al. Respiratory failure patient with lung cancer diagnosed by transesophageal bronchoscopic ultrasound-guided aspirates. Respirol Case Rep 2018;6:e00309. [Crossref] [PubMed]
- Nakashima K, Demura Y, Oi M, et al. Utility of Endoscopic Ultrasound with Bronchoscope-guided Fine-needle Aspiration for Detecting Driver Oncogenes in Non-small-cell Lung Cancer during Emergency Situations: Case Series. Intern Med 2021;60:1061-5. [Crossref] [PubMed]
- Hakrush O, Adir Y, Schneer S, et al. Per-Esophageal Needle Aspiration of Parenchymal Lung Lesions and Mediastinal Lymph Nodes Using an Endobronchial Ultrasound Bronchoscope. Isr Med Assoc J 2019;21:738-42. [PubMed]
- Torii A, Oki M, Yamada A, et al. EUS-B-FNA Enhances the Diagnostic Yield of EBUS Bronchoscope for Intrathoracic Lesions. Lung 2022;200:643-8. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. Transbronchial vs transesophageal needle aspiration using an ultrasound bronchoscope for the diagnosis of mediastinal lesions: a randomized study. Chest 2015;147:1259-66. [Crossref] [PubMed]
- Konge L, Vilmann P, Clementsen P, et al. Reliable and valid assessment of competence in endoscopic ultrasonography and fine-needle aspiration for mediastinal staging of non-small cell lung cancer. Endoscopy 2012;44:928-33. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- d'Hooghe JN, Eberl S, Annema JT, et al. Propofol and Remifentanil Sedation for Bronchial Thermoplasty: A Prospective Cohort Trial. Respiration 2017;93:58-64. [Crossref] [PubMed]
- Dhooria S, Sehgal IS, Gupta N, et al. Diagnostic Yield and Complications of EBUS-TBNA Performed Under Bronchoscopist-directed Conscious Sedation: Single Center Experience of 1004 Subjects. J Bronchology Interv Pulmonol 2017;24:7-14. [Crossref] [PubMed]
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005; [Crossref]
- Korevaar DA, Colella S, Spijker R, et al. Esophageal Endosonography for the Diagnosis of Intrapulmonary Tumors: A Systematic Review and Meta-Analysis. Respiration 2017;93:126-37. [Crossref] [PubMed]
- Bellinger CR, Khan I, Chatterjee AB, et al. Bronchoscopy Safety in Patients With Chronic Obstructive Lung Disease. J Bronchology Interv Pulmonol 2017;24:98-103. [Crossref] [PubMed]
- Neuman Y, Koslow M, Matveychuk A, et al. Increased hypoxemia in patients with COPD and pulmonary hypertension undergoing bronchoscopy with biopsy. Int J Chron Obstruct Pulmon Dis 2015;10:2627-32. [PubMed]
- Long Y, Liu HH, Yu C, et al. Pre-existing diseases of patients increase susceptibility to hypoxemia during gastrointestinal endoscopy. PLoS One 2012;7:e37614. [Crossref] [PubMed]
- Qadeer MA, Lopez AR, Dumot JA, et al. Hypoxemia during moderate sedation for gastrointestinal endoscopy: causes and associations. Digestion 2011;84:37-45. [Crossref] [PubMed]
- Casal RF, Lazarus DR, Kuhl K, et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration under general anesthesia versus moderate sedation. Am J Respir Crit Care Med 2015;191:796-803. [Crossref] [PubMed]
- Triantafillidis JK, Merikas E, Nikolakis D, et al. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol 2013;19:463-81. [Crossref] [PubMed]
- Midtling JI. Midazolam: a new drug for intravenous sedation. Anesth Prog 1987;34:87-9. [PubMed]
- Amornyotin S. Sedation-related complications in gastrointestinal endoscopy. World J Gastrointest Endosc 2013;5:527-33. [Crossref] [PubMed]


