
Echocardiographic features and influencing factors of pulmonary hypertension of total anomalous pulmonary venous connection in adults
Highlight box
Key findings
• This report presents the clinical and transthoracic echocardiographic manifestations of 16 adult patients with TAPVC and demonstrates that pregnancy affects the PASP of adult patients with TAPVC.
What is known and what is new?
• Patients with TAPVC rarely survive to adulthood, and the characteristics of TAPVC in adults remain unclear.
• This study summarized the echocardiographic of TAPVC in adults and evaluated the factors affecting PASP in these individuals.
What is the implication, and what should change now?
• This study enriches the number of reports describing TAPVC patients in their adulthood, increasing the understanding of this disease.
Introduction
Total anomalous pulmonary venous connection (TAPVC) accounts for 1.5–3.0% of all congenital heart diseases (CHDs) (1-4). Patients with TAPVC generally have symptoms, such as difficulty breathing, shortness of breath, and cyanosis, during the neonatal period and infancy. Moreover, for those with untreated TAPVC, 50% die within three months after birth, and increases to 80% die by the end of the first year after birth (5). In addition, increased pulmonary blood flow eventually leads to the muscularization of the pulmonary vascular bed, potentially causing irreversible pulmonary hypertension (PH). TAPVC can also lead to severe PH, which affects the treatment methods and prognosis. Thus, early detection and treatment are essential for optimal outcomes.
Patients with TAPVC rarely survive to adulthood, and the characteristics of TAPVC in adults remain unclear. Therefore, this study summarized the echocardiographic and clinical characteristics of TAPVC in adults and evaluated the factors affecting pulmonary artery systolic pressure (PASP) in these individuals. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1793/rc).
Methods
General data
Data from 16 adult patients with TAPVC diagnosed in Beijing Anzhen Hospital, China, between January 2002 to December 2020 were retrospectively collected. Patients with TAPVC with atrial septal defects (ASDs) were included, but those with CHDs other than ASD were excluded. Data from 32 patients diagnosed only with ASD were also collected as a control group; the ASD diameters of those in the ASD-only group were matched with the ASD diameters of those in the TAPVC group.
Anatomical features
TAPVC was classified as supra-cardiac, infracardiac, intracardiac, or mixed based on the drainage site of the abnormal veins as follows: (I) supra-cardiac type: the pulmonary vein (PV) was not connected to the left atrium (LA), forming a common cavity that drained into the innominate vein or superior vena cava through the vertical vein; (II) infracardiac type: a common cavity drained into the inferior vena cava or portal vein through the vertical vein; (III) intracardiac type: a common cavity converged with the coronary sinus or directly entered the right atrium (RA); and (IV) mixed type: the PV was connected to the RA or superior vena cava through different pathways.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Anzhen Hospital (Approval No. 2022186X), and individual consent for this retrospective analysis was waived.
Instruments and methods
Echocardiographic examinations were performed using a GE Vivid E9 ultrasound machine with an M5S probe (2–4 MHz; GE Healthcare, Chicago, IL, USA) or a Philips IE33 ultrasound machine with an S5-1 probe (2.5–3.5 MHz; Philips Healthcare, Andover, MA, USA).
Data collection
General clinical data were collected, including height, weight, body surface area (BSA), clinical symptoms, medical history, and the blood oxygen (SpO2) level. Data on echocardiographic parameters were collected, including left ventricular end-diastolic diameter (LVEDd); LA anteroposterior, right ventricle (RV) transverse, aortic (AO), pulmonary artery (PA), maximum ASD, RA longitudinal, and RA transverse diameters. The AO/PA ratio and ASD/atrial septum ratio were also calculated. All echocardiographic parameters were standardized by the BSA. Furthermore, the PASPs of five patients was measured by right cardiac catheterization, and the PASP of 11 patients was estimated by tricuspid regurgitation (TR). If there was no obstruction of the RV outflow tract, the PASP was equal to the square of the TR velocity plus the RA pressure. The LVEDd and LA anteroposterior and AO diameters were measured on the left ventricular long-axis view. The RV basal segment transverse and RA diameter were measured on the apical four-chamber view. The PA diameter was measured on the short-axis view of the major artery.
Patient groups
When the peak TR velocity was >3.4 m/s (PASP is ~60 mmHg), further intervention was needed (6). Thus, patients with TAPVC were divided into two groups: PASP <60 mmHg and PASP ≥60 mmHg; and patients with only ASD were accordingly divided into two groups: PASP <60 mmHg and PASP ≥60 mmHg.
Statistical analyses
SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Quantitative variables were expressed as means ± standard deviations, and qualitative variables as percentages. T-tests were performed for classifying data, chi-square tests for counting data, and binary logistic regression for multivariate analyses. A P value of <0.05 was considered reflective of statistical significance.
Results
General data
This study included 32 patients with only ASD [16 women and 16 men; mean age: 41.7±14.7 (range, 18–73) years] and 16 adults with TAPVC [11 women and five men; mean age: 32.2±9.5 (range, 16–47) years] (Table 1). Of those with TAPVC, 10 patients had moderate or severe TR, and six had a PASP of ≥60 mmHg, five of whom were women, all with a history of pregnancy. Nine patients with only ASD had a PASP of ≥60 mmHg.
Table 1
| Parameter | TAPVC | ASD | P |
|---|---|---|---|
| Age (years) | 32.2±9.5 | 41.7±14.7 | 0.020* |
| Number of patients | 16 | 32 | – |
| TAPVC type | – | ||
| Supra-cardiac | 8 | – | |
| Intracardiac | 4 | – | |
| Mixed | 4 | – | |
| Sex (male/female) | 5/11 | 16/16 | 0.175 |
| Tricuspid regurgitation | – | ||
| Severe | 7 | 7 | |
| Moderate | 3 | 10 | |
| Slight | 6 | 15 | |
| PASP ≥60 mmHg | 6 | 9 | – |
| BSA (m2) | 1.57±0.13 | 1.73±0.21 | 0.007* |
| Operation history | 13 | 32 | – |
| Pregnancy history (total) | 8 | 12 | – |
| SpO2 (%) | 88.6±4.2 | 97.3±1.3 | <0.001* |
Data are presented as mean ± standard deviation or number. *, P<0.05. TAPVC, total anomalous pulmonary venous connection; ASD, atrial septal defect; PASP, pulmonary artery systolic pressure; BSA, body surface area; SpO2, blood oxygen level.
Echocardiographic results
The AO inner diameter was significantly smaller than the PA diameter (Table 2).
Table 2
| Group | Diameter (mm) |
|---|---|
| AO | 25.91±5.22 |
| PA | 32.91±7.93 |
| t | –2.445 |
| P | 0.023 |
Data are presented as mean ± standard deviation. AO, aorta; PA, pulmonary artery; TAPVC, total anomalous pulmonary venous connection.
Echocardiographic parameters were compared between the ASD-only and TAPVC groups. The LA was smaller and the AO/PA ratio was lower in the TAPVC group than in the ASD-only group. Additionally, patients with TAPVC had larger RVds and RAs and were younger than those with only ASD (Tables 1,3).
Table 3
| Group | ASD (mm) | ASD/BSA (mm/m2) |
ASD/AS ratio | LA/BSA (mm/m2) |
LVEDd/BSA (mm/m2) | RV/BSA (mm/m2) |
RA trans-D/BSA (mm/m2) | RA long-D/BSA (mm/m2) | AO/PA ratio |
|---|---|---|---|---|---|---|---|---|---|
| ASD | 33.6±10.3 | 19.8±6.9 | 0.64±0.14 | 22.3±4.3 | 23.8±2.9 | 27.6±5.2 | 28.8±5.7 | 34.1±6.4 | 1.02±0.22 |
| TAPVC | 34.3±9.4 | 21.7±5.9 | 0.60±0.17 | 19.3±3.4 | 23.7±3.0 | 30.6±3.5 | 34.9±7.0 | 42.7±5.0 | 0.80±0.18 |
| t | −0.230 | −0.941 | 0.942 | 2.287 | 0.068 | −2.019 | −2.933 | −4.015 | 3.241 |
| P | 0.751 | 0.351 | 0.351 | 0.027* | 0.946 | 0.049* | 0.005* | <0.001* | 0.002* |
Data are presented as mean ± standard deviation. *, P<0.05. ASD, atrial septal defect; TAPVC, total anomalous pulmonary venous connection; BSA, body surface area; AS, atrial septum; LA, left atrium; LVEDd, left ventricular end-diastolic diameter; RV, right ventricle; RA trans-D, RA transverse diameter; RA long-D, RA longitudinal diameter; RA, right atrium; AO, aorta; PA, pulmonary artery.
Effects of the PASP
Echocardiographic parameters were compared between the PASP ≥60 and <60 mmHg groups in patients with TAPVC. The LA was smaller and the RVd was larger in patients with TAPVC with a PASP ≥60 mmHg (Table 4).
Table 4
| Group | SpO2 (%) | Age (years) | BSA (m2) | ASD/BSA (mm/m2) | ASD/AS ratio | LA/BSA (mm/m2) | LVEDd/BSA (mm/m2) | RV/BSA (mm/m2) | RA trans-D/BSA (mm/m2) | RA long-D/BSA (mm/m2) | AO/PA ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PASP ≥60 mmHg | 88.1±3.09 | 32.0±4.9 | 1.60±0.07 | 21.7±5.4 | 0.58±0.18 | 17.3±2.9 | 22.1±2.7 | 33.8±3.0 | 36.6±5.5 | 45.1±2.6 | 0.76±0.18 |
| PASP <60 mmHg | 88.9±4.78 | 32.3±11.7 | 1.56±0.15 | 21.6±6.5 | 0.62±0.18 | 20.9±3.0 | 25.1±2.7 | 28.7±2.2 | 32.9±8.7 | 39.8±6.0 | 0.83±0.18 |
| t | −0.308 | −0.059 | 0.700 | 0.018 | −0.348 | −2.213 | −2.012 | 3.938 | 0.856 | 1.951 | −0.614 |
| P | 0.763 | 0.954 | 0.495 | 0.986 | 0.735 | 0.049* | 0.069 | 0.001* | 0.414 | 0.083 | 0.552 |
Data are presented as mean ± standard deviation. *, P<0.05. PASP, pulmonary artery systolic pressure; TAPVC, total anomalous pulmonary venous connection; SpO2, blood oxygen level; BSA, body surface area; ASD, atrial septal defect; AS, atrial septum; LA, left atrium; LVEDd, left ventricular end-diastolic diameter; RV, right ventricle; RA trans-D, RA transverse diameter; RA long-D, RA longitudinal diameter; RA, right atrium; AO, aorta; PA, pulmonary artery.
Echocardiographic parameters were also compared between the ASD-only and TAPVC groups with a PASP of ≥60 mmHg. The RVd was larger and the LA was smaller in the TAPVC group than in the ASD-only group. The TAPVC group had more women than men, and the ASD-only group had more men than women (Table 5).
Table 5
| Group | Sex (male/female) | Age (years) | BSA (m2) | ASD/BSA (mm/m2) | ASD/AS ratio | LA/BSA (mm/m2) | LVEDd/BSA (mm/m2) | RV/BSA (mm/m2) | RA trans-D/BSA (mm/m2) | RA long-D/BSA (mm/m2) | AO/PA ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD | 7/2 | 49.7±12.2 | 1.73±0.21 | 23.3±4.0 | 0.71±0.05 | 24.5±3.4 | 23.5±2.7 | 29.9±2.7 | 33.8±5.1 | 39.7±5.7 | 0.92±0.18 |
| TAPVC | 1/5 | 32.0±4.9 | 1.60±0.07 | 21.7±5.4 | 0.58±0.18 | 17.3±2.9 | 22.1±2.7 | 33.7±3.0 | 36.6±5.5 | 46.1±2.6 | 0.76±0.18 |
| t | 5.402 | 2.390 | 1.407 | 0.637 | 2.131 | 4.210 | 1.007 | −2.542 | −1.037 | −2.083 | 1.614 |
| P | 0.020* | 0.020* | 0.183 | 0.535 | 0.053 | 0.001* | 0.332 | 0.025* | 0.318 | 0.059 | 0.133 |
Data are presented as mean ± standard deviation. *, P<0.05. PASP, pulmonary artery systolic pressure; ASD, atrial septal defect; TAPVC, total anomalous pulmonary venous connection; BSA, body surface area; AS, atrial septum; LA, left atrium; LVEDd, left ventricular end-diastolic diameter; RV, right ventricle; RA trans-D, RA transverse diameter; RA long-D, RA longitudinal diameter; RA, right atrium; AO, aorta; PA, pulmonary artery.
Factors affecting the PASP in patients with TAPVC
Pregnancy, including a prior history of pregnancy or currently pregnant status, was a significant influencing factor of the PASP (adjusted odds ratio: 15.000, 95% confidence interval: 1.031–218.300, P=0.047) (Table 6).
Table 6
| Variables | P | aOR | 95% CI |
|---|---|---|---|
| Pregnancy | 0.047 | 15.000 | 1.031–218.300 |
| Age | 0.607 | 0.972 | 0.872–0.083 |
PASP, pulmonary artery systolic pressure; aOR, adjusted odds ratio; CI, confidence interval.
Clinical manifestations
Eight patients with TAPVC recently had chest tightness, shortness of breath, and palpitations; of these, two had a PASP of ≥60 mmHg (68 and 101 mmHg with SpO2 values of 83% and 96%, respectively). One patient had hemoptysis due to chest tightness (PASP: 106 mmHg; SpO2: 85.7%). One patient was post-menopausal and had a PASP of ≥60 mmHg (PASP: 80 mmHg; SpO2: 90%). Physical examination indicated heart disease in one patient (SpO2: 93%). Furthermore, cyanosis occurred in five patients (SpO2: 84–88%) and PH in three patients (55, 68, and 90 mmHg).
History of pregnancy, surgery, and treatment among patients with TAPVS
In total, 13 patients underwent repair operation, of which three patients were treated with medication for PH prior to surgical repair. Seven patients had a history of pregnancy and were 3–10 years postpartum, of which three patients were pregnant for a second or third time at the time of the diagnosis. One patient received surgical treatment, amongst other treatments (e.g., lowering PH), three months after terminating the pregnancy.
Echocardiographic features
The echocardiographic features were as follows: (I) supra-cardiac type: the ASD and PV were not connected to the LA, forming a common cavity that drained into the innominate vein or superior vena cava through the vertical vein (Figures 1-3); (II) intracardiac type: the ASD and PV were not connected to the LA, forming a common cavity that converged with the coronary sinus or directly entered the RA (Figure 4); and (III) mixed type: the ASD and PV were not connected to the LA, and the PV was connected to the RA or superior vena cava through different pathways. No patients in this study had the infracardiac type.
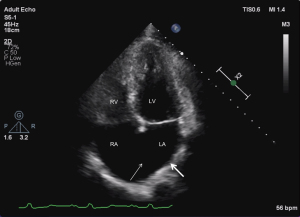
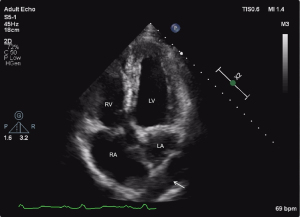
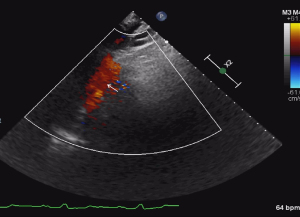

Misdiagnosis analysis
Two male patients were misdiagnosed. A large ASD was easily identified, but the connection between the PV and the common lumen structure was ignored. According to the analysis of the two patients, they were middle-aged men (44 and 47 years) with large ASDs, which explained the enlargement of the right heart. However, the doctor did not investigate the blood shunt direction at the ASD, nor locate the PV connection.
Discussion
CHDs are common congenital disabilities and the primary cause of infant death. However, TAPVC is a rare cyanotic CHD characterized by an abnormal connection between the PV and the RA, which refluxes hyper-oxygenated blood into the right cardiac system. TAPVC is further classified as supra-cardiac, infracardiac, intracardiac, or mixed based on the drainage site of the abnormal veins. To survive TAPVC, patent foramen ovale or ASDs must exist. Furthermore, surgical intervention is required when the RV volume overloads and the patient experiences clinical symptoms and cyanosis. For those who survive to adulthood, mild clinical symptoms appear relatively late.
To survive to adulthood, patients with TAPVC must have no obstruction between the PV and systemic vein or RA, and the ASD size must be appropriate (4,5,7,8). Non-restrictive ASD provides suitable conditions for long-term survival. PV obstruction is more common in patients with infracardiac and mixed TAPVC, which can lead to severe pulmonary edema and clinical decompensation (9,10). Therefore, these types of TAPVC usually develop into severe pulmonary vascular disease early in life; thus, the patient will not survive to adulthood (5). However, no one in this study had infracardiac TAPVC nor PV obstruction. To our knowledge, the oldest patient with TAPVC is a 70-year-old woman who survived because she had a large ASD, no obstruction, and no related deformities and underwent successful TAPVC repair (11). In this study, the oldest patient was a 47-year-old man with supra-cardiac TAPVC locally diagnosed as ASD (maximum ASD diameter: 47 mm; slight TR) by physical examination. The supra-cardiac TAPVC was diagnosed by echocardiography in our hospital, and surgical treatment was performed with good postoperative outcomes. Autopsies of infants who died from TAPVC occlusion showed an increased thickness of the artery’s middle layer, proliferation of the intima of the pre-sinus vein, and an abnormally small and thick wall of the external PV (12-14). This current study demonstrated that the cardiac structural changes in patients with TAPVC and only ASD are very similar; for example, as the right heart diameter increases, the left heart diameter and AO/PA ratio decrease: this relationship is also the primary reason for missed diagnosis of TAPVC. Notably, the LA and AO/PA ratio were smaller and the RVd and RA were larger in patients with TAPVC. The hemodynamic changes of patients with TAPVC are more obvious than those of patients with only ASD. Patients with TAPVC have a wide range of clinical manifestations because of the vast differences in anatomy and hemodynamics, ranging from asymptomatic to severe hypoxemia; thus, TAPVC is most easily misdiagnosed as a larger, secondary ASD (15). These patients present with gradual symptoms, such as shortness of breath, atypical chest pain, or atrial arrhythmia (15-17). However, most TAPVC cases were diagnosed by echocardiography during the initial visit or during physical examination (18).
Two men in our cohort came to our hospital for surgery because of an ASD diagnosis, but supra-cardiac TAPVC was identified during the preoperative examination. There are a couple of explanations for missed diagnoses and misdiagnoses. First, the common chamber position is relatively biased, and abnormal structures cannot be identified in the four-chamber view. Second, an ASD would explain the enlargement of the right heart; thus, if an ASD is present, the abnormalities of each structure are not always further explored, resulting in misdiagnosis. Transthoracic echocardiographic (TTE) is the preferred method for diagnosing isolated TAPVC; however, TTE may be considerably limited in its ability to assess PV abnormalities. Multidetector computed tomography (MDCT) (Figure 5) and magnetic resonance imaging (MRI) can also be used to accurately diagnose TAPVC. MDCT has 100% sensitivity and specificity for depicting the common PV drainage site, vertical vein stenosis, and atypical blood vessels entering the systemic veins. TTE also had 100% specificity for identifying the common PV drainage site, vertical vein stenosis, and atypical blood vessels entering the systemic veins, but the sensitivity of the common PV drainage site, vertical vein stenosis, and atypical blood vessels were 87%, 71%, and 0% respectively (18-20). Observing the direction of the blood shunt in the atrial septum and the PV connection is necessary if ASD is identified to avoid misdiagnosing TAPVC. However, when the specific return route is unclear, MDCT and MRI exams can provide more definite diagnostic information for the operation.
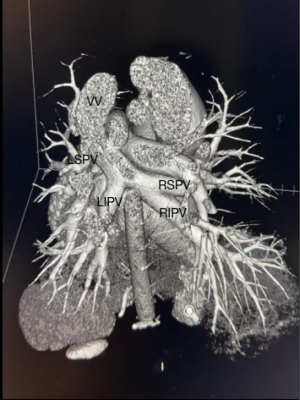
In this study, patients with TAPVC had a significantly smaller AO diameter than PA diameter, indicating that the increased volume of the right heart widened the PA. The RVd was larger and the LA was smaller in TAPCV patients with a PASP ≥60 mmHg than in those with a PASP <60 mmHg. Finally, of those with a PASP ≥60 mmHg, the RVd was larger and the LA was smaller in patients with TAPVC than in those with only ASD, and there were more women than men in the TAPVC group and more men than women in the ASD-only group. Pregnancy also significantly affected the PASP in patients with TAPVC. Maternal blood volume increases during pregnancy to meet the needs of the fetus; therefore, pregnancy dramatically affects the cardiovascular system, and the effects are sustained in the post-partum period (21). ASD size was not significant parameters related to PASP (Figure 6).

This study included a small number of patients, which is a limitation. However, the number of cases reported in the literature is also small because patients with TAPVC rarely live to adulthood. Notably, once TAPVC is diagnosed, it is surgically treated. Hence, relevant results on disease progression and changes cannot be obtained. Nevertheless, we plan to continue accumulating information on adult patients with TAPVC to study the related factors and disease changes of TAPVC-induced PH.
Conclusions
The structural changes in the heart of adult patients with TAPVC are similar to those of patients with ASD. Therefore, these two conditions must be differentiated based on the shunt at the atrial level and by identifying a common cavity. Furthermore, exploring the PV is very important for patients with ASD. Finally, pregnancy affected the PASP in patients with TAPVC, and the right heart was larger and the LA and the AO/PA ratios were smaller in patients with TAPVC than in those with only ASD.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. U21A20523), National Natural Science Foundation of China (Grant No. 82170301), Beijing Lab for Cardiovascular Precision Medicine (Grant No. PXM2018_014226_000013), and Beijing Key Laboratory of Maternal-Fetal Medicine in Fetal Heart Disease (Grant No. BZ0308).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1793/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1793/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1793/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1793/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Anzhen Hospital (Approval No. 2022186X), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu Y, Fan X, Chen L, et al. Emergency surgical treatment of total anomalous pulmonary venous connection. J Card Surg 2022;37:47-52. [Crossref] [PubMed]
- Harada T, Nakano T, Oda S, et al. Surgical results of total anomalous pulmonary venous connection repair in 256 patients. Interact Cardiovasc Thorac Surg 2019;28:421-6. [Crossref] [PubMed]
- Shaw FR, Chen JM. Surgical Considerations in Total Anomalous Pulmonary Venous Connection. Semin Cardiothorac Vasc Anesth 2017;21:132-7. [Crossref] [PubMed]
- Jian XH, Huang J, Ding Y, et al. Surgical outcome of isolated total anomalous pulmonary venous connection in adults: a 14-year experience. J Card Surg 2012;27:736-9. [Crossref] [PubMed]
- Bharati S, Lev M. Congenital anomalies of the pulmonary veins. Cardiovasc Clin 1973;5:23-41. [PubMed]
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023;61:2200879. [Crossref] [PubMed]
- Ishibe R, Arikawa K, Toyohira H, et al. Surgical repair of total anomalous pulmonary venous drainage--four adult cases of successful operation. Nihon Kyobu Geka Gakkai Zasshi 1993;41:199-204. [PubMed]
- Ross FJ, Joffe D, Latham GJ. Perioperative and Anesthetic Considerations in Total Anomalous Pulmonary Venous Connection. Semin Cardiothorac Vasc Anesth 2017;21:138-44. [Crossref] [PubMed]
- Xiang Y, Peng Y, Qiu J, et al. Echocardiographic evaluation of total anomalous pulmonary venous connection: Comparison of obstructed and unobstructed type. Medicine (Baltimore) 2022;101:e29552. [Crossref] [PubMed]
- Spigel ZA, Edmunds EE, Caldarone CA, et al. Total anomalous pulmonary venous connection: Influence of heterotaxy and venous obstruction on outcomes. J Thorac Cardiovasc Surg 2022;163:387-395.e3. [Crossref] [PubMed]
- Takagi S, Nakasu A, Yanagisawa J, et al. Late-onset total anomalous pulmonary venous connection in a 70-year-old woman. BMJ Case Rep 2021;14:e245186. [Crossref] [PubMed]
- Haworth SG. Total anomalous pulmonary venous return. Prenatal damage to pulmonary vascular bed and extrapulmonary veins. Br Heart J 1982;48:513-24. [Crossref] [PubMed]
- Shi G, Zhu Z, Chen J, et al. Total Anomalous Pulmonary Venous Connection: The Current Management Strategies in a Pediatric Cohort of 768 Patients. Circulation 2017;135:48-58. [Crossref] [PubMed]
- Paladini D, Pistorio A, Wu LH, et al. Prenatal diagnosis of total and partial anomalous pulmonary venous connection: multicenter cohort study and meta-analysis. Ultrasound Obstet Gynecol 2018;52:24-34. [Crossref] [PubMed]
- Nurkalem Z, Gorgulu S, Eren M, et al. Total anomalous pulmonary venous return in the fourth decade. Int J Cardiol 2006;113:124-6. [Crossref] [PubMed]
- Yalta K, Turgut OO, Yilmaz A, et al. Asymptomatic total anomalous pulmonary venous connection with double drainage in a young adult: a case report. Heart Surg Forum 2007;10:E211-2. [Crossref] [PubMed]
- Czekajska-Chehab E, Tomaszewski A, Adamczyk P, et al. Total anomalous pulmonary vein drainage in a 60-year-old woman diagnosed in an ECG-gated multidetector computed tomography - a case report and review of literature. Pol J Radiol 2018;83:e334-9. [Crossref] [PubMed]
- Files MD, Morray B. Total Anomalous Pulmonary Venous Connection: Preoperative Anatomy, Physiology, Imaging, and Interventional Management of Postoperative Pulmonary Venous Obstruction. Semin Cardiothorac Vasc Anesth 2017;21:123-31. [Crossref] [PubMed]
- Oh KH, Choo KS, Lim SJ, et al. Multidetector CT evaluation of total anomalous pulmonary venous connections: comparison with echocardiography. Pediatr Radiol 2009;39:950-4. [Crossref] [PubMed]
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563-645. [Crossref] [PubMed]
- Ashrafi R, Curtis SL. Heart Disease and Pregnancy. Cardiol Ther 2017;6:157-73. [Crossref] [PubMed]


