
The role of bronchoscopy in the multidisciplinary approach to benign tracheal stenosis
Introduction
In this article, we will provide a brief overview of tracheal stenosis symptoms, risk factors, and management strategies. We will focus on the role of bronchoscopic evaluation and management, specifically highlighting essential components of the bronchoscopic evaluation of patients with tracheal stenosis pertinent to deciding whether to proceed with endoscopic or open surgical treatment. Bronchoscopic evaluation of tracheal stenosis is critical to the assessment of the morphology, extent, and severity of stenosis, and is considered as the gold standard before pursuing any treatment options. We will describe and illustrate some of the fundamental therapeutic principles employed when addressing benign tracheal stenosis via bronchoscopy and summarize the relevant published data on such techniques.
Symptoms
Tracheal stenosis, a form of central airway obstruction (CAO), is frequently misdiagnosed as asthma or chronic obstructive pulmonary disease (COPD) (1). Patients with CAO share symptoms with other obstructive lung diseases and typically present with dyspnea, wheezing, cough, and even cough syncope. In cases of severe tracheal stenosis, patients can have stridor, dysphagia and dysphonia, especially for lesions involving the subglottis (2).
Risk factors
Benign tracheal stenosis has varied etiologies including gastro-esophageal reflux disease (GERD), airway infection, radiation, inhalation or chemical injury, systemic autoimmune disorders [granulomatosis with polyangiitis (GPA), sarcoidosis, relapsing polychondritis], and iatrogenic due to trauma from airway manipulation (endotracheal intubation or tracheostomy). A potential mechanism for the development of stenosis is an altered inflammatory response to injury, ischemia of the tracheal mucosa and excessive scar formation. Associated cartilage destruction leads to full thickness and/or concurrent tracheomalacia (Table 1) (1,3). Trauma due to endotracheal intubation or tracheostomy placement is the most common acquired cause of tracheal stenosis (4). Risk factors for post-intubation tracheal stenosis (PITS) include traumatic intubation, long duration of intubation (>14 days), prone positioning, and high cuff pressure in the endotracheal tube (ETT) cuff (>30 cmH2O), which is likely the main and the most frequent cause (5). Risk factors for post-tracheostomy tracheal stenosis (PTTS) include excessive force used during tracheostomy placement with cartilage fracture, regional ischemic necrosis, tracheostomy site infection, high tracheostomy site, and friction between the distal tracheostomy tube and the tracheal wall (6). Overall risk factors for both PITS and PTTS include previous radiation, obesity, GERD and diabetes (5,6). However, etiology for tracheal stenosis may remain undiagnosed in 18% of the cases (7).
Table 1
| Mechanism | Diagnosis |
|---|---|
| Local injury | Post-tracheostomy |
| Post-intubation | |
| Trauma | |
| Airway Burn Injury | |
| Chemical Inhalation injury | |
| Radiation injury | |
| Systemic disorders | Granulomatosis with polyangiitis |
| Relapsing polychondritis | |
| Sarcoidosis | |
| Amyloidosis | |
| Inflammatory bowel disease | |
| Infectious | Tuberculosis |
| Aspergillus | |
| Klebsiella rhinoscleromatis | |
| Staphylococcus Aureus | |
| Blastomycosis | |
| Miscellaneous | Tracheobronchopathia osteochondroplastica |
| Broncholithiasis | |
| Idiopathic | |
| Post-transplant anastomotic stenosis | |
| Idiopathic | No cause identified after a thorough work up and history |
Overview of management strategies
Definitive management of tracheal stenosis includes bronchoscopic interventions and open surgery, the latter having the potential of being curative in selected cases. Because of high GERD prevalence and low risk profile, most patients are being prescribed anti-reflux medications. Oral corticosteroids, and antibiotics for tracheal stenosis are usually reserved for acute decompensations in the setting of airway edema and infection, respectively. In cases where the tracheal stricture results from underlying connective tissue disorder, systemic immunosuppressive therapy, and endoscopic interventions remain central to management (8). Bronchoscopic interventions involve laser-assisted mechanical dilation (LAMD), electrosurgery (ES) knife or needle, mechanical dilation with rigid bronchoscopy, endoscopic balloon dilation, intralesional steroid injection or mitomycin C (MMC) application, and in refractory or complex lesions, silicone stent insertion may be considered (9-17). Tracheal resection of hypertrophic stenotic tissues is the procedure of choice in most patients with benign idiopathic or post intubation/tracheostomy tracheal strictures, with laryngotracheal reconstruction reserved for patients with laryngeal involvement (3). Surgery offers definitive treatment and has high success rates when performed by experienced operators (10,18-23). However, factors such as severe co-morbidities (obesity, diabetes), high subglottic stenosis (SGS), or tracheal stenosis with a long vertical extent (>4–6 cm) may preclude surgery. Furthermore, a definitive surgical interventional is typically deferred during the acute inflammatory phase and is considered once the stenosis has matured and inflammation has subsided. In these patients, bronchoscopic interventions are performed as a bridge to surgery or a long-term palliative solution (24-27). In addition, for patients with SGS, while cricotracheal resection (CTR) is more effective than endoscopic management with regards to recurrence, voice quality and vocal cord paralysis are more common with the surgical treatment, and endoscopic management with intralesional steroid injection remains a common practice (28).
Treatment decisions and clinical course are dictated by the disease acuity, patient’s co-morbidities, functional status, and importantly, the etiology, location, extent, morphology and degree of airway narrowing (1,29-34). A multi-disciplinary team (MDT) that involves the expertise and collaboration of otolaryngology, thoracic surgery, and interventional pulmonology helps facilitate the accurate and timely diagnosis of tracheal stenosis and enables evidence-based management (1). Whether the management is endoscopic or surgical will ultimately depend on stenosis and patient-related factors. Several of these factors are decided after thorough noninvasive and bronchoscopic evaluation.
Evaluation of tracheal stenosis
A complete workup for tracheal stenosis involves evaluating the etiology, assessing the mechanism of injury, and classifying the stenosis based on factors that impact management. This includes a series of non-invasive testing such as pulmonary function tests, serologies, and chest and neck imaging. In addition, associated symptomology including vocal cord function, swallowing dysfunction, and overall functional status should be noted (3,34-36).
Non-invasive evaluation of tracheal stenosis
Evaluation of tracheal stenosis starts with a thorough history and physical examination, which can help determine the etiology of the tracheal stenosis. Determining the patient’s functional status impacts treatment decisions. The patient’s voice and ability to swallow should be assessed as tracheal stenosis, especially with laryngeal involvement, may present with dysphonia and dysphagia. A serologic evaluation to establish whether there is an underlying systemic autoimmune disorder should be obtained (1). In these regards, a study of 92 patients with non-traumatic laryngotracheal stenosis (LTS) found that 25% of cases were due to GPA, with 75% being idiopathic, with all other serologic testing being equivocal (37). Of note, anti-neutrophil cytoplasmic antibody (ANCA) are positive in approximately 60% of patients with limited GPA, so a negative ANCA does not rule out GPA, and biopsies are still needed in such cases (38). Establishing a firm etiology is essential as recurrence rate for patients with GPA is high after surgical interventions. In one small study, 9% failed laryngotracheal resection and reconstruction (LTRR) and required tracheostomy, 55% required dilations after LTRR and 18% developed lower airway stenosis (39). These data suggest that LTTR should not be first choice in patients with GPA-related LTS.
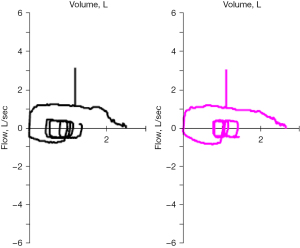
Spirometry can show the classic pattern of truncation of inspiratory and expiratory limbs on the flow volume loops; however, this pattern lacks sensitivity, and these findings can be appreciated only when the tracheal lumen is reduced to 6–8 mm (40). Furthermore, spirometry does not precisely localize strictures and is an insensitive test for mild to moderate narrowing and offers functional, not anatomical information (40,41) (Figure 1).
Computed tomography (CT) imaging of the neck and chest with or without 3D reconstruction can help determine the extent (length) of the tracheal stenosis and quantify the degree of airway narrowing (42,43). However, these findings are affected by the respiratory phase and the presence of secretions (40,44). Secretions can overestimate the degree of narrowing while a CT scan done at full inspiration can underestimate the degree of narrowing during tidal respiration. A dynamic CT neck and chest may help identify associated tracheomalacia or excessive dynamic airway collapse (Figure 2) (1).

Bronchoscopic evaluation of tracheal stenosis
Direct airway visualization via bronchoscopy remains the diagnostic gold standard for the workup of tracheal stenosis and is essential for determining patient candidacy for endoscopic or surgical treatments. It allows simultaneous assessment of vocal cord function, and quantitative measurement of the stenosis (length, location and degree of narrowing), determining the morphology (shape) of the narrowing, and identifying associated tracheomalacia (45). Endotracheal biopsies may help determine the etiology of the tracheal stenosis (1). Additionally, using the radial endobronchial ultrasound (r-EBUS) at 20 MHz frequency during bronchoscopy, may permit non-invasive bronchoscopic measurements of the tracheal diameter (46) and even assessment of the hypertrophic tissues and airway cartilage integrity (47).
If a tracheostomy tube is present during bronchoscopic evaluation, it should be removed to allow for complete airway inspection. If the patient is intubated, the endotracheal tube should be retracted as proximally as possible. In patients with high tracheal strictures, the use of a laryngeal mask airway (LMA) should be considered if patients require general anesthesia, but ideally, bronchoscopy should be performed with moderate sedation so patients cooperate with various speech and breathing maneuvers (2). However, in patients with suspected severe tracheal stenosis, the ideal choice of anesthesia for bronchoscopy (moderate anesthesia vs. general anesthesia) for airway evaluation should be selected after carefully assessing symptoms and pre-bronchoscopy imaging, when available. It is also essential to consider local expertise and availability of equipment for managing potential airway emergencies.
Several classification systems are available for grading laryngo-tracheal stenosis, mostly from otolaryngology literature. These include the Cotton-Myer scale, which grades the severity based on degree of airway narrowing (35), the Lano system, which grades the stenosis based on the number of locations involved (32), and the McCaffrey system, which grades the stenosis based on location and vertical extent of the stenosis (33). Bronchoscopic evaluation should be objective and assess the degree of narrowing, length of the stricture, morphology (circumferential, elliptical, crescent, triangular), complexity (with or without malacia), and precise location of the stricture in relation to the vocal cords, cricoid, and main carina (1,3,29,30,34-36,48). Different classification systems are as listed in Table 2.
Table 2
| Classification system study/year | Classification criteria | Comments |
|---|---|---|
| Myer et al./1994 | Grade 1: 0–50% obstruction | Based only on the degree of reduction in airway CSA |
| Grade 2: 51–70% obstruction | ||
| Grade 3: 71–99% obstruction | ||
| Grade 4: no detectable lumen | ||
| McCaffrey/1992 | Stage I: lesions are confined to the subglottis or trachea and <1 cm long | Based only on the vertical extent |
| Stage II: lesions are isolated to the subglottis and >1 cm long | Predicts tracheal decannulation based on anatomic location and extent of stenosis | |
| Stage III: subglottic/tracheal lesions not involving the glottis | 90% of stages I and II, 70% of stage III, and 40% of stage IV patients undergo decannulation successfully | |
| Stage IV: lesions involve the glottis | ||
| Lano et al./1998 | Stage I: one subsite involved | Based on subsites involved (glottis, subglottis, trachea) |
| Stage II: two subsites involved | Correlation between this staging and likelihood for successful decannulation | |
| Stage III: three subsites involved | Stage I: 94%, stage II: 78%, stage III: 20% | |
| Nouraei et al./2007 | Airway status | Comprehensive system used by otorhinolaryngologists |
| No airway prosthesis | Does not include extent and severity criteria (i.e., reduction in CSA) | |
| Intraluminal airway prosthesis (stent) | Designed for documenting functional outcomes of adult laryngotracheal stenosis | |
| Tracheostomy or tracheostomy-tube dependent, patient voices | ||
| Tracheostomy dependent, patient does not voice | ||
| Death because of a direct complication of airway disease | ||
| Dyspnea | ||
| Grade 1: “I only get breathless with strenuous exercise” | ||
| Grade 2: “I get short of breath when hurrying on level ground or walking up a slight hill” | ||
| Grade 3: “On level ground, I walk slower than people of the same age because of breathlessness, or have to stop for breath when walking at my own pace” | ||
| Grade 4: “I stop for breath after walking about 100 yards or after a few minutes on level ground” | ||
| Grade 5: “I am too breathless to leave the house or I am breathless when dressing” | ||
| Voice | ||
| No problems with voice | ||
| Some problems with my voice | ||
| Making voice is effortful and significant difficulties being heard or understood | ||
| Can only produce a weak voice or whisper | ||
| No voice | ||
| Swallowing | ||
| Eat and drink normally | ||
| Normal diet but with some difficulty swallowing | ||
| Significant swallowing difficulties | ||
| Serious problem swallowing (i.e., diet consists almost entirely of liquidized foods) | ||
| Unable to swallow | ||
| Freitag et al./2007 | Type | Designed for grading tracheal stenosis from pulmonologists’ perspective |
| Structural | ||
| Type 1: exophytic or intraluminal | The degree of severity criterion is not justified physiologically | |
| Type 2: extrinsic | ||
| Type 3: distortion | The structural types are not mutually exclusive | |
| Type 4: scar or stricture | ||
| Dynamic or functional | ||
| Type 1: damaged cartilage or malacia | ||
| Type 2: floppy membrane | ||
| Degree of stenosis | ||
| Code 0: no stenosis | ||
| Code 1: <25% | ||
| Code 2: 26–50% | ||
| Code 3: 51–75% | ||
| Code 4: 76–90% | ||
| Code 5: 91–100% | ||
| Location: | ||
| I: upper one-third of the trachea | ||
| II: middle one-third of the trachea | ||
| III: lower one-third of the trachea | ||
| IV: right main bronchus | ||
| V: left main bronchus | ||
| Ghorbani et al./2012 | Diameter of stricture | The degree of severity criterion is not justified physiologically |
| Score 0: stenosis rate between 0% and 25% | Terminology for types of stenosis is composed of pathophysiologic processes | |
| Score 1: stenosis rate between 26% and 50% | ||
| Score 2: stenosis rate between 51% and 75% | ||
| Score 3: stenosis rate between 76% and 90% | ||
| Score 4: stenosis rate 91% or higher | ||
| Type of stenosis | ||
| Score 1: granulation tissue | ||
| Score 2: granulation tissue, fibrosis, and inflammation | ||
| Score 3: fibrosis | ||
| Score 4: malacia | ||
| Clinical symptoms | ||
| Score 1: dyspnea only during intense activity | ||
| Score 2: dyspnea during normal activity but physical examination results are normal | ||
| Score 3: long inhalation and exhalation but with no stridor or retraction | ||
| Score 4: stridor and retraction |
Reprinted from (3), Copyright © 2016, with permission from Elsevier. CSA, cross-sectional area.
Extent and location
The extent, or vertical length of tracheal stenosis, is important to measure, as it determines whether surgical resection can be considered. While surgical resection is considered a definitive treatment for strictures that are 1–4 cm in length, endoscopic techniques are usually used for lesions that are <1 cm or >4 cm, although there are reports of longer tracheal segments (6 cm) being successfully resected (3). Some operators prefer to use the ratio of the stenotic segment extent to the length of the trachea as a criterion for surgical selection (i.e., stenosis involving >40% of tracheal length having high risk of complications). Lesions shorter than 1 cm tend to be simple, without cartilage involvement and often respond to mechanical dilation with or without using laser or ES for performing radial incisions. Lesions longer than 4–6 cm may not be amenable to tracheal resection after discussions with expert tracheal surgeons due to risks for tension and anastomotic complications. Therefore, precise measurement of the extent of tracheal stenosis is essential during bronchoscopic evaluation. One can measure the vertical length of the stenosis by placing the tip of the bronchoscope adjacent to the most distal aspect of the stenosis while pinching the bronchoscope at the proximal end of the endotracheal tube/nostril/teeth level (depending on the insertion route). The bronchoscope is then retracted proximally until the tip of the scope is adjacent to the proximal end of the stenosis. The distance between where the scope is pinched and the proximal end of the endotracheal tube/nostril/teeth level is the length of the stenosis. Of course, the extent can be measured on the CT as well, but inflammation or small fibrotic bands may be missed by the CT. When describing tracheal stenosis in bronchoscopy reports, it is essential to note its exact location in relation to the vocal cords, cricoid, and main carina. In particular, the distance should be measured between the distal edge of the stenosis to the main carina and between the proximal edge of the stenosis to the vocal cords (for lesions involving subglottis) or cricoid (for tracheal lesions only). Whether the stenosis involves the cricoid cartilage, and the subglottic larynx should be clearly noted, as surgical treatments depend on whether the subglottis or glottis are involved. The distribution of the stenosis and whether there is multifocal disease should also be identified (3).
Severity of airway narrowing
The severity of airway narrowing affects symptoms, especially dyspnea and is relevant to treatment decisions in patients with tracheal stenosis (32,33,35). Pressure drop along the stenosis, which affects the patient’s work of breathing, is primarily determined by the percentage reduction in airway caliber [i.e., cross-sectional area (CSA)] rather than the absolute decrease in the airway diameter (49). A physiologically abnormal obstruction is defined as a narrowing of the airway by >50%, as glottic opening results in a similar degree of pressure drop as a 50% tracheal stenosis (1,49).
When measuring the severity of airway narrowing, the bronchoscopist should measure and compare the CSA of the stenosis to a normal segment of the trachea {see below Eq. [1]}. Tracheal stenosis is categorized as mild (<50% reduction), moderate (51–70% reduction), or severe (71–100% reduction) based on the degree of airway narrowing and its impact on airflow limitation (see Table 2: Cotton-Myer’s classification). A study showed that even experienced bronchoscopist often misclassify the degree of airway narrowing when using still bronchoscopic images to subjectively assess strictures of benign etiology, when compared to using CSA that were objectively analyzed using morphometric bronchoscopy (48).
As mentioned, for mild stenosis (<50% reduction in CSA), the pressure gradient which develops across the stenosis is of similar magnitude as seen with a normal glottis opening. Therefore, patients with mild stenosis are typically asymptomatic, even with exertion (49,50). Significant pressure drop occurs at higher flow rates for moderate stenosis (51–70% reduction in CSA). Thus, these patients typically experience symptoms during exertion. For severe stenosis (71–100% reduction in CSA), there is a significant pressure gradient across the stenosis, even at low flow rates, leading to symptoms with mild exertion or even at rest (1,49,51). Thus, being precise in the assessment of airway narrowing severity is key in attributing symptoms to the actual stenosis itself. This is important as patients with tracheal stenosis may have comorbidities that also cause dyspnea.
Morphology (shape of stenosis)
Broadly, a stenotic area can be classified as simple or complex based not only on the extent but whether the cartilage is involved and if there is associated malacia. A simple stricture is defined as less than 1 cm in vertical extent and without malacia. A complex stricture is defined as longer than 1 cm in vertical extent or with associated malacia or full thickness tracheal wall injury. This classification for PITS is important as simple stenosis often responds to LAMD and stent insertion is avoided to limit further airway injury and potentially worsen a resectable disease. The associated chondritis predicts poor results and failure of endoscopic treatment (52). Idiopathic lesions, especially SGS, are characterized by hypertrophy of the mucosa and submucosa with intact cartilaginous wall and a stent insertion should not be performed in these cases. Thus, a thorough evaluation of stenosis complexity bronchoscopically, with a CT scan and EBUS should be performed. With the use of EBUS intra-operatively, a bronchoscopist can visualize hypertrophic tissue thickness and cartilage structure to determine integrity. Cartilage destruction determines the complexity of stenosis and could predict poor response to dilation alone. The location and thickness of stenotic tissues can also guide treatment strategies including choosing optimal site for laser or electrocautery radial incisions, or intralesional steroid injections.
In addition, the shape of the airway lumen at the stenotic level affects flow dynamics and symptoms. In a computer modeling study of tracheal stenosis lesion, morphology impacted flow velocity and the pressure drop across the stenosis, with triangular stenosis causing less pressure drop than elliptical ones for the same CSA (51). Morphology seems to also affect outcomes of endoscopic procedures. The completely circumferential strictures were shown to lead to poorer outcomes because they require more interventions when compared with eccentric lesions (52). Thus, describing the shape of the stenosis as well as associated malacia are important factors for treatment decisions and outcomes, and these factors can be assessed during a bronchoscopic evaluation under moderate sedation.
Bronchoscopic management of tracheal stenosis
The choice of intervention is generally dictated by all these factors reviewed above as well as operability, patient’s quality of life, and availability of technology or treatment modality (3). A multi-disciplinary airway team approach that includes otolaryngology, thoracic surgery, and interventional pulmonology is essential in determining treatment timing and modality. Parameters from classification systems pertinent to treatment are summarized in Table 3. Surgical resection of the stenotic area is the procedure of choice for most patients with benign tracheal stenosis, especially if complex in nature and not due to a systemic inflammatory disorder (e.g., GPA). However, co-morbidities such as diabetes, prior tracheostomy, prolonged steroid usage, high subglottic location, length of the lesion (>4–6 cm) can be prohibitive for resection. Mechanical dilation, laser therapy, ES, stent placement and adjuvant local pharmacologic therapy such as steroid injections and mitomycin application can be considered (53). Herein we describe and review the evidence for the endoscopic therapeutic options and their outcomes.
Table 3
| Criteria | Description |
|---|---|
| Functional class | Modified World Health Organization functional classification |
| 1 | Asymptomatic: ordinary physical activity does not cause symptoms |
| 2 | Symptomatic on exertion: there is no discomfort at rest, but normal physical activity causes increased symptoms |
| 3 | Symptomatic with daily activity: there is no discomfort at rest, but less than ordinary activity causes increased symptoms |
| 4 | Symptomatic at rest: symptoms may be present at rest and are increased by almost any physical activity |
| Extent | Location and distribution of the stenotic airway segment |
| Vertical length | (<1, 1–4, >4 cm) |
| Location | Glottic, subglottic, tracheal, or tracheobronchial |
| Morphology | Describes the shape of the airway lumen |
| Simple | Short-segment concentric stenosis, <1 cm in vertical length, without malacia |
| Complex | Long segments, >1 cm, with tracheal wall injury or associated malacia |
| Pseudoglottic | Refers to triangular stenosis |
| Eccentric | Refers to uneven distribution of the hypertrophic stenotic tissues |
| Circumferential | Refers to concentric (360°) distribution of the hypertrophic stenotic tissues |
| Voice quality | Describes presence of phonation-related symptoms |
| 1 | No problems related to voice |
| 2 | Some problems related to voice, needing repetition, closer proximity, or modulation |
| 3 | Significant problems related to being heard, needing significant augmentation |
| 4 | Problems with whispering |
| 5 | No voice |
| Origin | Describes the underlying cause responsible for the airway abnormality |
| Idiopathic | No underlying cause identified |
| Secondary | Secondary to known underlying processes or previous airway injury |
| Severity | Describes the degree of reduction in CSA |
| 1 | Normal: no reduction in CSA compared with normal airway caliber |
| 2 | Mild: reduction in CSA <50% |
| 3 | Moderate: reduction in CSA 51–70% |
| 4 | Severe: reduction in CSA ≥71% |
| Swallowing function | Describes the degree of swallowing impairment |
| 1 | No issues |
| 2 | Pain with swallowing, able to swallow liquids and solids |
| 3 | Pain with swallowing, able to swallow liquids only |
| 4 | Unable to swallow liquids or solids |
Reprinted from (3), Copyright © 2016, with permission from Elsevier. CSA, cross-sectional area.
LAMD
The laser cutting effects on tissues can be safely and effectively used in the endoscopic management of tracheal stenosis. Tissue vaporization (cutting) or photocoagulation are determined by power density, absorption, scattering, and delivery system. Power density depends on power setting, distance from the fiber to the target tissue and exposure time. Absorption and scattering make the difference between cutting and coagulation. For example, the CO2 laser has an infrared wavelength of 10,600 nm and is an excellent cutting tool due to its high absorption and low scattering. It is widely preferred in otolaryngology for treating SGS.
Laser bronchoscopy can be done both via flexible and rigid bronchoscopy and each modality carries its own advantages and disadvantages (54). At our institution, we mainly use rigid bronchoscopy to perform endoscopic laser treatments. For tracheal stenosis, we apply mucosal sparing technique, which involves two to three radial incisions by laser followed by gentle dilation with the rigid bronchoscope or by an inflatable endoscopic balloon (17). Laser treatments should not be circumferentially applied to the lesion as these risks inducing further stenosis due to secondary retraction and scarring of the mucosa. The incisions should be precise and without collateral thermal damage, which are best obtained with the CO2 or potassium titanyl phosphate (KTP) lasers (Figure 3A). Characteristics of lasers used in bronchoscopy are reviewed in Table 4 (55).

Table 4
| Type of laser used in airway | Wave length (nm) | Coagulation | Cutting precision | Vaporization | Absorption | Depth of penetration (mm) |
|---|---|---|---|---|---|---|
| Nd:YAG | 1,064 | ++ | + | +++ | Proteins of any opaque tissue | 5–15 |
| Nd:YAP | 1,340 | +++ | ++ | + | Water | 3 |
| Ho:YAG | 2,100 | + | +++ | ++ | Water | 0.5 |
| KTP | 532 | + | ++ | ++ | Hemoglobin | 1 |
| CO2 | 10,600 | + | +++ | +++ | Water | 0.1 |
| Thulium | 2,000 | +++ | +++ | + | Water | <1 |
| Diode | 1,318 | ++ | ++ | + | Water | 3–5 |
| Argon | 516 | +++ | + | + | Hemoglobin | 1 |
Reprinted with permission of the American Thoracic Society, Miller et al., 2013. Annals of American Thoracic Society. Copyright © 2022 American Thoracic Society. All rights reserved. +, low efficacy; ++, medium efficacy; +++, high efficacy. Nd:YAG, neodymium-doped yttrium aluminium garnet; Nd:YAP, neodymium-doped yttrium aluminium perovskite; Ho:YAG, holmium-doped yttrium aluminium garnet; KTP, potassium titanyl phosphate; CO2, carbon dioxide.
The best outcomes with laser therapy are noted in simple, web-like stenosis which can be potentially cured when combined with mechanical dilation. In PITS, the cure rate ranges from 60% to 95% after two to three treatment sessions (17,56,57). One study including 167 post-intubation and 34 post-tracheostomy stenoses, showed that 96% of cases with simple stenosis had a full resolution with endoscopic therapy alone when compared to 79% of cases with complex stenosis (58). In another study with long-term follow-up in 98 patients with a benign tracheal obstruction from various causes treated with laser therapy, only 60% showed complete resolution without needing another treatment modality (59). Literature on post infectious strictures is limited with the outcomes depending on associated chondritis (malacia). One retrospective study of post-tuberculosis airway stenosis showed that malacia and prior bronchoscopic laser-assisted resection were associated with less favorable outcomes, including symptom recurrence (60). In tracheal stenosis secondary to systemic inflammatory diseases (e.g., GPA), laser therapy has been used in a combined approach with systemic therapy (61).
Electrosurgical management
ES is available in monopolar and bipolar devices. In monopolar electrodes, current flows from the generator through the active electrode, into the tissue, through the patient, the dispersive electrode (grounding pad) and returns to the generator. In bipolar devices, the active and return electrodes are located at the target tissue site, typically within the instrument tip such as the forceps and current is limited to the tissue grasped (62). Monopolar ES is used for endoscopic cutting of fibrotic tissues in tracheal stenosis. Different types of monopolar electrodes are available (including blunt probe, knife, forceps and wire snare loops), but achieving contact of the probe with the mucosa at the site of treatment is necessary for this technique to be effective. Of note, argon plasma coagulation (APC) is a noncontact technique that utilizes ionized argon gas (plasma) to conduct monopolar electrical current to the nearest tissue. The temperature generated will determine the effect produced: coagulation (60–80 ℃), desiccation (>100 ℃), carbonization (>200 ℃) and vaporization (>300 ℃) but due to its diffuse tissue effects, APC should not be used for treatment of benign tracheal stenosis (63). ES knife or needle, in a contact mode, is the preferred ES modality for cutting through fibrotic tissues (Figure 3B). The depth of treatment from ES and histological effect can be assessed by visual feedback, unlike in laser therapy, where a tissue effect could go beyond the visual surface (64). ES is cheaper than laser and available in most hospitals.
In a study of 22 patients with benign tracheal stenosis, use of ES knife showed improvement in lung function and symptoms in 100% of the patients. Mean symptom free time was ~6 months and less than 50% of patients required second intervention (65). In a retrospective review of 15 patients with benign tracheal stenosis, ES was used in combination with rigid bronchoscopy and stent placement (80%) that showed a success rate of 87% with stent removed later in 55% patients (66). As demonstrated in another retrospective series, ES has advantages when combined with balloon dilation. A case series that included 43 patients with benign tracheal stenosis noted that an integrated approach of ES needle knife combined with balloon dilatation decreased the proportion of restenosis requiring treatment from 82% to 47% at 6 months and had a lower stenosis degree after treatment (67). The above studies highlight the additional value of combining ES along with other strategies such as dilation and potentially stent placement in improving symptoms.
Adjuvant pharmacologic management
A few pharmacologic options have been consistently used and reported in the literature for managing laryngotracheal stenoses, mainly involving intralesional steroid injection and MMC application. Wound healing and maturation are complex processes and these therapies are aimed at the inflammatory and proliferative phase of healing, respectively. The goal of these therapies is to suppress the propagation of inflammation and to modify the natural history of injury and wound formation (68).
MMC
MMC is an antimicrobial agent that carries additional antimetabolite and antiproliferative properties. It works as an alkylating agent inhibiting DNA synthesis and suppressing RNA and protein synthesis at higher concentrations. In airway pathology, it works as an inhibitor of fibroblast proliferation in wound healing processes. Topical MMC has been studied as adjuvant therapy in benign airway stenosis (69), in conjunction with endoscopic treatments such as radial incision with laser and dilation with MMC, and dilation alone with MMC. A meta-analysis of 15 studies with 387 patients showed that 70% of patients remained symptom-free after one year since the intervention (70). This analysis also showed that in studies that compared endoscopic treatment with and without MMC, a symptom-free period >1 year was four times higher in patients receiving MMC. A randomized, double-blind, placebo-controlled trial with 26 patients with mixed benign etiologies showed a relapse rate of 7%, 36% and 69% at the 1-, 3- and 5-year mark with two applications compared to 33%, 58% and 70% with one application of MMC, respectively. The study showed that the recurrence rate is similar in these groups at the 5-year mark; however, a dual application strategy delays the recurrence of symptomatic restenosis (11).
Steroid injection
Steroids are efficacious in the early inflammatory stage of injury mediated by prostaglandins, tumor growth factors, and interleukin-1 (71). Intralesional steroid injections have been shown to inhibit stricture formation by interfering with collagen synthesis, fibrosis, and chronic scarring. It has also been suggested that triamcinolone prevents the cross-linking of collagen that results in scar contracture so that if the scar is stretched and corticosteroid is injected into it, contracture will presumably not occur and causes a decrease in the fibrotic healing process (72).
Considering the etiology of injury/stenosis is essential and etiologies such as autoimmune diseases with active inflammation are known to benefit the most from intralesional steroid therapy. For example, a study evaluating effect of intralesional steroids in a total of 45 patients with autoimmune, idiopathic and traumatic etiology showed that 75% (9/12) of patients with autoimmune etiology achieved airway patency at 2 years; 3 patients who did not achieve benefit in the auto-immune group were noted to have long-term symptoms (7–18 months) and had tracheostomy at presentation (12). For SGS, endoscopic management with intra-lesion injection of steroids had a lower recurrence rate when compared with dilation alone and remains a preferred initial modality for managing SGS (28).
Airway stenting
In patients who are non-operative candidates (either due to lesion characteristics or comorbidities) or in patients with recurrent stenosis after endoscopic or surgical treatment, an airway stent placement should be considered for symptom palliation. In 2005, given the high number of complications reported with metallic stents, the Food and Drug Administration (FDA) published an advisory on their use in benign airway lesions. In addition, FDA noted that patients with benign airway stenosis have a greater risk of severe complications than those with malignant disorders since the metallic tracheal stent is left in place longer (73). Hence, silicone stents are the preferred type in benign airway stenosis and could be considered a long-term option for palliation in non-surgical candidates. A 7-year follow-up study after placement of Dumon silicone stents in 263 patients who underwent 419 silicone stent placements for benign tracheal stenosis showed that of the 117 patients in whom the stent was removed, no recurrence was noted in 64 (25% of all cases). The mean duration of stent placement in this study was 1.2 years (74).
Stents in post-intubation and post tracheostomy tracheal stenosis
Most studies reporting on stenting combine PTTS and PITS when describing outcomes, however, these entities have different mechanism of injury, characteristics of stenosis, and treatment-related outcomes. A study of 117 patients with PITS and 88 patients with PTTS showed that the success rate without surgery was higher in the PITS than in the PTTS group (76.9% vs. 63.6%, P<0.05). Additionally, successful airway stent or tracheostomy removal was achieved in 46.2% of patients in the PITS and in 33.0% of the PTTS group (P=0.06) (75). This is likely due to the complex nature of PTTS, with associated malacia due to chondritis. In addition, the outcomes of bronchoscopic interventions depend on the precise location of PTTS, with one study of 99 PTTS patients showing that silicone stents were required more in stomal-type stenosis compared to cuff-related or tip stenosis (76% vs. 55%, P=0.031). It was also noted that cuff type stenosis had a higher success rate with respect to removal of tracheostomy tube compared to the other types of PTTS (71% vs. 45%, P=0.012) (76). This is relevant for the bronchoscopist as describing the exact location of PTTS becomes relevant for predicting outcomes.
In a study focused on PITS, among 60 complex stenosis patients, 47 patients (78%) required stent placement. Of these, 47% had their stent removed after one year and did not require further therapy. This study’s mean duration of stenting was 11.6±4.6 months (16). Another study focusing on PITS showed a similar period of stent placement of 12 months in 59 patients, of whom 22 (40%) had the stent removed and required no further intervention (25).
Thus, airway stents, when necessary, do not have to be a life-long intervention and based on the above data, they can be removed after a period of approximately 12 months or if a patient were to become a surgical candidate. Indeed, in some instances, airway remodeling can occur, resulting in the removal of stents and no stenosis recurrence. Based on the available literature, a stent removal should be attempted to evaluate airway patency as regeneration of the tracheal cartilage is possible and patency may be restored (Figure 4). While the optimal time frame for a trial of stent removal is unknown, a higher rate of success (46.8%) was described when stents remained in place for a longer time (mean of ~12 months) (16). A recent study of 128 subjects with complex tracheal stenosis secondary to PITS and PTTS treated with protocolized duration for silicone airway stenting showed that restenosis at 1 year after stent removal was seen in 58% of the patients, with no difference between PITS and PTTS. In this study, the stent was removed after 6 months during the first 8 years and after 4 months during the next 8 years (77). Based on the available data, it appears that for a successful removal of the stent with lower risk of restenosis, stents should be in place for a minimum of 12 months.

Stenting in post-infectious stenoses
A wide variety of infectious etiologies result in airway stenosis such as tuberculosis, fungal (Aspergillus, Blastomycosis), viral (SARS-COV2) or bacterial tracheitis (Klebsiella rhinoscleromatis or Staphylococcus aureus tracheitis). In tuberculosis-endemic areas, airway complications are frequently encountered and seen in up to 20% of patients. A retrospective series of 71 patients with stent placement for stenosis secondary to endobronchial tuberculosis showed successful removal in 56% patients with a median duration of 12.5 months for stent placement; 38% of patients required permanent stent placement (78). In another retrospective series, 75 of 80 (94%) required airway stenting to maintain patency. There was an immediate improvement in lung function and symptoms in 88% post-stenting. Stent was successfully removed in 72% of patients after a median of 14 months with recurrence noted in 9% of patients requiring repeat stent placement (79). Granulation tissue (76%) and migration (70%) were the commonest complications in this group (80). Based on these data, we suggest that for complex post-tuberculosis (post-TB) strictures, stents should be in place for a minimum period of 12 months for eventual successful removal with decreased restenosis rates.
Use of self-expanding metallic stents in benign tracheal stenosis
Recently, a third generation of metallic stents which are fully covered and self-expandable are being considered in patients with benign tracheal stenosis. A study of 30 patients with 40 stents showed a clinical success rate of 40% (no additional interventions after elective stent removal) but 50% of the stents had to be removed secondary to stent related complications after a median 77 days (81). Another study of 19 patients with fully covered self expandable metallic stent (SEMS) for benign tracheal stenosis had stents removed in all patients secondary to complications at a median of 3 months (82). Based on the available limited studies on long term efficacy and safety, fully covered SEMS should not be considered as the first choice of stent in management of inoperable benign tracheal stenosis but are an option in scenarios where a rigid bronchoscopy cannot be performed for inserting silicone stents. These patients should be closely followed up for potential complications.
Multi-disciplinary management of tracheal stenosis
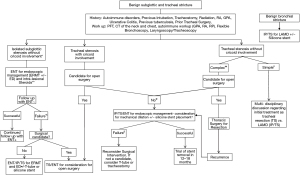
Patients with tracheal stenoses of all types benefit from a collaboration within a multidisciplinary team of physicians with expertise in different endoscopic and surgical techniques. At a minimum, this team should involve otolaryngologists, thoracic surgeons, and interventional pulmonologists. In multidisciplinary airway meetings and clinics, the team discusses and considers different methods for management of tracheal diseases including conservative medical management alone, endoscopic incisions and dilation, stenting, and open surgical resection. Algorithms of multidisciplinary care, based on lesion location, etiology, extent and patient operability have been proposed and used in practice (Figure 5). At our institution, multidisciplinary management of complex airway cases includes regular conferences to discuss patients and review imaging, laryngoscopy, and bronchoscopy videos. This is often followed by shared clinics for further evaluation and joint procedures in the operating room. Notably, collaborative management in the operating room is also frequently utilized for both airway assessment and therapeutic management. In some cases, airway patency must be restored emergently via rigid bronchoscopy, but an evaluation by a surgeon can be performed simultaneously, especially for patients who are known to have a complex stenosis and are otherwise surgical candidates. Other times, the otolaryngologists manage the subglottic disease while the interventional pulmonologists address the tracheal component. Another function of the multidisciplinary complex airway care team is to manage patients who develop short or long-term tracheostomy-related adverse events, including but not limited to stomal strictures, SGS, stenosis/granulation distal to the tracheostomy and tracheoesophageal fistulas. For inpatients, allied healthcare providers (nurses, physician assistants, or respiratory therapists) with specialized training in tracheostomy management should be involved for consistent follow up and early detection of any tracheostomy-related issues and to assure an optimal post procedure management (downsizing the tracheostomy tube, capping trials, and decannulation). Speech and language pathologists are integral members helping patients to resume speech and swallowing function. Studies have described the approach to implementation of tracheostomy specialists and have demonstrated improved outcomes with fewer complications and critical incidents. Tracheostomy specialists can also help to educate other staff members in tracheostomy management. Since the beginning of COVID-19 pandemic, there has been even more of a need for careful tracheostomy management from multidisciplinary specialized care teams (83,84).
Conclusions
Benign tracheal stenosis requires a comprehensive evaluation and multidisciplinary management. Short of true airway emergencies, we recommend an inspection bronchoscopy prior to any intervention to assess lesion characteristics that impact decision regarding endoscopic or surgical management. For inoperable patients, there are several bronchoscopic techniques including laser, electrosurgical, mechanical dilation, stenting, as well as local application of mitomycin or intralesional steroid injection. We recommend a multidisciplinary approach integrating thoracic surgery, otolaryngology, and interventional pulmonology to ensure optimal and timely care for patients with tracheal stenosis.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1734/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1734/coif). SM has acted as a paid educational consultant for Olympus, Medtronic, Boston Scientific, and Johnson and Johnson. He is a course director for bronchoscopy courses at CHEST, and is AABIP President and a BOD member. EH is an educational consultant for Intuitive, Olympus, and Biodesix. AW has been a consultant for Noah Medical and Ambu. He is an educational consultant for Medtronic. He is a Speaker for Biodesix. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agrawal A, Baird BJ, Madariaga MLL, et al. Multi-disciplinary management of patients with benign airway strictures: A review. Respir Med 2021;187:106582. [Crossref] [PubMed]
- Taylor L, Mitchell JD. Open Surgical Approach to Tracheal Stenosis. WABIP Newsletter. 2022;10:7-9.
- Murgu SD, Egressy K, Laxmanan B, et al. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Aravena C, Almeida FA, Mukhopadhyay S, et al. Idiopathic subglottic stenosis: a review. J Thorac Dis 2020;12:1100-11. [Crossref] [PubMed]
- Songu M, Ozkul Y. Risk Factors for Adult Postintubation Tracheal Stenosis. J Craniofac Surg 2019;30:e447-50. [Crossref] [PubMed]
- Li M, Yiu Y, Merrill T, et al. Risk Factors for Posttracheostomy Tracheal Stenosis. Otolaryngol Head Neck Surg 2018;159:698-704. [Crossref] [PubMed]
- Gelbard A, Francis DO, Sandulache VC, et al. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope 2015;125:1137-43. [Crossref] [PubMed]
- Lally L, Lebovics RS, Huang WT, et al. Effectiveness of rituximab for the otolaryngologic manifestations of granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2014;66:1403-9. [Crossref] [PubMed]
- Wang H, Wright CD, Wain JC, et al. Idiopathic Subglottic Stenosis: Factors Affecting Outcome After Single-Stage Repair. Ann Thorac Surg 2015;100:1804-11. [Crossref] [PubMed]
- Feinstein AJ, Goel A, Raghavan G, et al. Endoscopic Management of Subglottic Stenosis. JAMA Otolaryngol Head Neck Surg 2017;143:500-5. [Crossref] [PubMed]
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009;119:272-83. [Crossref] [PubMed]
- Wierzbicka M, Tokarski M, Puszczewicz M, et al. The efficacy of submucosal corticosteroid injection and dilatation in subglottic stenosis of different aetiology. J Laryngol Otol 2016;130:674-9. [Crossref] [PubMed]
- Bertelsen C, Shoffel-Havakuk H, O’Dell K, et al. Serial In-Office Intralesional Steroid Injections in Airway Stenosis. JAMA Otolaryngol Head Neck Surg 2018;144:203-10. [Crossref] [PubMed]
- Hoffman MR, Coughlin AR, Dailey SH. Serial office-based steroid injections for treatment of idiopathic subglottic stenosis. Laryngoscope 2017;127:2475-81. [Crossref] [PubMed]
- Gelbard A, Shyr Y, Berry L, et al. Treatment options in idiopathic subglottic stenosis: protocol for a prospective international multicentre pragmatic trial. BMJ Open 2018;8:e022243. [Crossref] [PubMed]
- Cavaliere S, Bezzi M, Toninelli C, et al. Management of post-intubation tracheal stenoses using the endoscopic approach. Monaldi Arch Chest Dis 2007;67:73-80. [PubMed]
- Mehta AC, Lee FY, Cordasco EM, et al. Concentric tracheal and subglottic stenosis. Management using the Nd-YAG laser for mucosal sparing followed by gentle dilatation. Chest 1993;104:673-7. [Crossref] [PubMed]
- Maurizi G, Vanni C, Rendina EA, et al. Surgery for laryngotracheal stenosis: Improved results. J Thorac Cardiovasc Surg 2021;161:845-52. [Crossref] [PubMed]
- D’Andrilli A, Maurizi G, Andreetti C, et al. Long-term results of laryngotracheal resection for benign stenosis from a series of 109 consecutive patients. Eur J Cardiothorac Surg 2016;50:105-9. [Crossref] [PubMed]
- Bagheri R, Majidi M, Khadivi E, et al. Outcome of surgical treatment for proximal long segment post intubation tracheal stenosis. J Cardiothorac Surg 2013;8:35. [Crossref] [PubMed]
- Yamamoto K, Kojima F, Tomiyama K, et al. Meta-analysis of therapeutic procedures for acquired subglottic stenosis in adults. Ann Thorac Surg 2011;91:1747-53. [Crossref] [PubMed]
- Wright CD, Li S, Geller AD, et al. Postintubation Tracheal Stenosis: Management and Results 1993 to 2017. Ann Thorac Surg 2019;108:1471-7. [Crossref] [PubMed]
- Rea F, Callegaro D, Loy M, et al. Benign tracheal and laryngotracheal stenosis: surgical treatment and results. Eur J Cardiothorac Surg 2002;22:352-6. [Crossref] [PubMed]
- Dutau H. Airway stenting for benign tracheal stenosis: what is really behind the choice of the stent? Eur J Cardiothorac Surg 2011;40:924-5. [PubMed]
- Lim SY, Kim H, Jeon K, et al. Prognostic factors for endotracheal silicone stenting in the management of inoperable post-intubation tracheal stenosis. Yonsei Med J 2012;53:565-70. [Crossref] [PubMed]
- Jeong BH, Um SW, Suh GY, et al. Results of interventional bronchoscopy in the management of postoperative tracheobronchial stenosis. J Thorac Cardiovasc Surg 2012;144:217-22. [Crossref] [PubMed]
- Noirclerc MJ, Metras D, Vaillant A, et al. Bilateral bronchial anastomosis in double lung and heart-lung transplantations. Eur J Cardiothorac Surg 1990;4:314-7. [Crossref] [PubMed]
- Gelbard A, Anderson C, Berry LD, et al. Comparative Treatment Outcomes for Patients With Idiopathic Subglottic Stenosis. JAMA Otolaryngol Head Neck Surg 2020;146:20-9. [Crossref] [PubMed]
- Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J 2007;30:7-12. [Crossref] [PubMed]
- Ghorbani A, Dezfouli AA, Shadmehr MB, et al. A proposed grading system for post-intubation tracheal stenosis. Tanaffos 2012;11:10-4. [PubMed]
- Grundfast KM, Morris MS, Bernsley C. Subglottic stenosis: retrospective analysis and proposal for standard reporting system. Ann Otol Rhinol Laryngol 1987;96:101-5. [Crossref] [PubMed]
- Lano CF Jr, Duncavage JA, Reinisch L, et al. Laryngotracheal reconstruction in the adult: a ten year experience. Ann Otol Rhinol Laryngol 1998;107:92-7. [Crossref] [PubMed]
- McCaffrey TV. Classification of laryngotracheal stenosis. Laryngoscope 1992;102:1335-40. [Crossref] [PubMed]
- Nouraei SA, Nouraei SM, Upile T, et al. A proposed system for documenting the functional outcome of adult laryngotracheal stenosis. Clin Otolaryngol 2007;32:407-9. [Crossref] [PubMed]
- Myer CM 3rd, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol 1994;103:319-23. [Crossref] [PubMed]
- Herrington HC, Weber SM, Andersen PE. Modern management of laryngotracheal stenosis. Laryngoscope 2006;116:1553-7. [Crossref] [PubMed]
- Hall SR, Allen CT, Merati AL, et al. Evaluating the utility of serological testing in laryngotracheal stenosis. Laryngoscope 2017;127:1408-12. [Crossref] [PubMed]
- Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with wegener’s granulomatosis†. Laryngoscope 1992;102:1341-5. [Crossref] [PubMed]
- Costantino CL, Niles JL, Wright CD, et al. Subglottic Stenosis in Granulomatosis With Polyangiitis: The Role of Laryngotracheal Resection. Ann Thorac Surg 2018;105:249-53. [Crossref] [PubMed]
- Miller RD, Hyatt RE. Evaluation of obstructing lesions of the trachea and larynx by flow-volume loops. Am Rev Respir Dis 1973;108:475-81. [PubMed]
- Hyatt RE, Black LF. The flow-volume curve. A current perspective. Am Rev Respir Dis 1973;107:191-9. [PubMed]
- Hoppe H, Walder B, Sonnenschein M, et al. Multidetector CT virtual bronchoscopy to grade tracheobronchial stenosis. AJR Am J Roentgenol 2002;178:1195-200. [Crossref] [PubMed]
- De Wever W, Vandecaveye V, Lanciotti S, et al. Multidetector CT-generated virtual bronchoscopy: an illustrated review of the potential clinical indications. Eur Respir J 2004;23:776-82. [Crossref] [PubMed]
- Polverosi R, Vigo M, Baron S, et al. Evaluation of tracheobronchial lesions with spiral CT: comparison between virtual endoscopy and bronchoscopy. Radiol Med 2001;102:313-9. [PubMed]
- Murgu S, Colt HG. Morphometric bronchoscopy in adults with central airway obstruction: case illustrations and review of the literature. Laryngoscope 2009;119:1318-24. [Crossref] [PubMed]
- Husein M, Manoukian JJ, Platt R, et al. Ultrasonography and videobronchoscopy to assess the subglottic diameter in the paediatric population: a first look. J Otolaryngol 2002;31:220-6. [Crossref] [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest 1999;115:1500-6. [Crossref] [PubMed]
- Murgu S, Colt H. Subjective assessment using still bronchoscopic images misclassifies airway narrowing in laryngotracheal stenosis. Interact Cardiovasc Thorac Surg 2013;16:655-60. [Crossref] [PubMed]
- Brouns M, Jayaraju ST, Lacor C, et al. Tracheal stenosis: a flow dynamics study. J Appl Physiol (1985) 2007;102:1178-84. [Crossref] [PubMed]
- Brouns M, Verbanck S, Lacor C. Influence of glottic aperture on the tracheal flow. J Biomech 2007;40:165-72. [Crossref] [PubMed]
- Nishine H, Hiramoto T, Kida H, et al. Assessing the site of maximal obstruction in the trachea using lateral pressure measurement during bronchoscopy. Am J Respir Crit Care Med 2012;185:24-33. [Crossref] [PubMed]
- Simpson GT, Strong MS, Healy GB, et al. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol 1982;91:384-8. [Crossref] [PubMed]
- Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9. [Crossref] [PubMed]
- Cavaliere S, Dumon JF. Laser Bronchoscopy. Interventional Bronchoscopy 2000;30:108-19. [Crossref]
- Miller RJ, Murgu SD. Bronchoscopic resection of an exophytic endoluminal tracheal mass. Ann Am Thorac Soc 2013;10:697-700. [Crossref] [PubMed]
- Baugnée PE, Marquette CH, Ramon P, et al. Endoscopic treatment of post-intubation tracheal stenosis. Apropos of 58 cases. Rev Mal Respir 1995;12:585-92. [PubMed]
- Arami S, Jabbardarjani H, Masjedi M. Treatment of post-intubation tracheal stenosis with the Nd-YAG laser at the NRITLD. Crit Care 2005;9:121. [Crossref]
- Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg 2009;35:429-33; discussion 933-4. [Crossref] [PubMed]
- Rahman NA, Fruchter O, Shitrit D, et al. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg 2010;5:2. [Crossref] [PubMed]
- Lee KCH, Tan S, Goh JK, et al. Long-term outcomes of tracheobronchial stenosis due to tuberculosis (TSTB) in symptomatic patients: airway intervention vs. conservative management. J Thorac Dis 2020;12:3640-50. [Crossref] [PubMed]
- Catano J, Uzunhan Y, Paule R, et al. Presentation, Diagnosis, and Management of Subglottic and Tracheal Stenosis During Systemic Inflammatory Diseases. Chest 2022;161:257-65. [Crossref] [PubMed]
- Alkatout I, Schollmeyer T, Hawaldar NA, et al. Principles and safety measures of electrosurgery in laparoscopy. JSLS 2012;16:130-9. [Crossref] [PubMed]
- Barlow DE. Endoscopic applications of electrosurgery: a review of basic principles. Gastrointest Endosc 1982;28:73-6. [Crossref] [PubMed]
- van Boxem TJ, Westerga J, Venmans BJ, et al. Tissue effects of bronchoscopic electrocautery: bronchoscopic appearance and histologic changes of bronchial wall after electrocautery. Chest 2000;117:887-91. [Crossref] [PubMed]
- Amat B, Esselmann A, Reichle G, et al. The electrosurgical knife in an optimized intermittent cutting mode for the endoscopic treatment of benign web-like tracheobronchial stenosis. Arch Bronconeumol 2012;48:14-21. [Crossref] [PubMed]
- Park JW, Ko Y, Kim C. Use of an Insulation-Tipped Knife during Rigid Bronchoscopic Treatment of Benign Tracheobronchial Stenosis. Medicina (Kaunas) 2021;57:251. [Crossref] [PubMed]
- Bo L, Li C, Chen M, et al. Application of Electrocautery Needle Knife Combined with Balloon Dilatation versus Balloon Dilatation in the Treatment of Tracheal Fibrotic Scar Stenosis. Respiration 2018;95:182-7. [Crossref] [PubMed]
- Hirshoren N, Eliashar R. Wound-healing modulation in upper airway stenosis-Myths and facts. Head Neck 2009;31:111-26. [Crossref] [PubMed]
- Ward RF, April MM. Mitomycin-C in the treatment of tracheal cicatrix after tracheal reconstruction. Int J Pediatr Otorhinolaryngol 1998;44:221-6. [Crossref] [PubMed]
- Queiroga TLO, Cataneo DC, Martins RHG, et al. Mitomycin C in the Endoscopic Treatment of Laryngotracheal Stenosis: Systematic Review and Proportional Meta-Analysis. Int Arch Otorhinolaryngol 2020;24:e112-24. [Crossref] [PubMed]
- Ashcraft KW, Holder TM. The xperimental treatment of esophageal strictures by intralesional steroid injections. J Thorac Cardiovasc Surg 1969;58:685-91 passim. [Crossref] [PubMed]
- Mendelsohn HJ, Maloney WH. The treatment of benign strictures of the esophagus with cortisone injection. Ann Otol Rhinol Laryngol 1970;79:900-4. [Crossref] [PubMed]
- FDA Public Health Notification: Complications from Metallic Tracheal Stents in Patients with Benign Airway Disorders. 2005.
- Dumon JF, Cavaliere S, Diaz-Jimenez JP, et al. Seven-Year Experience with the Dumon Prosthesis. J Bronchology Interv Pulmonol 1996;3:6-10.
- Shin B, Kim K, Jeong BH, et al. Clinical significance of differentiating post-intubation and post-tracheostomy tracheal stenosis. Respirology 2017;22:513-20. [Crossref] [PubMed]
- Shin B, Kim K, Jeong BH, et al. Clinical implications of differentiating between types of post-tracheostomy tracheal stenosis. J Thorac Dis 2017;9:4413-23. [Crossref] [PubMed]
- Chrissian AA, Diaz-Mendoza J, Simoff MJ. Restenosis Following Bronchoscopic Airway Stenting for Complex Tracheal Stenosis. J Bronchology Interv Pulmonol 2023;30:268-76. [Crossref] [PubMed]
- Lim SY, Park HK, Jeon K, et al. Factors predicting outcome following airway stenting for post-tuberculosis tracheobronchial stenosis. Respirology 2011;16:959-64. [Crossref] [PubMed]
- Ryu YJ, Kim H, Yu CM, et al. Use of silicone stents for the management of post-tuberculosis tracheobronchial stenosis. Eur Respir J 2006;28:1029-35. [Crossref] [PubMed]
- Verma A, Um SW, Koh WJ, et al. Long-term tolerance of airway silicone stent in patients with post-tuberculosis tracheobronchial stenosis. ASAIO J 2012;58:530-4. [Crossref] [PubMed]
- Fortin M, Lacasse Y, Elharrar X, et al. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017;93:430-5. [Crossref] [PubMed]
- Jeong BH, Ng J, Jeong SH, et al. Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina (Kaunas) 2020;56:367. [Crossref] [PubMed]
- Meister KD, Pandian V, Hillel AT, et al. Multidisciplinary Safety Recommendations After Tracheostomy During COVID-19 Pandemic: State of the Art Review. Otolaryngol Head Neck Surg 2021;164:984-1000. [Crossref] [PubMed]
- Lamb CR, Desai NR, Angel L, et al. Use of Tracheostomy During the COVID-19 Pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology Program Directors Expert Panel Report. Chest 2020;158:1499-514. [Crossref] [PubMed]


