Surgical treatment of synchronous multiple primary lung cancers: a retrospective analysis of 122 patients
Introduction
Since synchronous multiple primary lung cancers (SMPLC) were discovered by Beyreuther in 1924, they have become an increasingly recognized. The incidence rate of SMPLC ranges from 0.2% to 20% and is increasing as the result of the widespread use of multi-slice spiral computed tomography (CT) and positron emission tomographic/computed tomographic (PET/CT) (1-4). Surgical resection becomes necessary to prolong patient’s survival (4-7).However, controversies related to diagnosis, patient selection, treatment and outcome still exist. The aim of this retrospective study is to assess the surgical treatment of SMPLC and to investigate risk factors that may affect the outcomes.
Methods
Patients
The study was approved by Ethics Committee of Shanghai Pulmonary Hospital and consent was given by all participants before their clinical records were used in this study. From 1990 to 2010, 13,587 cases of surgically treated lung cancer patients were registered in Department of Thoracic Surgery at Shanghai Pulmonary Hospital. Patients were re-staged according to the 7th TNM classification guideline. Pathological subtypes of adenocarcinoma were classified according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society (IASLC/ATS/ERS) guidelines. We used the following modified criteria for the diagnosis of SMPLC:
- Tumors with different histology or different subtype;
- Tumors with same histology:
- no distant metastasis;
- no mediastinal lymph node metastasis;
- different molecular genetic characteristics.
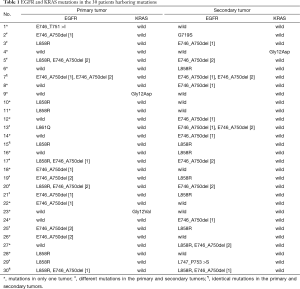
Altogether, 145 patients were diagnosed as having multifocal lung cancers. It was difficult to distinguish primary lung cancers from metastases in 50 patients because tumors were all diagnosed as adenocarcinoma and the predominant histological subtype was identical. EGFR mutations in exon 18–21 and KRAS mutations in codons 12 and 13 were detected in tumors of these 50 patients. We compared EGFR and KRAS mutations between each primary and secondary tumor, and classified the results to three different patterns: pattern A: mutations in only one tumor (17 cases); pattern B: different mutations in the primary and secondary tumors (10 cases); pattern C: identical mutations (3 cases) and no mutation (20 cases) in the primary and secondary tumors. Patterns A and B were considered as SMPLC. EGFR and KRAS mutations are shown in Table 1.
Full table
A total of 122 patients with SMPLC at the time of diagnosis were enrolled into current study. Prospectively collected demographic variables included age, gender, smoking status, location, tumor number, tumor size, stage, resection type, histology, and tumor density. We examined the ratio of maximum diameter of consolidation to maximum tumor diameter from lung window. Consolidation tumor ratio (CTR) was used in this study according to published studies and patients were divided into ground glass opacity (GGO) group (CRT ≤0.5) and mixed group (CRT >0.5) (8-10). Unsuitable patients for surgery and that with advanced disease were excluded from preoperative examination, and patients with typical carcinoid tumors were not included in the present study.
Statistics
Surgical mortality was death occurring within 30 days of surgery or death directly related to the procedure. The overall survival was defined as the time interval between the date of first surgery and the date of death or the date of most recent follow-up. Overall survival rates were calculated using the Kaplan–Meier method, and statistical comparisons between survival curves were performed using a log rank test. Multivariable analysis was done using Cox proportional hazard regression model. Modeling starts with all variables, variables not significantly associated with overall survival (P>0.05) were removed from the model by means of a step-down procedure. All analysis was performed on SPSS for Windows (Version 13.0, Chicago, IL, USA). Quantitative variables were expressed as mean ± standard deviation; P values less than 0.05 were considered to be statistically significant.
Results
Baseline information
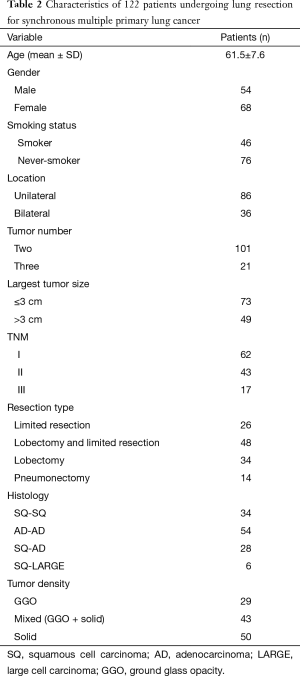
The characteristics of 122 patients are shown in Table 2. The study included 68 women and 54 men aged between 28 and 76. The median age was 61.5. A total of 46 patients were current-smokers or former-smokers and 76 patients were never-smokers. Eight patients had previous histories of additional extrathoracic malignancies including thyroid cancer, breast cancer and rectal cancer. These patients were completely cured and had no evidence of disease at the time of diagnosis of SMPLC. On pulmonary function testing, the mean forced expiratory volume in one second was 1.98 L (77.5% of predicted). A total of 265 separate tumors were identified among 122 patients. The median of tumor size was 2.2 (0.4 to 5.0) cm. Forty-nine patients have the tumor greater than 3 cm, 73 patients have tumor size 3 cm or less. Tumors histology were same among 88 patients, adenocarcinoma is the predominant histological type and occurs among 54 patients. Among patients with same tumor histology, there are 52 stage I cases and 36 stage II cases. Among those with different histology, there are 10 cases of stage I disease, 7 cases of stage II disease and 17 cases of stage III. The highest pathologic stage of tumors was used as the stage of SMPLC.
Full table
Surgery
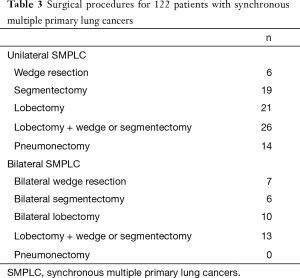
The details of surgical procedures are shown in Table 3. The most frequent complications were arrhythmia, prolonged air leak, and pneumonia. Atelectasis, hemothorax and empyema were seldom seen—each occurred once. The operative mortality was 3.3% and the causes of death were respiratory failure in two patients, pulmonary embolism in one patient, and heart failure in one patient.
Full table
Survival
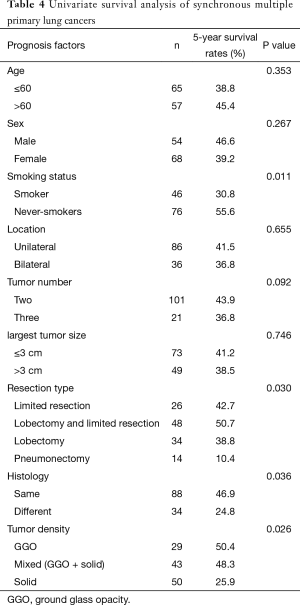
The median follow-up was 60.3±6.3 months and the 5-year overall survival rate was 40.5%. The survival rates of patients are shown in Table 4. There was a significant difference in the 5-year survival rate between smokers and nonsmokers (Figure 1A). A significant difference was also found between patients with same tumor histology and those with different tumor histology (Figure 1B). The 5-year survival rate of patients with solid nodule was 25.9%, which was worse than those with GGO and mixed nodule (Figure 1C). The 5-year survival rates of patients treated with pneumonectomy was 10.4%, which was worse than those who did not receive pneumonectomy (Figure 1D). There were no difference in survival among patients with other clinicopathological characteristics, including age, gender, tumor size, tumor number, and unilateral or bilateral location.
Full table
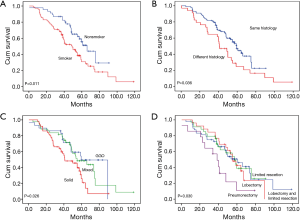
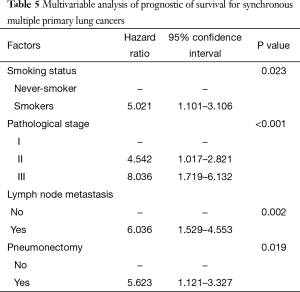
Multivariable analysis (Table 5) indicates four independent prognostic factors attributed to patient’s overall survival: smoking status (P=0.023), pathological stage (P<0.001), lymph node metastasis (P=0.002), and pneumonectomy (P=0.019). Age, gender, location, tumors with same or different histology, tumor size, tumor number and tumor density had no predictive value on survival.
Full table
Discussion
The criteria used to determine SMPLC were initially based on tumor locations and histological findings. It was difficult to validate the clinicopathological assessment and further used in distinguishing primary lung cancer from pulmonary metastasis. With the recent advance of molecular biology, researchers have assessed molecular genetic characteristics using various markers (11-13). EGFR and KRAS mutations, which were observed in a mutually exclusive manner, have been proven powerful in distinguishing primary lung cancers from metastases. Takamochi and his colleagues determined the molecular genetic characteristics of 82 multifocal lung adenocarcinomas from 36 patients and drew the conclusion that EGFR and KRAS may be useful for making decisions regarding treatment strategies for patients with multifocal lung adenocarcinomas (14). Chang et al. (12) also reported that EGFR mutation was a useful marker of the clonal origin of multiple lung cancers, especially in cases with same histology features. Therefore, the most commonly accepted criteria of SMPLC was outlined by Martini and Melamed and modified by Antakli (15,16). In 2013, the American College of Chest Physicians updated the diagnostic criteria, by adding molecular genetic characteristics (17). Based on their molecular genetic characteristics, we identified 27 cases as SMPLC from 50 cases, which tumors were diagnosed as adenocarcinoma with identical predominant histological subtypes. Patients without mutations were excluded from our study because the undetermined mutations among 20 cases. A study reported that there was no difference of survival in same, different and undetermined molecular genetic characteristics based on EGFR and KRAS mutations (14). However, due to intratumor heterogeneity which could not be avoided in PCR or DNA sequencing analyses, the diagnosis of SMPLC cannot completely rely on molecular genetic characteristics.
Multiple primary lung cancers are potentially curable by surgical resection, especially in patients without lymph node involvement. Because of the difficulty in establishing SMPLC diagnosis and the heterogeneity of therapeutic methods, there was considerable variation in the 5-year survival rates, ranging from 20% to 70%, with surgical mortality ranged from 5% to 7.6% (6,18,19). Surgical procedures were determined according to tumor’s size and location, and cardiopulmonary function. The surgical mortality in our study was 3.3% and the causes of death were respiratory failure in two patients, pulmonary embolism in one patient, and heart failure in one patient. More procedures performed with a longer surgical time and synchronous bilateral resection might increase the risk of surgery. Our results clearly showed that pneumonectomy had a major adverse and independent impact on survival. Pneumonectomy was associated with a high risk of postoperative respiratory failure and should be avoided whenever possible, even for patients with ipsilateral tumors located in different lobes. Despite the fact that limited resection has been associated with increased local recurrence rates, segmentectomy or wedge resection remains a good alternative when the patient is unable to tolerate a more extensive resection because of compromised pulmonary function (20,21).
The survival of patients with same tumor histology was relatively favorable or not statistically different compared to those with different histology (4,18,22). The pathological diagnosis of the patients with the same tumor histology showed minimally invasive adenocarcinoma which is previously called “well differentiation” adenocarcinoma. Studies reported an excellent prognosis for patients with malignant pure GGO who underwent surgical resection (23,24). Our study demonstrated that surgery for multiple GGO patients can be conducted in a safe manner. However, the five-year survival of patients with multiple GGO was 50.4%, which was worse when compared to the published survival rates of for single GGO. As the high proportion of GGO in lung adenocarcinoma is well known to be an indicator of better prognosis (25), difference of criteria might be able to explain the results. GGO group in our study has CRT ≤0.5, which include more solid part than that using criteria of CRT ≤0.25 or less. Although we distinguished primary lung cancer from metastasis by molecular genetic characteristics and histological findings, it was quite possible that some cases of metastatic disease mixed into the study unnoticed. Further studies with stratification according to tumor number, tumor size, and histologic subtypes would be helpful.
In the patients with different tumor histology, 17 (50%) patients with stage III disease all have mediastinal lymph node metastasis. Conversely, there are more early stage cases within the same tumor histology group. Multivariable analysis showed pathological stage and lymph node metastases were independent prognostic factors, but tumor histology was not. It indicated that the difference in survival between patients with the same tumor histology and those with different tumor histology could be caused by pathological stage and lymph node status.
In conclusion, our study demonstrated that EGFR and KRAS mutations could be used for assessing molecular genetic characteristics in diagnosis of SMPLC. Surgical treatment is a safe manner for selected patients who are likely to have a favorable outcome. However, a pneumonectomy should not be performed given the likelihood of a poor post-operative prognosis.
Acknowledgements
Funding: This work was supported by the Foundation for Youths of Shanghai Municipal Health Bureau (grant number 20134Y112093), and Wu Jieping Medical Foundation (grant number 320.6700.09028).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wu SC, Lin ZQ, Xu CW, et al. Multiple primary lung cancers. Chest 1987;92:892-6. [Crossref] [PubMed]
- Deschamps C, Pairolero PC, Trastek VF, et al. Multiple primary lung cancers. Results of surgical treatment. J Thorac Cardiovasc Surg 1990;99:769-77; discussion 777-8. [PubMed]
- Carey FA, Donnelly SC, Walker WS, et al. Synchronous primary lung cancers: prevalence in surgical material and clinical implications. Thorax 1993;48:344-6. [Crossref] [PubMed]
- Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer 2011;73:237-42. [Crossref] [PubMed]
- Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg 2007;133:1193-200. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Luzzi L, et al. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg 2010;37:1198-204. [Crossref] [PubMed]
- Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. [Crossref] [PubMed]
- Shimada Y, Saji H, Otani K, et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer 2015;88:174-80. [Crossref] [PubMed]
- Matsuguma H, Oki I, Nakahara R, et al. Comparison of three measurements on computed tomography for the prediction of less invasiveness in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2013;95:1878-84. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Wang X, Wang M, MacLennan GT, et al. Evidence for common clonal origin of multifocal lung cancers. J Natl Cancer Inst 2009;101:560-70. [Crossref] [PubMed]
- Chang YL, Wu CT, Lin SC, et al. Clonality and prognostic implications of p53 and epidermal growth factor receptor somatic aberrations in multiple primary lung cancers. Clin Cancer Res 2007;13:52-8. [Crossref] [PubMed]
- Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 2009;33:1752-64. [Crossref] [PubMed]
- Takamochi K, Oh S, Matsuoka J, et al. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K-ras. Lung Cancer 2012;75:313-20. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Antakli T, Schaefer RF, Rutherford JE, et al. Second primary lung cancer. Ann Thorac Surg 1995;59:863-6; discussion 867. [Crossref] [PubMed]
- Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e191S-210S.
- Riquet M, Cazes A, Pfeuty K, et al. Multiple lung cancers prognosis: what about histology? Ann Thorac Surg 2008;86:921-6. [Crossref] [PubMed]
- Kocaturk CI, Gunluoglu MZ, Cansever L, et al. Survival and prognostic factors in surgically resected synchronous multiple primary lung cancers. Eur J Cardiothorac Surg 2011;39:160-6. [Crossref] [PubMed]
- Mathisen DJ, Jensik RJ, Faber LP, et al. Survival following resection for second and third primary lung cancers. J Thorac Cardiovasc Surg 1984;88:502-10. [PubMed]
- Jensik RJ, Faber LP, Kittle CF, et al. Survival following resection for second primary bronchogenic carcinoma. J Thorac Cardiovasc Surg 1981;82:658-68. [PubMed]
- Battafarano RJ, Meyers BF, Guthrie TJ, et al. Surgical resection of multifocal non-small cell lung cancer is associated with prolonged survival. Ann Thorac Surg 2002;74:988-93; discussion 993-4. [Crossref] [PubMed]
- Kohno T, Fujimori S, Kishi K, et al. Safe and effective minimally invasive approaches for small ground glass opacity. Ann Thorac Surg 2010;89:S2114-7. [Crossref] [PubMed]
- Kim TJ, Goo JM, Lee KW, et al. Clinical, pathological and thin-section CT features of persistent multiple ground-glass opacity nodules: comparison with solitary ground-glass opacity nodule. Lung Cancer 2009;64:171-8. [Crossref] [PubMed]
- Kim HS, Lee HJ, Jeon JH, et al. Natural history of ground-glass nodules detected on the chest computed tomography scan after major lung resection. Ann Thorac Surg 2013;96:1952-7. [Crossref] [PubMed]


