
Marginal calcification of thymoma: differences in the location of calcification indicate differences in the characteristics of thymomas
Highlight box
Key findings
• Calcification of thymoma could be classified into inner calcification and marginal calcification. The differences in the location of calcification indicate differences in the characteristics of thymomas.
What is known and what is new?
• In low-risk thymomas, especially type AB thymoma, marginal calcification was observed more frequently than in other lesions.
• In group I, younger patients and patients with advanced-stage disease were included.
What is the implication, and what should change now?
• The location of calcification should be a point of focus in thymomas.
Introduction
Thymomas are the most common tumor of the anterior mediastinum. Surgical resection has been recommended as the principal treatment, and the completeness of resection is considered to be the most important determinant of long-term survival in these patients (1-3).
Recently, an increased incidence of small-sized thymic epithelial tumors (TETs) has been reported. The differential diagnosis of thymoma and thymic carcinoma in small-sized TETs has not been easy preoperatively (4,5). TETs have been classified by their pathological results into three subgroups with increasing grades of malignancy: low-risk thymoma [World Health Organization (WHO) histological classification A, AB and B1], high-risk thymoma (B2 and B3), and thymic carcinoma (6). It would be beneficial if the malignancy grade of TETs were possible using usual radiological examinations without a tumor biopsy.
Calcification is reported in 10–40% of thymomas (7-10). The incidence of calcification might differ according to the WHO histological classification. In type B thymomas in particular, calcification may be found more frequently than in other types (8). Interestingly, the pattern, size, and location of calcification vary among lesions. However, while the presence of calcification reportedly indicates the tumor invasiveness of thymomas or the stage, we are unsure of whether or not all types of calcification indicate an invasive behavior, as we have experienced noninvasive thymoma cases in which calcification was located at the marginal zone.
In the present retrospective study, 77 thymomas were reviewed for their location, size, and patterns of calcification to determine the relationships between clinicopathological factors and prognosis and calcification. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-164/rc).
Methods
A retrospective review of clinical and pathologic data from all patients undergoing surgery for thymomas at Aichi Medical University Hospital was conducted between January 2012 and May 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) of Aichi Medical University Hospital (No. 2022-702). As opt-out consent procedures have been adapted and the details of the study were shown on the website of the institution, written informed consent was not obtained from the patients.
We included only operated cases pathologically diagnosed with thymoma based on surgically resected samples. Patients without surgical resection were not included in the present study. Preoperative computed tomography (CT) findings were examined in all patients, and the pattern, size, and location of calcification were determined by two radiologists who specialized in diagnosing mediastinal lesions. Calcification was categorized by its location into two groups: those with inner calcifications of the tumor (group I) and those with marginal calcification (group M). Of note, only calcification located clearly at the marginal zone of the tumor was defined as marginal calcification. In addition, cases with both obvious marginal calcification and inner calcification were included in group M. Cases in group I were further categorized by the size of calcification as stippled calcification (0.1–0.3 cm), nodular calcification (0.4–1.9 cm), and large calcification (≥2.0 cm), while cases in group M were further categorized by the pattern of calcification as focal or multifocal stippled calcification, dot-lined calcification, and ring calcification. Cases without calcification were defined as group N.
Both the Masaoka-Koga staging system (1,11) and T factor of the tumor-node-metastasis (TNM) staging system (12) were used for staging thymomas in the present study. For all groups, the clinicopathological factors and prognosis were analyzed. For the prognosis, only the recurrence-free portion was evaluated, as the survival time after recurrence in thymoma cases is long, and the impact of death from other causes becomes too large to compensate for.
Statistical analyses
The EZR software program was used to perform the statistical analyses (13). Values are presented as the mean ± standard deviation (SD) and were analyzed by the nonpaired t-test and one-way analysis of variance with multiple comparisons. The Masaoka-Koga stages and T factor of the TNM staging system were analyzed by nonparametric analysis and pairwise comparisons using the Kruskal-Wallis test and Mann-Whitney U test. The significance of differences between categorized groups was evaluated using Pearson’s χ2 test. The survival analysis was performed by the Kaplan-Meier method and a univariate log-rank test. P values of <0.05 were considered to indicate statistical significance.
Results
Radiological characteristics of calcification
Seventy-seven thymomas were reviewed to analyze the presence of calcification and its pattern, size, and location. Calcification was recognized in 13 tumors (16.9%) that were categorized into groups I (n=8) and M (n=5). The typical figures of calcifications are shown in Figure 1. In group I, stippled calcification was recognized in two cases (Figure 1A), nodular calcification in five cases (Figure 1B), and large calcification in one case (Figure 1C). In group M, focal or multifocal calcification was recognized in three cases (Figure 1D), dot-lined calcification in one case (Figure 1E), and ring calcification in one case (Figure 1F).

Case presentation
Case 1 (Figures 1E,2A,2B)
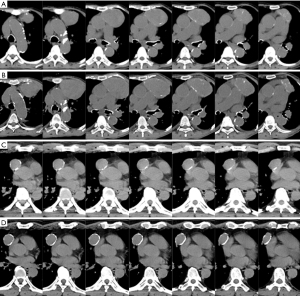
A 71-year-old woman with dilated cardiomyopathy and chronic kidney disease requiring hemodialysis. Although an anterior mediastinal tumor had been noted 20 years ago, she had been followed up without surgical resection due to comorbidities. Anti-AChR antibody was negative. Anemia appeared and progressed, and transfusion was repeated. She was diagnosed with pure red cell aplasia and referred to the Department of Thoracic Surgery. The maximum diameter of the tumor increased from 2.5 to 8.4 cm over 20 years. Marginal calcification was recognized on CT taken 2 years ago (Figure 2A), and the calcifications became clearer over 2 years (Figure 2B). Thoracoscopic thymectomy using the subxiphoid approach (14) was performed with an operation time of 190 min and blood loss of 10 g. The postoperative condition was excellent, and she was discharged on the fifth postoperative day. The pathological diagnosis was type AB thymoma with Masaoka-Koga stage I and T1aN0M0 TNM classification. She is alive without recurrence of thymoma at 17 months after the operation.
Case 2 (Figures 1F,2C,2D)
A 65-year-old man underwent open abdominal surgery due to traffic trauma. At that time, an anterior mediastinal tumor had been pointed out incidentally by CT (Figure 2C). Following a 7-year follow-up period without enlargement of the tumor, he was referred to the Department of Thoracic Surgery (Figure 2D). The maximum diameter of the anterior mediastinal tumor was 3.0 cm. During this period, ring formation of calcification became more continuous, and calcification thickened partially. Anti-AChR antibody (3.6 nmol/dL) was positive, but symptoms of myasthenia gravis (MG) were not recognized. Thoracoscopic thymectomy using the subxiphoid approach was performed with an operation time of 104 min and 5 g of blood loss. The postoperative condition was excellent, and he was discharged on the third postoperative day. The pathological diagnosis was type AB thymoma with Masaoka-Koga stage I and T1aN0M0 TNM classification. He is alive without recurrence of thymoma at 16 months after the operation.
Clinical and pathologic data (Tables 1,2)
Table 1
| Characteristics | All cases | Calcification: + (n=13)/− (n=64) | P value |
|---|---|---|---|
| Age (years) | Range: 28–83 (mean: 61) | 57/60 | 0.451 |
| Gender | Female (n=45) | 9/36 | 0.54 |
| Male (n=32) | 4/28 | ||
| Maximal tumor diameter (cm) | 3.9±2.0 | Mean: 4.8/3.8 | 0.078 |
| WHO pathological classification | A (n=12) | 0/12 | 0.439 |
| AB (n=19) | 5/14 | ||
| B1 (n=10) | 2/8 | ||
| B2 (n=26) | 5/21 | ||
| B3 (n=7) | 1/6 | ||
| Others (n=3) | 0/3 | ||
| MG | + (n=17) | 5/12 | 0.146 |
| − (n=60) | 8/52 | ||
| Non-MG autoimmune disease | + (n=5) | 2/3 | 0.196 |
| − (n=72) | 11/61 | ||
| Masaoka stage | I (n=42) | 6/36 | 0.143 |
| II (n=28) | 5/23 | ||
| III (n=4) | 0/4 | ||
| IVA (n=3) | 2/1 | ||
| T factor | T1a (n=57) | 9/48 | 0.335 |
| T1b (n=3) | 0/3 | ||
| T2 (n=12) | 2/10 | ||
| T3 (n=4) | 1/3 | ||
| T4 (n=1) | 1/0 | ||
| Cystic lesions | + (n=6) | 1/5 | 1 |
| − (n=71) | 12/59 | ||
| Recurrence | + (n=4) | 1/3 | 0.531 |
| − (n=73) | 12/61 | ||
| Recurrence-free period (months) | 42.3±35.3 | Mean: 45.2/41.7 | 0.746 |
Continuous values are presented as mean ± standard deviation. WHO, World Health Organization; MG, myasthenia gravis.
Table 2
| Characteristics | All cases | Calcification: group I (n=8)/group M (n=5)/group N (n=64) | P value |
|---|---|---|---|
| Age (years) | Range: 28–83 (mean: 61) | Mean: 48/71/60 | 0.0097 |
| I vs. M: 0.0098 | |||
| I vs. N: 0.053 | |||
| M vs. N: 0.241 | |||
| Gender | Female (n=45) | 6/3/36 | 0.733 |
| Male (n=32) | 2/2/28 | ||
| Maximal tumor diameter (cm) | 3.9±2.0 (cm) | Mean: 5.1/4.6/3.8 | 0.2 |
| WHO pathological classification | A (n=12) | 0/0/12 | 0.201 |
| AB (n=19) | 1/4/14 | ||
| B1 (n=10) | 1/1/8 | ||
| B2 (n=26) | 5/0/21 | ||
| B3 (n=7) | 1/0/6 | ||
| Others (n=3) | 0/0/3 | ||
| MG | + (n=17) | 4/1/12 | 0.132 |
| − (n=60) | 4/4/52 | ||
| Non-MG autoimmune disease | + (n=5) | 1/1/3 | 0.196 |
| − (n=72) | 7/4/61 | ||
| Masaoka stage | I (n=42) | 2/4/36 | 0.020 |
| II (n=28) | 4/1/23 | I vs. M: 0.044 | |
| III (n=4) | 0/0/4 | I vs. N: 0.034 | |
| IVA (n=3) | 2/0/1 | M vs. N: 1.00 | |
| T factor | T1a (n=57) | 4/5/48 | 0.236 |
| T1b (n=3) | 0/0/3 | ||
| T2 (n=12) | 2/0/10 | ||
| T3 (n=4) | 1/0/3 | ||
| T4 (n=1) | 1/0/0 | ||
| Cystic lesions | + (n=6) | 1/0/5 | 0.684 |
| − (n=71) | 7/5/59 | ||
| Recurrence | + (n=4) | 1/0/3 | 0.53 |
| − (n=73) | 7/5/61 | ||
| Recurrence-free period (months) | 42.3±35.3 | Mean: 53.3/32.3/41.7 | 0.558 |
Continuous values are presented as the mean ± standard deviation. Group I: those with inner calcification of the tumor. Group M: those with marginal calcification. Group N: those without any calcification. WHO, World Health Organization; MG, myasthenia gravis.
There were 45 women and 32 men, with a mean age of 61 years old, ranging between 28 and 83 years old. The mean maximum tumor diameter (MD) ± SD was 3.9±2.0 cm. The pathological diagnoses of thymomas were type A (n=12), type AB (n=19), type B1 (n=10), type B2 (n=26), type B3 (n=7), and others (n=3), including micronodular thymoma with lymphoid stroma (n=2) and metaplastic thymoma (n=1). Seventeen of the 77 patients showed symptoms of MG. As with other autoimmune diseases, pure red cell aplasia, systemic lupus erythematosus (SLE), Issacs syndrome, and chronic thyroiditis were diagnosed in one non-MG patient each. Polymyositis and carditis were observed as complications in an MG patient. The Masaoka-Koga stages were I (n=42), II (n=28), III (n=4), and IVA (n=3). The T factors of the TNM classification were T1a (n=57), T1b (n=3), T2 (n=12), T3 (n=4), and T4 (n=1). There were no significant differences in any factors between cases with and without calcification.
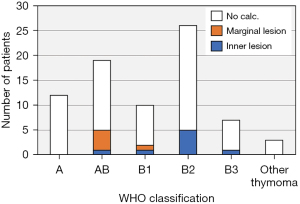
In the analysis among groups I, M, and N, there were significant differences in age (P=0.0097) and Masaoka-Koga stage (P=0.020). In group M, older patients and patients with early-stage disease accounted for the majority. In contrast, in group I, younger patients and patients with advanced-stage disease were included. There was no significant difference in the WHO classification among the groups (Figure 3). However, when thymomas were divided into low-risk thymomas (types A, AB, and B1) and high-risk thymomas (types B2 and B3), there was a significant difference among the groups (P=0.030), and in low-risk thymomas, especially type AB thymomas, marginal calcification was observed more frequently than in other types.
The prognosis (Figure 4)
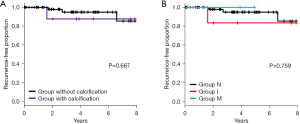
The average observation period was 42.9 (range, 0.1–133.0) months. There were 4 cases with recurrence and no deaths during the observation period. Only the Masaoka stage and TNM stage classification were prognostic factors for recurrence. There was no significant difference in the recurrence-free duration between the groups with and without calcification (P=0.667) (Figure 4A). There were also no significant differences in the recurrence-free duration between groups I, M, and N (P=0.759) (Figure 4B). There were many cases with a short observation period in all groups. However, recurrence has not been recognized in any cases of group M thus far.
Pathological characteristics of calcification
In group I, multifocal and linear calcifications were identified in hyalinized stroma (Figure 5A,5B). These pathological photographs were of the case described in Figure 1B. In group M, linear calcification was identified in the thick capsule of the tumor (Figure 5C,5D). These pathological photographs were of the case described in Figure 1F.

Discussion
In the present study, we were able to categorize the calcification of thymomas into inner calcification and marginal calcification based on the location of calcification. In addition, inner calcification was subclassified into stippled calcification, nodular calcification, and large calcification based on the maximum size of the calcification. Marginal calcification was similarly subclassified into focal or multifocal calcification, dot-lined calcification, and ring calcification based on the calcification pattern. Of note, marginal calcification of TETs predicted low invasiveness of tumors and a low-risk pathology of thymoma, especially type AB thymoma. We were able to pathologically confirm the difference between inner calcification and marginal calcification. The presence of calcification in the thick and firm capsule of the tumor in the marginal calcification group may therefore suggest a slow-growing tumor.
With the increasingly frequent performance of CT examinations, reports of the calcification of thymomas have been growing. The incidence of calcification ranges from 10–40% among thymomas (7-10). Tomiyama et al. reported a higher incidence of calcification in type B2 (61%) and B3 thymomas (75%) (8) than in other types of thymoma and further found that invasive thymomas had a higher prevalence of foci of calcification than noninvasive thymomas (15). Yoshida et al. also reported that calcification in thymomas was more frequent in high-risk thymomas and those with an advanced Masaoka stage than in others (7), and the findings of Jeong et al. were consistent with this (low-risk thymoma, 10% vs. high-risk thymoma, 31%) (9). The presence of calcification in thymomas is now commonly considered to indicate tumor invasiveness and high-risk thymomas.
However, previous studies did not discuss the location of calcification. We experienced a case with dot-lined calcification (Figure 1E) and another case with ring calcification (Figure 1F) in group M. These cases initially inspired us to attempt the present study. We reviewed recent CT findings of thymomas and hypothesized the transition beginning from focal stippled calcification via multifocal stippled calcification, dot-lined calcification, to ring calcification and came to the conception of a group with marginal calcification. We also subclassified the cases with inner calcification based on the size of the calcification. We adopted 3 mm as the boundary between stippled calcification and nodular calcification, as reported by Yoshida et al. (7), and newly adapted a boundary of 2.0 cm to separate nodular calcification from large calcification. The validity of this subclassification is unclear.
Only a few cases involving ring calcification have been described. In the case reported by Yoshida et al., the ring calcification was located at the marginal zone of the tumor, as in our case. However, in the case reported by Sano et al., ring formation was incomplete, and it appeared that part of the tumor had been destroyed and overcame the calcification to infiltrate into the mediastinum (16). Siraj et al. reported a B3 thymoma case with ring calcification that seemed to be completely contained inside the tumor (17). Given these findings, the appearance of ring calcification may not necessarily indicate low invasiveness of a tumor or a low-risk pathology of thymoma. Whether the ring calcification is located at the marginal zone of the tumor may in fact be more important for indicating the low invasiveness of tumors or a low-risk pathology in such cases.
In the present study, old patients were more common in group M than in the other groups. In our study setting, the patients with Masaoka stage IV disease were significantly younger (38 years old) than those with Masaoka stage I, II, and III disease (60, 61, and 60 years old, respectively; P=0.0036, 0.022, and 0.155, respectively). The lack of patients with Masaoka stage IV disease in group M may have led to the patients in this group being older than those in the other groups.
While the results of this study are encouraging, any conclusions should be tempered by the limitations. The single-institution setting, small number of cases, short observation period, and inclusion of only operated cases may limit the validity of the present results. We are planning our next study to involve multiple institutions with a large number of thymoma patients.
Conclusions
We categorized thymoma cases based on the location of calcification and indicated details of thymoma cases with calcifications. Differences in the location of calcification indicated differences in the characteristics of thymomas. Inner calcifications seemed to indicate more invasive characteristics than marginal calcifications. The location of calcification should therefore receive focus when evaluating thymomas.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-164/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-164/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-164/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-164/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Aichi Medical University Hospital (No. 2022-702). As opt-out consent procedures have been adapted and the details of the study were shown on the website of the institution, written informed consent was not obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Wright CD, Wain JC. Acute presentation of thymoma with infarction or hemorrhage. Ann Thorac Surg 2006;82:1901-4. [Crossref] [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Clinicopathological analysis of small-sized anterior mediastinal tumors. Surg Today 2014;44:1817-22. [Crossref] [PubMed]
- Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. [Crossref] [PubMed]
- Yoshida M, Kondo K, Miyamoto N, et al. Calcification in thymomas can predict invasiveness to surrounding organs. Thorac Cancer 2021;12:1857-63. [Crossref] [PubMed]
- Tomiyama N, Johkoh T, Mihara N, et al. Using the World Health Organization Classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol 2002;179:881-6. [Crossref] [PubMed]
- Jeong YJ, Lee KS, Kim J, et al. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol 2004;183:283-9. [Crossref] [PubMed]
- Jung KJ, Lee KS, Han J, et al. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol 2001;176:433-9. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Yano M, Moriyama S, Haneda H, et al. Thymectomy using the subxiphoid approach. J Thorac Cardiovasc Surg 2016;152:278-9. [Crossref] [PubMed]
- Tomiyama N, Müller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr 2001;25:388-93. [Crossref] [PubMed]
- Sano A, Kawashima M. Thymoma with ring calcification. Ann Thorac Surg 2014;98:2202-4. [Crossref] [PubMed]
- Siraj F, Dhawan S, Jain D. Invasive thymoma with osseous metaplasia and cystic change in a case of myasthenia gravis: a rare presentation. Gen Thorac Cardiovasc Surg 2011;59:583-6. [Crossref] [PubMed]


