
Video-assisted thoracic surgery for spontaneous pneumothorax under two-lung ventilation and single-lumen endotracheal tube intubation
Highlight box
Key findings
• The pneumothorax can be operated with two lung ventilation through single-lumen endotracheal tube intubation, and we obtained satisfactory results.
What is known and what is new?
• A representative way to maintain two-lung ventilation is CO2 gas insufflation, and the other is to keep the ventilator at a low tidal volume. We operated without CO2 insufflation and, if possible, did not change the tidal volume.
What is the implication, and what should change now?
• Regarding various complications and costs, surgery using tidal volume control and two-lung ventilation is advantageous for patients. In the future, it will be necessary to investigate the recurrence rate of pneumothorax according to the anesthesia method to determine the usefulness of surgery using two-lung ventilation.
Introduction
Video-assisted thoracic surgery (VATS) is a standard technique for thoracic surgery. During VATS, one-lung ventilation (OLV) is performed using a double-lumen endotracheal tube (DLET) intubation (1,2). Although lung isolation using OLV provides an excellent surgical view for the thoracic surgeon to perform the planned surgery, it has many disadvantages (3-8). First, intubation using a DLET should be performed by a skilled anesthesiologist. In addition, the location of the tube must be confirmed using fiber-optic bronchoscopy (FOB). This process adds cost to the patient. It also increases the preparation time before surgery (3,9). Moreover, it is associated with complications such as hoarseness, tracheobronchial injury, and vocal cord injury due to DLET (5,10-12). Problems such as ventilation/perfusion (V/Q) mismatch, hypoxemia, re-expansion pulmonary edema, and oxidative stress due to OLV have also been frequently reported (6,13-16).
Due to these problems, some researchers have applied two-lung ventilation (TLV) using single-lumen endotracheal tube (SLET) intubation to simple surgeries such as pleural biopsy and talc pleurodesis (3). Recently, TLV has been actively used for surgery related to pneumothorax. Kim et al. have performed VATS with TLV for pneumothorax with excellent results (9,17).
We can consider two methods to proceed with VATS while maintaining TLV. One is to lay low tidal volume. The other is to make artificial pneumothorax using CO2 insufflation. However, since CO2 has various disadvantages (18), it seems wise to avoid it as much as possible. It is also desirable to apply a low tidal volume. Therefore, the purpose of this study was to confirm the feasibility of TLV using SLET intubation in VATS for pneumothorax. The study focused mainly on the time required for procedures and operations and compared it with OLV using DLET intubation to confirm the value. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1413/rc).
Methods
Patient population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of the St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea (No. VC19RESI0150), which did not require individual consent for this retrospective study. From January 2016 to December 2019, 344 patients with a spontaneous pneumothorax who underwent surgery at the St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea were reviewed. Patients underwent preoperative oxygen therapy or tube thoracostomy if indicated. In addition, we performed high-resolution computed tomography (HRCT) scan for all patients before surgery. A slice thickness of 1 mm with section spacing of 5 mm was used. Surgical treatment was actively performed for patients with prominent bulla or bleb observed in HRCT, even for the first episode of pneumothorax. Other surgical indications included air leakage over 4 days after tube thoracostomy, hydropneumothorax, and recurrent or tension pneumothorax.
Anesthetic techniques
In the past, OLV was applied to all patients. Still, after TLV was applied, OLV was applied to severe cases of multifocal pleural adhesion, those with a bullous emphysematous lung on CT, or those with a recurrence in the ipsilateral side. However, depending on the operator’s propensity, TLV might be applied to all patients. After general anesthesia was induced, intubation was performed for each group using a SLET (Shiley taperguard oral/nasal tracheal tube, 7 mm ID for females and 7.5 mm ID for males; Covidien, Mansfield, MA, USA) or a DLET (Shiley endobronchial tube, 11.7 mm/35 Fr for females and 12.3 mm/37 Fr for males). Intraoperative ventilator settings for the TLV group and the OLV group were as follows: respiratory rate (RR), 12 and 12–15 breaths/min; tidal volume, 8 mL/kg and 6–8 mL/kg; and inspiratory oxygen fraction (FiO2), 0.5 and 0.5, respectively. For the OLV group, bronchoscopy was used to confirm the location of the DLET. It was performed by a trainee or a professor of anesthesiology. For the TLV group, if the ventilated lung obstructed the visual field during surgery, we could improve the problem by inducing a temporary apnea or reducing the tidal volume to 4 mL/kg.
Operative techniques
Except for the method of anesthesia, the operative technique was almost the same for the two groups. Patient’s posture was a lateral decubitus position. Skin preparation and draping were then performed. Surgery was performed with conventional three ports located at the third intercostal space of a mid-axillary line, the fourth intercostal space of a posterior-axillary line, and the sixth intercostal space of a mid-axillary line. In the OLV group, the camera port entered the thoracic cavity after starting the OLV. After checking the degree of deflation of the lung and the presence of pleural adhesion, the other two ports were inserted.
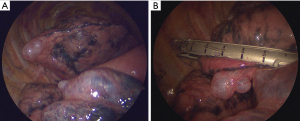
On the other hand, for the TLV group, the camera port entered the thoracic cavity after reducing the tidal volume or inducing an apnea state. After placing all three ports, we first looked closely at the thoracic cavity. We then checked bleb or bulla in the lung (Figure 1A). In the TLV group, if the operator did not obtain a sufficient field of view, the anesthesiologist reduced the tidal volume sufficiently or a temporary apnea was made. Pleural adhesion was dissected using electrocautery. We used energy devices for severe adhesion. When the lesion was identified, resection was performed using an endostapler (Figure 1B). Both groups received reinforced stapled lines using an absorbable polyglycolic acid sheet (Neoveil, Gunze Ltd., Kyoto, Japan) and fibrin glue. We did not perform pleurodesis or pleurectomy on primary pneumothorax. However, chemical pleurodesis was performed for patients with secondary pneumothorax and severe bullous emphysematous changes. After the surgery, the chest tube was placed.
Data collection and statistical analysis
We collected data of all patients retrospectively from medical records. Collected information included age, gender, body height, body weight, body mass index (BMI) [weight (kg)/height (m2)], smoking habit, underlying lung disease, Blebs/bulla on HRCT, preoperative treatments, number of episodes of pneumothorax, recurrence of pneumothorax, duration of anesthesia, course of operation, number of specimens resected, time of chest drainage, length of stay, complications, and duration of follow-up.
We used SPSS ver 11.0 software (SPSS Inc., Chicago, IL, USA) for all statistical analyses. Descriptive data are expressed as mean ± standard deviation (SD). Student’s t-test was used to assess differences in continuous variables and chi-square test or Fisher’s exact test was used to compare categorical variables. A P value less than 0.05 was considered statistically significant.
Results
Of the 344 patients, 200 were in the TLV group and 144 were in the OLV group. There was no case of conversion to OLV or insufflation of CO2 gas during surgery in the TLV group. There was no conversion to open thoracotomy either during surgery.
The mean age of patients was 25.9±14.4 years in the TLV group and 30.7±19.3 years in the OLV group (P=0.013). There were more current smokers in the OLV group than in the TLV group [n=36 (25.0%) vs. n=30 (15.0%), P=0.02]. However, there was no significant difference in gender [male, n=184 (92.0%) vs. n=133 (92.4%)], BMI (19.8±2.8 vs. 19.4±3.2 kg/m2), or smoking history (15.1±15.0 vs. 17.0±17.0 pack-year) between the two groups. There was no difference in the proportion of patients indicated for surgery because pneumothorax recurred on the ipsilateral side in each group. The proportion of patients who underwent surgery without tube thoracostomy did not differ between the two groups either (Table 1).
Table 1
| Characteristics | Group | P value | |
|---|---|---|---|
| TLV (n=200) | OLV (n=144) | ||
| Age (years), mean ± SD | 25.9±14.4 | 30.7±19.3 | 0.013 |
| Gender (male), n (%) | 184 (92.0) | 133 (92.4) | 0.902 |
| Height (cm), mean ± SD | 174.7±6.9 | 174.4±6.9 | 0.695 |
| Weight (kg), mean ± SD | 60.7±9.8 | 59.7±9.7 | 0.355 |
| BMI (kg/m2), mean ± SD | 19.8±2.8 | 19.4±3.2 | 0.231 |
| Smoker, n (%) | 30 (15.0) | 36 (25.0) | 0.02 |
| Smoking (pack-year), mean ± SD | 15.1±15.0 | 17.0±17.0 | 0.628 |
| 1st episode, n (%) | 167 (83.5) | 123 (85.4) | 0.63 |
| Operation site (Rt.), n (%) | 84 (42.0) | 75 (52.1) | 0.064 |
| Initial therapy (operation), n (%) | 97 (48.5) | 61 (42.4) | 0.26 |
TLV, single lumen endotracheal tube-two lung ventilation; OLV, double lumen endotracheal tube-one lung ventilation; SD, standard deviation; BMI, body mass index; Rt., right.
Total anesthesia time was 72.6±17.8 min in the TLV group and 89.9±24.3 min in the OLV group (P<0.001). The operation time was 42.1±16.2 min in the TLV group and 54.7±23.8 min in the OLV group (P<0.001, Table 2). The time from the start of endotracheal intubation to the beginning of the incision was 23.6±7.0 min in the TLV group and 27.6±9.5 min in the OLV group (P<0.001). During surgery, hemodynamic changes were minimally monitored. The two groups showed significant differences in SBP and SPO2. Fluctuation ranges of systolic blood pressure (TLV vs. OLV: 41.6±17.5 vs. 46.4±19.2, P=0.017) and SPO2 (TLV vs. OLV: 0.7±6.5 vs. 1.9±4.1, P=0.046) were more prominent in the OLV group. There was no significant difference in diastolic blood pressure, heart rate, or ETCO2 between the two groups. The number of patients with pleural adhesion was 45 (22.5%) in the TLV group and 52 (36.1%) in the OLV group (P=0.006). The number of endo-staplers used and the number of resected specimens did not differ between the two groups. The most used thoracoscope’s diameter was 2.9 mm [n=159 (81.1%)] for the TLV group and 5 mm [n=108 (78.3%)] for the OLV group (P<0.001) (Table 2). The chest tube indwelling time was 1.6±1.1 days for the TLV group and 2.3±3.6 days for the OLV group (TLV vs. OLV, P=0.017). The length of stay was 3.8±1.5 days for the TLV group and 4.7±3.8 days for the OLV group (TLV vs. OLV, P=0.01) (Table 3).
Table 2
| Variables | Group | P value | |
|---|---|---|---|
| TLV (n=200) | OLV (n=144) | ||
| Total anesthetic time (min), mean ± SD | 72.6±17.8 | 89.9±24.3 | <0.001 |
| Operative time (min), mean ± SD | 42.1±16.2 | 54.7±23.8 | <0.001 |
| Time from intubation to incision (min), mean ± SD | 23.6±7.0 | 27.6±9.5 | <0.001 |
| Difference in fluctuations, mean ± SD | |||
| Systolic blood pressure (mmHg) | 41.6±17.5 | 46.4±19.2 | 0.017 |
| Diastolic blood pressure (mmHg) | 35.3±13.0 | 36.4±13.1 | 0.461 |
| Heart rate | 29.1±12.2 | 30.9±12.1 | 0.173 |
| SPO2 | 0.7±6.5 | 1.9±4.1 | 0.046 |
| ETCO2 | 3.7±3.0 | 4.3±3.3 | 0.082 |
| Adhesion, n (%) | 45 (22.5) | 52 (36.1) | 0.006 |
| Thoracoscope’s diameter (mm), n (%) | <0.001 | ||
| 2 | 6 (3.1) | 6 (4.3) | |
| 2.9 | 159 (81.1) | 16 (11.6) | |
| 5 | 28 (14.3) | 108 (78.3) | |
| 10 | 3 (1.5) | 8 (5.8) | |
| Endostapler used (n), mean ± SD | 3.5±1.8 | 3.5±2.1 | 0.957 |
| Specimen resected (n), mean ± SD | 2.1±1.3 | 2.1±1.3 | 0.958 |
TLV, single lumen endotracheal tube-two lung ventilation; OLV, double lumen endotracheal tube-one lung ventilation; SD, standard deviation; SPO2, saturation of percutaneous oxygen; ETCO2, end tidal CO2.
Table 3
| Variables | Group | P value | |
|---|---|---|---|
| TLV (n=200) | OLV (n=144) | ||
| Chest tube indwelling time (days), mean ± SD | 1.6±1.1 | 2.3±3.6 | 0.017 |
| Time from operation to discharge (days), mean ± SD | 2.7±1.2 | 3.2±2.3 | 0.009 |
| Length of stay (days), mean ± SD | 3.8±1.5 | 4.7±3.8 | 0.01 |
TLV, single lumen endotracheal tube-two lung ventilation; OLV, double lumen endotracheal tube-one lung ventilation; SD, standard deviation.
Discussion
OLV is still a standard procedure in VATS. OLV has various advantages, creating a surgical environment that is also beneficial to surgeons. Once lungs are isolated, the surgeon can avoid unintended surgical injuries to the lungs and obtain sufficient surgical space under good vision. However, compared to TLV, OLV has several disadvantages (3-16). Especially, in addition to the price of the DLET itself, the cost is also increased due to the procedure for checking the endotracheal tube’s location (3,9). To maintain the OLV, the location of the DLET must be appropriate and the process of confirming it by performing a bronchoscopy is necessary. To avoid such disadvantages of OLV, studies have applied TLV to relatively simple surgery for problems such as pneumothorax and pleural effusion without needing access to the hilum, showing good results (3,17,19). Cerfolio et al. have successfully performed thoracoscopic pleural biopsy, drainage of pleural effusion, and talc pleurodesis using TLV (3). They also noted that a sufficient field of view could be obtained with TLV using SLET intubation in VATS. Results of their study laid the foundation for thoracoscopic wedge resection using TLV.
In VATS using TLV, there are two representative options for a better surgical field. One is to use CO2 insufflation. The other is to adjust the tidal volume of a ventilator. Several studies have performed VATS using TLV, with most studies securing the surgical field of view using CO2 insufflation. However, CO2 insufflation also has its drawbacks (18,20). Lee et al. have reported that CO2 insufflation does not have a superior effect in securing the surgical field except at the beginning of the surgery (20). CO2 insufflation can increase PaCO2, peak airway pressure, and operation time. In addition, CO2 insufflation can lead to complications such as cardiovascular response associated with arterial hypercapnia, CO2 embolism, and hypotension due to impaired venous return.
Therefore, some studies have applied other options to maintain TLV without CO2 insufflation (17,21-23). Kim et al. have reported no technical problem applying TLV and low tidal volume in patients with pneumothorax (17). During VATS, it was necessary to study the optimal RR to maintain TLV and low tidal volume while minimizing physiological adverse effects on patients. Lee et al. have published an optimal RR for maintaining TLV and low tidal volume in thoracoscopic bleb resection (21). TLV, by applying a low tidal volume of 5 mL/kg and an RR of 15 cycles/min, can adequately promote CO2 excretion while meeting the patient’s oxygen demand. When this optimal setting is applied, minute ventilation can be reduced compared to the conventional ventilator setting, making the surgeon more comfortable. At the same time, no physiological problems related to hypoventilation occurred. These results showed a difference in ‘RR’ from the ventilator setting of patients applied with TLV in this study. But, as mentioned by Lee et al., whether RR of 15 cycles/min could shorten the surgical time or reduce the number of endostaplers used has not been demonstrated yet.
The reason why we applied TLV rather than OLV was that when firing was performed while the lung was completely collapsed, it was estimated that air leakage might occur due to tension applied to the staple margin during lung inflation. If the thickness of the tissue between the jaw and cartridge of the selected stapler is too thin or too thick, it may cause air leakage after firing (24). Therefore, the authors performed surgery while avoiding complete isolation of the lung and maintaining ventilation of the operated lung using TLV. However, firing with lung inflation itself can cause excessive compression in the tissue, which might be another cause of air leakage. One of the limitations of this study is that a clear answer to this has not yet been prepared. When firing an endostapler, it is believed that there is still no conclusion on whether the lung will collapse completely or maintain partial inflation. The authors believe that the pressure applied to the staple line during re-expansion after the lung completely collapses will be more substantial and cause air leakage. However, since we could not prove this in this study, further research on related content is needed in the future.
This study has some limitations. Because it was a retrospective study, there were cases in which it was difficult to obtain complete information related to data collection, especially anesthesia. It was difficult to obtain data other than basic ventilator settings such as tidal volume and RR. In addition, four surgeons operated on the patients in this study. Each surgeon selected the anesthesia method according to their preference, which inevitably confused some results. In this study, the actual tension applied to the staple line of patients who underwent surgery using OLV and TLV could not be verified. Future research on this is necessary. Future studies should also determine whether it is possible to expand the basis of surgery using TLV.
Conclusions
Still, even a relatively simple VATS for pneumothorax tends to favor OLV. However, as can be seen from the results of this study, VATS using TLV was able to proceed without any problems in primary and secondary pneumothorax, and it has sufficient merit regarding the time required. Therefore, we believe VATS with TLV for pneumothorax is well worth it.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1413/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1413/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1413/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of the St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea (No. VC19RESI0150), which did not require individual consent for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Campos J. Lung Isolation. In: Slinger P. editor. Principles and Practice of Anesthesia for Thoracic Surgery. New York, NY, USA: Springer Publisher; 2011:227-246.
- Cohen E. Methods of lung separation. Minerva Anestesiol 2004;70:313-8. [PubMed]
- Cerfolio RJ, Bryant AS, Sheils TM, et al. Video-assisted thoracoscopic surgery using single-lumen endotracheal tube anesthesia. Chest 2004;126:281-5. [Crossref] [PubMed]
- Horswell JL. Anesthetic techniques for thoracoscopy. Ann Thorac Surg 1993;56:624-9. [Crossref] [PubMed]
- Knoll H, Ziegeler S, Schreiber JU, et al. Airway injuries after one-lung ventilation: a comparison between double-lumen tube and endobronchial blocker: a randomized, prospective, controlled trial. Anesthesiology 2006;105:471-7. [Crossref] [PubMed]
- Misthos P, Katsaragakis S, Milingos N, et al. Postresectional pulmonary oxidative stress in lung cancer patients. The role of one-lung ventilation. Eur J Cardiothorac Surg 2005;27:379-82; discussion 382-3. [Crossref] [PubMed]
- Kurihara N, Imai K, Minamiya Y, et al. Hoarseness caused by arytenoid dislocation after surgery for lung cancer. Gen Thorac Cardiovasc Surg 2014;62:730-3. [Crossref] [PubMed]
- Toolabi K, Aminian A, Javid MJ, et al. Minimal access mediastinal surgery: One or two lung ventilation? J Minim Access Surg 2009;5:103-7. [Crossref] [PubMed]
- Kim H, Kim HK, Kang DY, et al. A comparative study of two- versus one-lung ventilation for needlescopic bleb resection. Eur Respir J 2011;37:1183-8. [Crossref] [PubMed]
- Chu CP, Chen PP. Tracheobronchial injury secondary to blunt chest trauma: diagnosis and management. Anaesth Intensive Care 2002;30:145-52. [Crossref] [PubMed]
- Kim HY, Baek SH, Kim KH, et al. Endobronchial hemorrhage after intubation with double-lumen endotracheal tube in a patient with idiopathic thrombocytopenic purpura for minimally invasive cardiac surgery: a case report. Korean J Anesthesiol 2014;66:59-63. [Crossref] [PubMed]
- Pandey V, Meena DS, Choraria S, et al. Tracheobronchial Injury caused by Blunt Trauma: Case Report and Review of Literature. J Clin Diagn Res 2016;10:UD01-3. [Crossref] [PubMed]
- Cohen E. Physiology of the lateral position and one-lung ventilation. Chest Surg Clin N Am 1997;7:753-71. [PubMed]
- Dunn PF. Physiology of the lateral decubitus position and one-lung ventilation. Int Anesthesiol Clin 2000;38:25-53. [Crossref] [PubMed]
- Katz Y, Zisman E, Isserles SA, et al. Left, but not right, one-lung ventilation causes hypoxemia during endoscopic transthoracic sympathectomy. J Cardiothorac Vasc Anesth 1996;10:207-9. [Crossref] [PubMed]
- Frölich MA, Janelle GM. Postoperative atelectasis after one-lung ventilation with the Univent tube in a child. J Clin Anesth 2003;15:159-63. [Crossref] [PubMed]
- Kim H, Kim HK, Choi YH, et al. Thoracoscopic bleb resection using two-lung ventilation anesthesia with low tidal volume for primary spontaneous pneumothorax. Ann Thorac Surg 2009;87:880-5. [Crossref] [PubMed]
- Suarez-Pierre A, Terasaki Y, Magruder JT, et al. Complications of CO(2) insufflation during endoscopic vein harvesting. J Card Surg 2017;32:783-9. [Crossref] [PubMed]
- Cheng YL, Huang TW, Lee SC, et al. Video-assisted thoracoscopic surgery using single-lumen endotracheal tube anaesthesia in primary spontaneous pneumothorax. Respirology 2010;15:855-9. [Crossref] [PubMed]
- Lee DK, Kim H, Kim HK, et al. CO2 during single incisional thoracoscopic bleb resection with two-lung ventilation. J Thorac Dis 2018;10:5057-65. [Crossref] [PubMed]
- Lee DK, Kim HK, Lee K, et al. Optimal Respiratory Rate for Low-Tidal Volume and Two-Lung Ventilation in Thoracoscopic Bleb Resection. J Cardiothorac Vasc Anesth 2015;29:972-6. [Crossref] [PubMed]
- Schultz MJ, Haitsma JJ, Slutsky AS, et al. What tidal volumes should be used in patients without acute lung injury? Anesthesiology 2007;106:1226-31. [Crossref] [PubMed]
- Kilpatrick B, Slinger P. Lung protective strategies in anaesthesia. Br J Anaesth 2010;105:i108-16. [Crossref] [PubMed]
- Wu Y, Perlman CE. In situ methods for assessing alveolar mechanics. J Appl Physiol 1985;2012:519-26. [PubMed]


