
Rapid deployment versus its conventional counterpart in aortic valve replacement: comparison of early hemodynamic outcomes
Highlight box
Key findings
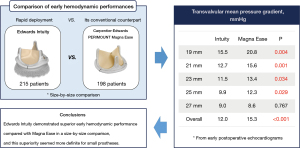
• Edwards Intuity showed superior early hemodynamic performance compared with PERIMOUNT Magna Ease in a size-by-size comparison.
What is known and what is new?
• There have been many comparative studies reporting that Intuity valve demonstrated superior hemodynamics compared with conventional bioprosthetic valves
• The present study is the first study that directly compared the hemodynamics between Intuity valve and its conventional counterpart, Magna Ease valve, as a control prosthesis in a size-by-size fashion.
What is the implication, and what should change now?
• Superior hemodynamics with comparable clinical outcomes of Intuity over Magna Ease may help guide device selection in small aortic roots.
Introduction
The introduction of a rapid-deployment (RD) valve into clinical practice has expanded the already rich portfolio of aortic valve substitutes for patients undergoing aortic valve replacement (AVR). Both European and American trials (TRITON and TRANSFORM trials) (1,2) and many consecutive studies demonstrated excellent clinical outcomes after AVR using a RD valve.
RD valves are known to have several advantages compared with conventional bioprostheses. It allows for a shorter aortic cross-clamp time (2,3), thus shortening the overall procedural time. It also simplifies and facilitates minimally invasive AVR. Furthermore, the hemodynamic performance of RD valves are reported to be better than that of conventional bioprostheses (4,5).
Edwards Intuity (Edwards Lifesciences, Irvine, California, USA) is a rapid-deployment prosthesis that is constructed on the platform of the Carpentier-Edwards PERIMOUNT Magna Ease (Edwards Lifesciences, Irvine, CA, USA) and incorporates a balloon expandable, stainless-steel cloth-covered inflow frame for a subannular fixation system. Magna Ease valve/prosthesis is a bovine, stented, supra-annular aortic valve bioprosthesis based on the designs of the well-established PERIMOUNT and Magna valves with proven long-term durability (6).
Although the Intuity valve has been shown to perform well in many studies (1,7,8), few studies have explored size-by-size comparisons of the hemodynamic performance of this valve to its conventional counterpart, Magna Ease valve. Because these two valves are based on identical functional components except for the subvalvular stent frame, the comparison of these two valves can preclude any confounding factors originating from different prosthetic materials and valve construction, and enable us to focus only on the effect of the subvalvular system on valve hemodynamics.
The aim of this study was to compare the early hemodynamic profile of the Intuity valve with that of the Magna Ease valve stratified by prosthesis size. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-318/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Seoul National University Hospital (No. H-2109-010-1251) and individual consent for this retrospective analysis was waived.
Of 780 patients who underwent aortic valve surgery between June 2016 and July 2021 in our institution, AVR was performed in 768 patients, and AVR using a bioprosthetic valve was performed in 551 patients. Among these patients, 215 patients who received Edwards Intuity for aortic valve substitute and 198 patients who received Carpentier-Edwards PERIMOUNT Magna Ease valve for aortic valve substitute were enrolled in this study (Figure 1). Both Intuity valve and Magna Ease valve were introduced to our center around the same time, and each valve was regularly used during the study period.
Surgical techniques and strategy
The surgical procedures and strategies of RDAVR have been illustrated in previous study (9). All operations were conducted under median sternotomy. Cardiopulmonary bypass with mild or moderate hypothermia and cardioplegic arrest was also used in all patients. After aortotomy, aortic valve excision, and annular decalcification were performed, the valve replica was always simulated to the annulus; we found that the semilunar design of the replica does not perfectly fit to the native annulus in many cases (actually in all cases of bicuspid valves). We focused on these discrepancies; 3 guiding sutures and several additional sutures, which was our modification of the original manufacturer’s instructions for use, were placed at the surgeon’s discretion after careful inspection of the annular geometry to prevent incomplete fitting after valve deployment. After parachuting the valve into the annulus, the delivery system was temporarily removed from the valve holder. Then, a 5-mm videoscope was inserted through the central hole of the holder for evaluation of the fit from the inside. The position of the valve at the left ventricular outflow tract (LVOT), spatial relationship with the anterior leaflet of the mitral valve, and any loosening or displacement of the guiding sutures were carefully examined under direct vision. After the delivery system was reassembled, balloon expansion was performed with 4.5 or 5.0 atm for 10 seconds, following the instructions. After balloon expansion, the videoscope was reinserted, and checked for adequate subannular expansion, accurate prosthesis position, and any related abnormalities. After confirmation, the guiding sutures were tied, and the aortotomy was closed using a typical double-layer technique (or replaced with a graft in cases of concomitant ascending aorta replacement).
In cases of AVR with conventional stented bioprostheses, operations were performed under median sternotomy or upper partial sternotomy. After the excision of diseased AV, the annulus was prepared for placing the prosthesis, the valve size was selected, and the prosthesis was implanted. In most patients, non-everting mattress sutures which were buttress-reinforced with polytetrafluoroethylene as a tubule were used. Everting or non-everting mattress sutures with polytetrafluoroethylene as a usual pledget, instead of a tubule, were occasionally used as needed. Continuous suture technique was used in 11 patients (5.1%). Knot-tying was performed manually or with an automated knot-fastener.
Prosthesis selection between Intuity valve and other bioprostheses including Magna Ease valve was largely determined by the surgeon’s preference. Intuity valve was used exclusively by a single surgeon, whereas Magna Ease valve was used by all surgeons in our institution.
Evaluation of early clinical outcomes
Operative mortality was defined as any death within 30 days. Continuous electrocardiography monitoring was applied to all patients until discharge, and the detection of any short runs of atrial fibrillation was regarded as an occurrence of postoperative atrial fibrillation. Low cardiac output was defined as a cardiac index <2.0 L/min/m2, a systolic arterial pressure <90 mmHg requiring inotropic support (dopamine or dobutamine) of >5 mg/kg/min or a mechanical circulatory support (e.g., intra-aortic balloon pump). Acute kidney injury was defined as a two-fold increase in serum creatinine level from the preoperative value, glomerular filtration rate decrease by 50%, urine output <0.5 mL/kg/h for 12 hours or a need for renal replacement therapy regardless of serum creatinine level. Respiratory complications included prolonged mechanical ventilation over 48 hours postoperatively, pneumonia, or the need for tracheostomy.
Evaluation of early hemodynamic outcomes
Early postoperative echocardiography was performed in 99.0% (409 out of 413) of the study patients at median 6 days (interquartile range, 5–7) after surgery, except for a few mortality cases. The echocardiographic parameters of the prostheses included the transvalvular mean pressure gradient (PG) and effective orifice area (EOA). The measurements of the parameters were performed according to the recommendations for the imaging assessment of prosthetic heart valves (10). Transvalvular mean PG, EOA, and EOA index (EOAI) were compared between the groups stratified by prosthesis size.
Statistical analysis
Statistical analysis was performed using SPSS statistics software version 25.0 (IBM Inc., Armonk, New York, USA), and SAS software, version 9.4 (SAS Institute, Cary, North Carolina, USA). Continuous variables are presented as mean ± standard deviation for normally distributed data or median with interquartile range for not normally-distributed data. Categorical variables are presented as the number and percentage of the subjects. Comparisons of baseline characteristics, operative data, early clinical outcomes, and early hemodynamic outcomes were performed using the chi-square test or Fisher’s exact test for categorical variables, and Student’s t-test or Mann-Whitney test for continuous variables, as appropriate. All tests were two-tailed, and a P value <0.05 was considered statistically significant.
Results
Preoperative characteristics
There was no difference in the proportion of female, body mass index, or body surface area between the groups, but the patients who received Magna Ease valve were older than those who received Intuity valve (68.6±10.5 vs. 71.9±9.2 years old in Intuity vs. Magna Ease, P=0.001). Risk factors showed no significant difference between the groups, except for chronic kidney disease (20.5% vs. 28.8% in Intuity vs. Magna Ease, P=0.049) and reoperation (6.0% vs. 12.1% in Intuity vs. Magna Ease, P=0.031) (Table 1).
Table 1
| Variable | Intuity (n=215) | Magna Ease (n=198) | P value |
|---|---|---|---|
| Sex (female), n (%) | 93 (43.3) | 89 (44.9) | 0.729 |
| Age, years | 68.6±10.5 | 71.9±9.2 | 0.001 |
| Body mass index, kg/m2 | 24.2±3.4 | 24.0±3.5 | 0.541 |
| Body surface area, m2 | 1.67±0.19 | 1.65±0.18 | 0.233 |
| Risk factors, n (%) | |||
| Diabetes mellitus | 51 (23.7) | 60 (30.3) | 0.132 |
| Hypertension | 124 (57.7) | 128 (64.6) | 0.147 |
| Dyslipidemia | 93 (43.3) | 77 (38.9) | 0.368 |
| COPD | 18 (8.4) | 11 (5.6) | 0.263 |
| Stroke | 23 (10.7) | 23 (11.6) | 0.767 |
| Chronic kidney disease | 44 (20.5) | 57 (28.8) | 0.049 |
| Renal replacement therapy | 8 (3.7) | 9 (4.5) | 0.673 |
| Coronary artery disease | 49 (22.8) | 45 (22.7) | 0.988 |
| PAOD | 12 (5.6) | 13 (6.6) | 0.675 |
| Infective endocarditis | 3 (1.4) | 10 (5.1) | 0.046 |
| Atrial fibrillation | 25 (11.6) | 26 (13.1) | 0.643 |
| Reoperation | 13 (6.0) | 24 (12.1) | 0.031 |
| EuroSCORE II | 3.09±4.53 | 3.17±3.40 | 0.836 |
| NYHA class, n (%) | 0.646 | ||
| I | 51 (23.7) | 37 (18.7) | |
| II | 121 (56.3) | 121 (61.1) | |
| III | 36 (16.7) | 33 (16.7) | |
| IV | 7 (3.3) | 7 (3.5) | |
| Etiology, n (%) | |||
| Degenerative | 70 (32.6) | 94 (47.5) | 0.002 |
| Bicuspid | 113 (52.6) | 60 (30.3) | <0.001 |
| Rheumatic | 8 (3.7) | 10 (5.1) | 0.509 |
| Infectious | 3 (1.4) | 9 (4.5) | 0.078 |
| Prosthetic valve failure | 1 (0.5) | 5 (2.5) | 0.109 |
| Pure aortic regurgitation | 20 (9.3) | 20 (10.1) | 0.784 |
| Emergency operation, n (%) | 4 (1.9) | 4 (2.0) | >0.999 |
Continuous variables are presented as mean ± standard deviation. COPD, chronic obstructive pulmonary disease; EuroSCORE, European System for Cardiac Operative Risk Evaluation, NYHA, New York Heart Association; PAOD, peripheral arterial occlusive disease.
Operative data
A prosthesis size of 21 mm was the most frequently implanted in both groups (29.3% and 39.9% in Intuity and Magna Ease valves, respectively), followed by 23 mm (23.7% and 31.8% in Intuity and Magna Ease valves, respectively).
Isolated AVR was performed in 32.6% and 56.6% of the Intuity and Magna Ease groups, respectively (P<0.001). In the overall population, concomitant procedures included mitral valve surgery (16.9%), tricuspid valve surgery (6.3%), coronary artery bypass grafting (8.7%), arrhythmia surgery (8.2%), and aorta surgery (27.6%). Concomitant procedures were more frequently performed in the Intuity group (67.4% vs. 43.4%, P<0.001), especially mitral valve surgery (22.3% vs. 11.1%, P<0.001) and aorta surgery (42.3% vs. 11.6%, P<0.001) (Table 2).
Table 2
| Variable | Intuity (n=215) | Magna Ease (n=198) | P value |
|---|---|---|---|
| Valve size, n (%) | – | ||
| 19 mm | 35 (16.3) | 36 (18.2) | |
| 21 mm | 63 (29.3) | 79 (39.9) | |
| 23 mm | 51 (23.7) | 63 (31.8) | |
| 25 mm | 44 (20.5) | 13 (6.6) | |
| 27 mm | 22 (10.2) | 7 (3.5) | |
| Isolated AVR, n (%) | 70 (32.6) | 112 (56.6) | <0.001 |
| Concomitant procedures, n (%) | 145 (67.4) | 86 (43.4) | <0.001 |
| Mitral valve surgery | 48 (22.3) | 22 (11.1) | 0.002 |
| Tricuspid valve surgery | 15 (7.0) | 11 (5.6) | 0.552 |
| CABG | 9 (4.2) | 27 (13.6) | 0.001 |
| Arrhythmia surgery | 15 (7.0) | 19 (9.6) | 0.333 |
| Aorta surgery | 91 (42.3) | 23 (11.6) | <0.001 |
| Procedural times | |||
| CPB time, min | 166 (145, 202) | 139 (105, 193) | <0.001 |
| ACC time, min | 113 (96, 138) | 87 (71, 121) | <0.001 |
Continuous variables are presented as medians with interquartile ranges. AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp.
Early clinical outcomes
Operative mortality was 1.7% (7 out of 413), and there was no significant difference between the groups. Common postoperative complications included postoperative atrial fibrillation (39.2%), acute kidney injury (10.9%), and respiratory complications (12.8%). There were no significant differences in the incidences of postoperative complications including permanent pacemaker implantation (1.4% vs. 1.0% in Intuity vs. Magna Ease, P>0.999) between the 2 groups (Table 3).
Table 3
| Variable | Intuity (n=215) | Magna Ease (n=198) | P value |
|---|---|---|---|
| Operative mortality, n (%) | 6 (2.8) | 1 (0.5) | 0.124 |
| Postoperative complication, n (%) | |||
| Postoperative atrial fibrillation | 86 (40.0) | 76 (38.4) | 0.737 |
| Low cardiac output | 8 (3.7) | 5 (2.5) | 0.579 |
| Permanent pacemaker implantation | 3 (1.4) | 2 (1.0) | >0.999 |
| Acute kidney injury | 27 (12.6) | 28 (14.1) | 0.636 |
| Bleeding reoperation | 7 (3.3) | 7 (3.5) | 0.875 |
| Stroke | 7 (3.3) | 3 (1.5) | 0.342 |
| Respiratory complication | 24 (11.2) | 27 (13.6) | 0.445 |
| Mediastinitis | 3 (1.4) | 1 (0.5) | 0.624 |
| Infective endocarditis | 0 (0.0) | 0 (0.0) | – |
Early hemodynamic outcomes
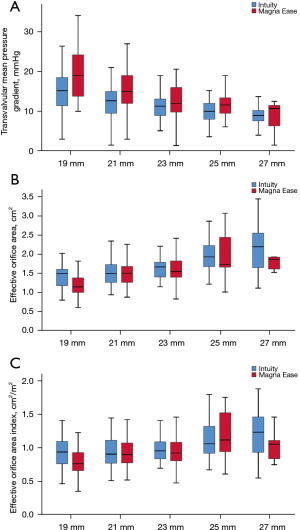
Transvalvular mean PG in the overall population was significantly lower in Intuity valve than in Magna Ease valve (12.0±4.3 vs. 15.3±6.8 mmHg, P<0.001). When stratified by prosthesis size, transvalvular mean PGs were also significantly lower in Intuity valve with 19 mm (15.5±5.0 vs. 20.8±9.1 mmHg, P=0.004), 21 mm (12.7±4.2 vs. 15.6±5.3 mmHg, P=0.001), 23 mm (11.5±3.3 vs. 13.4±5.8 mmHg, P=0.034) and 25 mm (9.9±3.1 vs. 12.3±4.0 mmHg, P=0.029) prostheses, but not the 27 mm prosthesis.
EOA in the overall population was significantly larger in Intuity valve than in Magna Ease valve (1.73±0.52 vs. 1.53±0.41 cm2, P<0.001). When stratified by prosthesis size, no significant differences of EOAs were found between the groups in patients with 21, 23, 25 and 27 mm prostheses. However, in patients with 19 mm prostheses, the EOA was significantly larger in Intuity valve than in Magna Ease valve (1.45±0.38 vs. 1.19±0.2 cm2, P=0.002).
EOAI demonstrated similar results to EOA that it was significantly larger in Intuity valve than in Magna Ease valve only with 19 mm prosthesis (0.96±0.26 vs. 0.80±0.20 cm2/m2, P=0.005) (Table 4 and Figure 2).
Table 4
| Variable | Intuity | Magna Ease | P value | |||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | |||
| Mean PG (mmHg) | ||||||
| 19 mm | 35 | 15.5±5.0 | 35 | 20.8±9.1 | 0.004 | |
| 21 mm | 62 | 12.7±4.2 | 79 | 15.6±5.3 | 0.001 | |
| 23 mm | 51 | 11.5±3.3 | 63 | 13.4±5.8 | 0.034 | |
| 25 mm | 42 | 9.9±3.1 | 13 | 12.3±4.0 | 0.029 | |
| 27 mm | 22 | 9.0±2.8 | 7 | 8.6±4.1 | 0.767 | |
| Overall | 212 | 12.0±4.3 | 197 | 15.3±6.8 | <0.001 | |
| EOA (cm2) | ||||||
| 19 mm | 35 | 1.45±0.38 | 35 | 1.19±0.28 | 0.002 | |
| 21 mm | 61 | 1.55±0.38 | 79 | 1.51±0.32 | 0.474 | |
| 23 mm | 49 | 1.68±0.33 | 63 | 1.63±0.36 | 0.469 | |
| 25 mm | 42 | 2.03±0.56 | 13 | 1.97±0.60 | 0.634 | |
| 27 mm | 22 | 2.22±0.72 | 7 | 1.90±0.43 | 0.328 | |
| Overall | 209 | 1.73±0.52 | 197 | 1.53±0.41 | <0.001 | |
| EOA index (cm2/m2) | ||||||
| 19 mm | 35 | 0.96±0.26 | 35 | 0.80±0.20 | 0.005 | |
| 21 mm | 61 | 0.98±0.25 | 79 | 0.94±0.22 | 0.601 | |
| 23 mm | 49 | 1.00±0.22 | 63 | 0.94±0.20 | 0.259 | |
| 25 mm | 42 | 1.13±0.31 | 13 | 1.16±0.39 | 0.782 | |
| 27 mm | 22 | 1.25±0.44 | 7 | 1.02±0.24 | 0.206 | |
| Overall | 209 | 1.04±0.30 | 197 | 0.93±0.24 | <0.001 | |
SD, standard deviation; PG, pressure gradient; EOA, effective orifice area.
Dimensionless parameter [Doppler velocity index (DVI)] and LVOT hemodynamic parameters [LVOT velocity time integral (VTI) and peak velocity] also demonstrated significant differences between the groups in the overall population. When stratified by prosthesis size, a trend of superior hemodynamics was observed in Intuity valve, although it failed to prove statistical significance in some subgroups of patients (Table S1).
Discussion
The present study demonstrated 3 main findings. First, the early hemodynamic performances of Edwards Intuity were superior to those of Carpentier-Edwards PERIMOUNT Magna Ease for all prosthesis sizes. Second, this superior hemodynamic performance of Intuity valve was more prominent in the smaller prostheses. Third, there were no differences between Intuity and Magna Ease valves in the early clinical outcomes after AVR, including the need for permanent pacemaker implantation (Figure 3).
The excellent hemodynamics of RD valve has been reported in previous observational and prospective studies (1,2,7,11). Comparative studies of RD valve versus conventional bioprosthetic valves have also consistently suggested that RD valve demonstrated better hemodynamics than conventional bioprosthetic valves (4). Rahmanian and colleagues (5) similarly analyzed 163 patients who received either an Intuity valve or a PERIMOUNT Magna valve (Edwards Lifesciences, Irvine, CA, USA) with propensity score-matching, and showed lower transvalvular gradients and higher EOAI in the Intuity valve. The CADENCE-MIS (12), which enrolled minimally invasive RDAVR patients, also demonstrated that significantly lower peak gradients after 1 year, a trend toward lower mean gradients, and a significantly greater EOA compared with the control group were observed, but it compared the RD valve with a range of different conventional prostheses. Another previous study (13) comparing the Intuity valve with the PERIMOUNT Magna valve demonstrated a superior hemodynamics in the Intuity valve with significantly lower transprosthetic gradients, but it made comparisons for the whole population and for the combined data of the 21 and 23 mm valves as a subgroup analysis. A propensity-score matched study was performed to compare RDAVR from the TRITON cohort and conventional AVR from the Magna Ease postmarket study and it revealed that RDAVR patients showed significantly lower mean and peak gradients than conventional AVR patients (14).
In contrast to previous studies, this was the first study that directly compared the hemodynamics between the RD valve and its conventional counterpart as a control prosthesis in a size-by-size fashion. In addition, the assessment of hemodynamic performance by echocardiographic measurements could be affected by interobserver differences, and most of the previous multicenter studies might have this potential bias. However, since the present study was conducted on a single-center basis during a contemporary period, the comparison of echocardiographic measurements regarding valve hemodynamics would be more reliable than in other multicenter studies. The Intuity valve was introduced in our institution in 2016 and Magna Ease valve was also introduced around the same time. After introduction, both valves were steadily used during the period covered by this study.
Considering the identical valvular structures of the Magna Ease valve and its rapid-deployment successor, the subannular fixation components of Intuity valve may play an important role in the lowering transvalvular gradient. It is convincing that the subvalvular stent frame reshapes the LVOT, which consequently reduces turbulent flow and optimizes the hemodynamic performance of the bioprosthesis. Intuity valve is also free of the bulky pledget material, which is commonly used to fix the prosthesis in conventional valves, and induces turbulent flow and subclinical inflow obstruction (15,16). In an experimental investigation (17), an aortic root model was created using 3-dimensional printing, and the superior performance of the Intuity valve over Magna Ease valve was proven, showing that peak systolic flow across the Intuity valve was accompanied by a significantly lower maximum velocity, less turbulent shear stress, and less turbulent kinetic energy than flow across the Magna Ease valve. Another in vitro study (18) conducted using cadaveric human heart and micro-computed tomography revealed that the RD valve formed a larger and more circular LVOT than the control valve and demonstrated an increase in the LVOT hydraulic diameter, which was maintained consistently across the LVOT. The in vivo hemodynamic outcomes in our study are in good agreement with the results from these in vitro studies. Considering that subannular pledget placement is rarely performed in conventional AVR in our institution, it is certain that the subvalvular stent frame itself can produce a positive effect on valve hemodynamics. It should also be noted that although the superiority of Intuity valve is observed in patients with narrow LVOT, some patients require LVOT resection to relieve outflow obstruction and perform suitable AVR.
Although RDAVR has demonstrated promising clinical outcomes, conduction and the consequent requirement for permanent pacemaker implantation after RDAVR have been the Achilles’ heel of the valve (19-21). The incidence of permanent pacemaker implantation has been reported to range from 5% to 15% (21-23). However, we previously reported an overall permanent pacemaker implantation rate of 1.8% (3 out of 167 patients) after RDAVR in our institution (9), which was similar to the outcomes after conventional AVR (1.5–3.9%) (21). This might be attributed to our procedure modification of using additional anchoring sutures and 5-mm videoscope to achieve ‘complete annulus fitting’. Even in cases of aortic valves with elliptical opening, Intuity valve can be well-fixed and takes best advantage of our modified technique. Distorted geometry of native annulus was frequently observed, particularly in bicuspid aortic valves, and with additional anchoring sutures, the sewing ring of Intuity valve could be completely fitted to the native annulus (24). The skirt portion of Intuity valve also have advantages in reducing turbulent flow and optimizing the hemodynamic performance by reshaping the left ventricular outflow tract, particularly in patients with elliptical or distorted LVOT.
If the issue about the permanent pacemaker implantation after RDAVR can be overcome, Intuity valve can be the optimal choice of bioprosthesis for patients with a small aortic annulus to overcome prosthesis-patient mismatch after AVR. Ghoneim and colleagues (25) analyzed 4 choices to address the small aortic annulus (stented valve, stentless valve, sutureless valve, and root enlargement techniques), showing that stentless valves and the Trifecta prosthesis (St. Jude Medical, St. Paul, Minnesota, USA) produced the best hemodynamics in these cases. However, there are also reports about intraoperative malfunction (26) or early degeneration in cases of the Trifecta prosthesis because of leaflet dysfunction with calcification, fibrous thickening, or pannus formation (27,28). In the same study, the Perceval sutureless valve (LivaNova, London, UK) showed comparable hemodynamics to conventional stented valves in patients with a small aortic annulus. Shrestha and colleagues (29) compared the hemodynamic performance of sutureless and conventional bioprostheses in geriatric patients with an annulus <22 mm, and there was no significant difference in terms of mean gradients and EOA between groups. Especially in these geriatric patients and small-sized aortic annuli, transcatheter aortic valve implantation (TAVI) should be considered a reasonable treatment option. Other techniques, including use of stentless valves, aortic root enlargement, complete aortic root replacement with homografts or a Ross procedure, would be more technically demanding and limited to a small subset of patient populations (30). In these circumstances, Intuity valve could be considered an alternative option to provide the best postoperative hemodynamics in small aortic roots.
Several shortcomings of Intuity valve reported in the previous studies should be recognized. The number of new postoperative conduction disorders, especially left bundle branch block, remains high during follow-up, although the long-term clinical significance was undetermined (31). There are also concerns that the existence of the subvalvular structure might cause anatomical changes in the aortic-mitral fibrous continuity, thus resulting in the alteration of mitral annular motion (32).
Limitations
There are several limitations that should be noted. First, this study was a retrospective single-center study with a small sample size although it would be advantageous to compare echocardiographic measurements of valve hemodynamics on a single-center basis. Second, the hemodynamic outcomes could be influenced by many factors, including body surface area, anemia, inflammation, and other medical conditions, but adjustments for these confounding factors were not performed in this study. The implantation technique of the Magna Ease valve, which varied among surgeons, might also confound the hemodynamic outcomes. Third, the present study only reported early hemodynamic profiles, whereas it has been recommended to evaluate the hemodynamic performance of AV prostheses at 6 months to 1 year after surgery (33-35). Also, data regarding the long-term durability beyond 5 years and incidence of structural valve deterioration, which would be of great importance, was not investigated. Follow-up investigation for any possible changes in valve hemodynamics beyond 1 year is needed. Fourth, all RDAVRs were performed with median sternotomy in this study, not like in other studies in which minimally invasive procedures were frequently used. The responsible surgeon put more value on lower incidence of paravalvular leakage and lower incidence of permanent pacemaker implantation by secure procedure with standard sternotomy than the advantages obtained by minimally invasive procedures.
Conclusions
Edwards Intuity demonstrated superior early hemodynamic performance compared with Magna Ease, and this superiority was more definite for small prostheses. This finding may help guide device selection in patients with small aortic roots.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-318/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-318/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-318/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-318/coif). KHK is an official proctor for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Seoul National University Hospital (No. H-2109-010-1251) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [Crossref] [PubMed]
- Barnhart GR, Accola KD, Grossi EA, et al. TRANSFORM (Multicenter Experience With Rapid Deployment Edwards INTUITY Valve System for Aortic Valve Replacement) US clinical trial: Performance of a rapid deployment aortic valve. J Thorac Cardiovasc Surg 2017;153:241-51.e2. [Crossref] [PubMed]
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25. [Crossref] [PubMed]
- Ferrari E, Roduit C, Salamin P, et al. Rapid-deployment aortic valve replacement versus standard bioprosthesis implantation. J Card Surg 2017;32:322-7. [Crossref] [PubMed]
- Rahmanian PB, Kaya S, Eghbalzadeh K, et al. Rapid Deployment Aortic Valve Replacement: Excellent Results and Increased Effective Orifice Areas. Ann Thorac Surg 2018;105:24-30. [Crossref] [PubMed]
- Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg 2015;99:831-7. [Crossref] [PubMed]
- Haverich A, Wahlers TC, Borger MA, et al. Three-year hemodynamic performance, left ventricular mass regression, and prosthetic-patient mismatch after rapid deployment aortic valve replacement in 287 patients. J Thorac Cardiovasc Surg 2014;148:2854-60. [Crossref] [PubMed]
- Schlömicher M, Haldenwang PL, Moustafine V, et al. Minimal access rapid deployment aortic valve replacement: initial single-center experience and 12-month outcomes. J Thorac Cardiovasc Surg 2015;149:434-40. [Crossref] [PubMed]
- Sohn SH, Kim KH, Kang Y, et al. Recovery From Conduction Abnormalities After Aortic Valve Replacement Using Edwards Intuity. Ann Thorac Surg 2021;112:1356-62. [Crossref] [PubMed]
- Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:589-90. [Crossref] [PubMed]
- Young C, Laufer G, Kocher A, et al. One-year outcomes after rapid-deployment aortic valve replacement. J Thorac Cardiovasc Surg 2018;155:575-85. [Crossref] [PubMed]
- Borger MA, Dohmen PM, Knosalla C, et al. Haemodynamic benefits of rapid deployment aortic valve replacement via a minimally invasive approach: 1-year results of a prospective multicentre randomized controlled trial. Eur J Cardiothorac Surg 2016;50:713-20. [Crossref] [PubMed]
- Andreas M, Wallner S, Habertheuer A, et al. Conventional versus rapid-deployment aortic valve replacement: a single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact Cardiovasc Thorac Surg 2016;22:799-805. [Crossref] [PubMed]
- Wahlers TCW, Andreas M, Rahmanian P, et al. Outcomes of a Rapid Deployment Aortic Valve Versus Its Conventional Counterpart: A Propensity-Matched Analysis. Innovations (Phila) 2018;13:177-83. [Crossref] [PubMed]
- Capelli C, Corsini C, Biscarini D, et al. Pledget-Armed Sutures Affect the Haemodynamic Performance of Biologic Aortic Valve Substitutes: A Preliminary Experimental and Computational Study. Cardiovasc Eng Technol 2017;8:17-29. [Crossref] [PubMed]
- Tasca G, Vismara R, Fiore GB, et al. Does the type of suture technique affect the fluid-dynamic performance of bioprostheses implanted in small aortic roots? Results from an in vitro study. J Thorac Cardiovasc Surg 2015;149:912-8. [Crossref] [PubMed]
- Ai L, Chen H, Lin V, et al. Rapid Deployment Aortic Valves Deliver Superior Hemodynamic Performance In Vitro. Innovations (Phila) 2017;12:338-45. [Crossref] [PubMed]
- Sadri V, Bloodworth CH 4th, Madukauwa-David ID, et al. A mechanistic investigation of the EDWARDS INTUITY Elite valve's hemodynamic performance. Gen Thorac Cardiovasc Surg 2020;68:9-17. [Crossref] [PubMed]
- Coti I, Schukro C, Drevinja F, et al. Conduction disturbances following surgical aortic valve replacement with a rapid-deployment bioprosthesis. J Thorac Cardiovasc Surg 2021;162:803-11. [Crossref] [PubMed]
- Mogilansky C, Balan R, Deutsch C, et al. New postoperative conduction abnormalities after the implantation of a rapid-deployment aortic valve prosthesis. Interact Cardiovasc Thorac Surg 2019;28:581-6. [Crossref] [PubMed]
- Herry M, Laghlam D, Touboul O, et al. Pacemaker implantation after aortic valve replacement: rapid-deployment Intuity® compared to conventional bioprostheses. Eur J Cardiothorac Surg 2020;58:335-42. [Crossref] [PubMed]
- Andreas M, Coti I, Rosenhek R, et al. Intermediate-term outcome of 500 consecutive rapid-deployment surgical aortic valve procedures. Eur J Cardiothorac Surg. 2019;55:527-33. [Crossref] [PubMed]
- Romano MA, Koeckert M, Mumtaz MA, et al. Permanent Pacemaker Implantation After Rapid Deployment Aortic Valve Replacement. Ann Thorac Surg 2018;106:685-90. [Crossref] [PubMed]
- Yun T, Kim KH, Sohn SH, et al. Rapid-Deployment Aortic Valve Replacement in a Real-World All-Comers Population. Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Ghoneim A, Bouhout I, Demers P, et al. Management of small aortic annulus in the era of sutureless valves: A comparative study among different biological options. J Thorac Cardiovasc Surg 2016;152:1019-28. [Crossref] [PubMed]
- Kataoka H, Tanaka H, Toshida T, et al. Intraoperative Trifecta Valve Malfunction. Ann Thorac Surg 2021;112:e107-9. [Crossref] [PubMed]
- Campisi S, Camilleri L, Innorta A, et al. Early failures of Trifecta aortic bioprosthesis. J Thorac Cardiovasc Surg 2014;148:e133-4. [Crossref] [PubMed]
- Schaefer AK, Kocher A, Laufer G, et al. Cusp Tear of Trifecta Aortic Bioprosthesis Resulting in Acute Heart Failure. J Heart Valve Dis 2017;26:592-594. [PubMed]
- Shrestha M, Maeding I, Höffler K, et al. Aortic valve replacement in geriatric patients with small aortic roots: are sutureless valves the future? Interact Cardiovasc Thorac Surg 2013;17:778-82; discussion 782. [Crossref] [PubMed]
- Oeser C, Uyanik-Uenal K, Kocher A, et al. Long-term performance of pulmonary homografts after the Ross procedure: experience up to 25 years. Eur J Cardiothorac Surg 2019;55:876-84. [Crossref] [PubMed]
- Mogilansky C, Massoudy P, Czesla M, et al. Conduction Disorders after Surgical Aortic Valve Replacement Using a Rapid Deployment Aortic Valve Prosthesis: Medium-Term Follow-Up. J Clin Med 2023;12:2083. [Crossref] [PubMed]
- Hori D, Nomura Y, Taniguchi Y, et al. The effect of balloon-expandable stent and self-expanding stent on changes in mitral annular motion after aortic valve replacement in patients with aortic stenosis. J Artif Organs 2023; Epub ahead of print. [Crossref] [PubMed]
- Lee H, Hwang HY, Sohn SH, et al. Hemodynamic Performance of Pericardial Bioprostheses in the Aortic Position. Korean J Thorac Cardiovasc Surg 2020;53:285-90. [Crossref] [PubMed]
- Bleiziffer S, Eichinger WB, Hettich I, et al. Prediction of valve prosthesis-patient mismatch prior to aortic valve replacement: which is the best method? Heart 2007;93:615-20. [Crossref] [PubMed]
- Pibarot P, Dumesnil JG. Hemodynamic and clinical impact of prosthesis-patient mismatch in the aortic valve position and its prevention. J Am Coll Cardiol 2000;36:1131-41. [Crossref] [PubMed]



