Co-morbid psychological dysfunction is associated with a higher risk of asthma exacerbations: a systematic review and meta-analysis
Introduction
Asthma is a serious global health problem affecting 1–18% of the population, and is a significant financial burden because of the healthcare costs and productivity loss (1). The heavy burden of asthma appears to be related to poor asthma control, and the cost could be greatly decreased if disease control was improved (2). According to the American Thoracic Society and European Respiratory Society, asthma control should include good symptom control and normal activity level, referred to as “clinical asthma control”, and should minimize the further risk of exacerbations, fixed airflow limitation, and side-effects (1,3).
The prevention of asthma exacerbations (AE) is an important component of optimal asthma control. It could be argued that AE are the most important outcome of asthma, because they are the key cost driver in asthma management, cause the greatest risk to patients, and increase the mortality of asthma (3). AE are common clinical incidents in patients with severe asthma and are often defined as hospitalizations, emergency department (ED) visits, systemic corticosteroid (SCS) use, or unscheduled doctor visits related to asthma (3). Achieving optimal asthma control relies on a good partnership between the individual with asthma and the healthcare provider and good self-management skills including effective communication, therapy adherence, self-monitoring, and regular visits to a care provider (2).
Psychological dysfunction (PD), including anxiety and depression, is more common in individuals with asthma than in healthy individuals, and asthma patients who experienced asthma symptoms had a significantly higher risk prevalence of depression than asthma patients who did not experience asthma symptoms (4,5). Psychological or emotional factors may impact the behavioral precautions taken by patients with asthma (6). PD is significantly associated with poor asthma control or intractability in asthma, damaged quality of life, more hospitalized days, and frequent AE (7-9). However, existing studies of the relation between PD and asthma outcomes are mainly cross-sectional or retrospective. Published prospective cohort studies of the relation between PD and AE have conflicting results (10-12). The results of pooled analyses of the effectiveness of psychological interventions for individuals with asthma are also inconsistent (13-15). Therefore, we undertook a systematic review and meta-analysis of prospective cohort studies to explore the influence of co-morbid PD on AE, and to determine whether different kinds of PD differentially affect AE.
Methods
Our systematic review and meta-analysis conformed to standard methodological guidelines for meta-analysis of observational studies (16).
Search strategy and inclusion criteria
A study protocol was formulated before starting the systematic review. The following databases were searched using a highly sensitive search filter: PubMed (1966 to January 2016), Cochrane library (up to January 2016), Web of Science (1994 to January 2016), Embase (1974 to January 2016), and Ovid (1946 to January 2016). The keywords used in the search were “asthma”, “psychological dysfunction”, and “asthma exacerbations” and their variations. The detailed search strategy is described in the Supplementary materials.
Studies were eligible for inclusion if they were prospective cohort studies that reported the influence of PD on AE, with adequately available data on either the number of individuals with AE during the follow-up period or the relative risk estimates (RRs) such as risk ratio, incidence rate ratio, hazard ratio, or odds ratio with 95% confidence interval (95% CI), and if PD exposure was measured by a relevant psychometric scale or diagnosed by a psychological specialist. Disagreements were solved by a third reviewer (GW).
Data extraction and quality assessment
A standardized data extraction form was completed prior to the commencement of data extraction. The full text of all articles that definitely or possibly met the inclusion criteria was accessed independently by two reviewers, and the relevant details were extracted. Study quality was assessed as described in our previous study (17). The data extraction and quality assessment are described in detail in the Supplementary materials.
Primary and secondary outcomes
AE or RRs of AE in subjects with PD were specified a priori as the primary outcome. The details of AE such as hospitalization, ED visit, unscheduled doctor visit, and SCS use were secondary outcomes (3). Where available, adjusted RRs of AE with 95% CI were also extracted and the adjusted confounding factors were noted.
According to the American Thoracic Society and European Respiratory Society (3), SCS use and hospitalizations or ED visits because of asthma are clinical indicators of severe AE. If a study reported either SCS use or hospitalizations or ED visits because of asthma as outcomes rather than AE directly, we interpreted these outcomes as AE. If a study reported two or even three of these events as outcomes, we first used the outcome of SCS use, and then ED visits because of asthma, and finally hospitalizations because of asthma. If different kinds of PD were defined, we selected one set of data to represent the effect of PD on AE, but performed sensitivity analyses for the other sets of data.
Statistical analysis and quality of the evidence assessment
The number of subjects who experienced AE during the follow-up period was treated as a dichotomous variable, and the pooled relative risk (RR) with 95% CI was calculated. Summary RR for the association between PD and different outcomes (AE, hospitalization because of asthma, ED visit because of asthma, unscheduled doctor visit because of asthma, and SCS use) were calculated using Stata Version 11.0 (Stata Corp. LP, College Station, TX). Heterogeneity in every effect estimate was assessed using the I2 statistic and the Cochran Q method, as in our previous studies (17,18). A random-effects meta-analysis model was used to calculate pooled estimates when substantial heterogeneity was observed (I2>50.0% and P<0.10), otherwise a fixed effects meta-analysis model was used. Sensitivity analysis was performed on the study population and quality of the included studies to assess the robustness of the results. Where original data had been adjusted for potential confounding factors, adjusted RR (RRadj) with 95% CI was also pooled. In the subgroup analysis, trials were further stratified by the type of PD. The time-dependent response of AE to PD exposure was also plotted. To flexibly plot the relation between RR and duration of PD exposure, we divided the included studies into three groups according to the duration of the follow-up period (≤1, >1 year and <2, ≥2 years). A two-sided P value <0.05 was considered statistically significant throughout the analysis.
The quality of the evidence was evaluated according to the suggestions of the Grading of Recommendations Assessment, Development, and Evaluation Working Group using GradePro software (Version 3.6) (19).
Results
Characteristics and quality of included studies
Our search strategy initially identified 4,585 studies. Ten of these were eligible prospective cohort studies and were included in the analysis (10-12,20-26). The flowchart for screening studies is shown in Figure 1. Table S1 shows the characteristics of the included studies. All the included studies were published between 2001 and 2014, and the duration of the follow-up period ranged from 6.0 to 86.4 months. There were 31,432 adults with asthma included, and 175 of these were pregnant women. In the study by Sumino et al. (20), the sample was divided into three independent groups by age (18–45, 46–64, and ≥65 years), and the adjusted risk ratio for AE and asthma-related hospitalizations in each group was calculated.
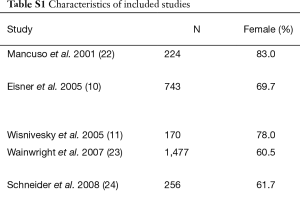
Full table
Most of the included studies were of high quality with Newcastle-Ottawa Quality Assessment Scale scores ranging from 5 to 9 (mean and standard deviation: 8.10±1.32; Tables S1, S2).

Full table
Exposure to psychological dysfunction (PD)
In the included studies, exposure to PD was ascertained according to one of eight psychometric tools (10,12,21-26), the International Classification of Diseases, 9th Revision, diagnosis of co-morbidities (21), or patient self-report (11). The eight psychometric tools used were all produced in the 1900s. Two were diagnostic tools (the Diagnostic and Statistical Manual of Mental Disorders, 4th edition and the Primary Care Evaluation of Mental Disorder) (21,23) and six were screening tools. Most have good reliability and validity, but they have varying specificity and sensitivity in different populations with different time frames (Table S3). The Diagnostic and Statistical Manual of Mental Disorders, 4th edition, is the standard classification of mental disorders used by mental health professionals for patient diagnosis and treatment in the United States and is widely used as the criterion standard in validity studies for many other psychometric scales (21,24,25). The Primary Care Evaluation of Mental Disorder is the origin of several other psychometric scales (21,27,28). It is composed of a one-page patient questionnaire and a 12-page clinician evaluation guide. The patient questionnaire should be completed by the patient before seeing the doctor, and the clinician evaluation guide is a structured interview that the physician uses to follow up on positive responses on the patient questionnaire (29).

Full table
Primary outcome
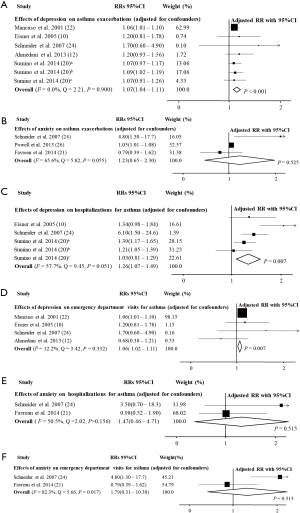
In the study by Schneider et al. (24), the impact of panic disorder and depression on hospitalizations and ED visits because of asthma was reported separately. When the data of the effect of depression was included, the pooled estimates showed that asthma patients with co-morbid PD had increased risk of AE (RR =1.07, 95% CI: 1.05–1.10, P<0.001, I2=38.9%, Q=18.01, P=0.081; RRadj =1.06, 95% CI: 1.04–1.09, P<0.001, I2=0.0%, Q=3.52, P=0.940; Figure 2 and Table 1), and when the data about the effect of panic disorder was included instead, the result maintained the same (RR =1.11, 95% CI: 1.05–1.17, P<0.001, I2=50.7%, Q=22.33, P=0.022; RRadj =1.06, 95% CI: 1.04–1.09, P<0.001, I2=0.0%, Q=7.88, P=0.546) (10-12,20-26). Subgroup analyses to investigate the effects of depression and anxiety on AE indicated that depression was associated with a higher risk of AE (RRadj =1.07, 95% CI: 1.04–1.11, P<0.001, I2=0.0%, Q=2.21, P=0.900) (10,12,20,22,24) but anxiety was not (RRadj =1.23, 95% CI: 0.65–2.30, P=0.525, I2=65.6%, Q=5.82, P=0.055; Figure 3) (21,24,26).
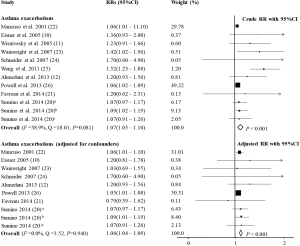

Full table
Secondary outcomes
In patients with asthma, PD significantly increased the risk of hospitalizations (RR =1.25, 95% CI: 1.14–1.37, P<0.001, I2=3.0%, Q=5.15, P=0.397; RRadj =1.22, 95% CI: 1.12–1.34, P<0.001, I2=12.3%, Q=5.70, P=0.337; Figure 4 and Table 1) (10,20,21,23), unscheduled doctor visits (RR =4.26, 95% CI: 2.52–7.19, P<0.001) (25), and ED visits because of asthma (RR =1.36, 95% CI: 0.86–2.14, P=0.185, I2=82.0%, Q=22.26, P<0.001; RRadj =1.06, 95% CI: 1.01–1.10, P=0.009, I2=8.7%, Q=3.29, P=0.350; Figure 4 and Table 1) (10,12,21,22,25), but did not increase the risk of SCS use (RR =1.17, 95% CI: 0.97–1.41, P=0.110; RRadj =1.20, 95% CI: 0.93–1.56, P=0.160) (12).
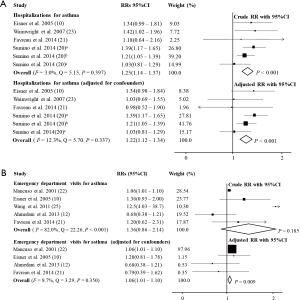
In the subgroup analysis, depression significantly increased the risk of hospitalizations (RRadj =1.26, 95% CI: 1.07–1.49, P=0.007, I2=57.7%, Q=9.45, P=0.051) (10,20,24) and ED visits because of asthma (RRadj =1.06, 95% CI: 1.02–1.11, P=0.007, I2=12.2%, Q=3.42, P=0.332; Figure 3) (10,12,22,24). However, anxiety did not significantly increase the risk of hospitalizations (RRadj =1.47, 95% CI: 0.46–4.71, P=0.515, I2=50.5%, Q=2.02, P=0.156) (21,24) or ED visits because of asthma (RRadj =1.79, 95% CI: 0.31–10.38, P=0.515, I2=82.3%, Q=5.66, P=0.017; Figure 3) (21,24).
Time-dependent response to psychological dysfunction (PD)
For studies with exposure time ≤1 year, the relation between PD and AE was not significant (RR =1.24, 95% CI: 0.96–1.59, P=0.095, I2=82.8%, Q=11.65, P=0.003) (11,25,26). For studies with exposure time >1 year, <2, and ≥2 years, the relation between PD and AE was significant, but there was no specific time-dependent response [RRadj =1.07, 95% CI: 1.02–1.11, P=0.003, I2=0.0%, Q=1.22, P=0.544 (10,12,22) and RRadj =1.08, 95% CI: 1.02–1.14, P=0.011, I2=0.0%, Q=0.88, P=0.927 (20,21,23), respectively; Figure 5].
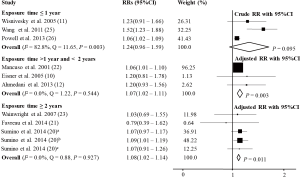
Sensitivity analyses
The effects of PD on AE were unchanged statistically when the low-quality study by Wainwright et al. (23) was excluded (RR =1.07, 95% CI: 1.05–1.10, P<0.001; I2=37.6%, Q=14.43, P=0.108; RRadj =1.06, 95% CI: 1.04–1.09, P<0.001; I2=0.0%, Q=2.72, P=0.909) and when the study of pregnant women by Powell et al. (26) was excluded (RR =1.09, 95% CI: 1.05–1.12, P<0.001; I2=44.7%, Q=16.26, P=0.062; RRadj =1.07, 95% CI: 1.03–1.11, P<0.001; I2=0.0%, Q=2.19, P=0.948).
Quality of evidence
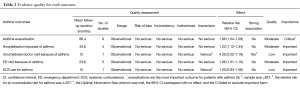
Table 2 shows the quality of evidence for the relation between PD and AE extrapolated according to the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation Working Group. In adults with asthma, the evidence was of low or moderate quality for every asthma outcome.
Full table
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis of prospective cohort studies on the effects of PD on AE. Our results indicated that subjects with co-morbid asthma and PD were at higher risk of AE, presenting as hospitalizations, unscheduled doctor visits, and ED visits because of asthma. The subgroup analysis indicated that depression was significantly associated with higher risk of AE, presenting as hospitalizations, unscheduled doctor visits, and ED visits because of asthma, but anxiety only increased the risk of AE in pregnant women. There was a significant effect of PD on AE only when the exposure time of PD exceeded 1 year.
It should be noted that, in our analysis, anxiety did not have a significant influence on hospitalizations or ED visits because of asthma, and this is inconsistent with our prior study (25). The current analysis on anxiety was limited by a small amount of data, and the effects of anxiety on AE should be further considered.
Contrary to our conclusions, several prior cross-sectional studies showed that probable PD, including anxiety or depression symptoms, was not significantly associated with higher levels of medical services for asthma (10,30,31). However, the cross-sectional study design and possible recall bias may weaken the validity of these cross-sectional studies. The current analysis was based on prospective cohort studies that can measure events in chronological order.
An interesting result in our subgroup analysis was that the influence of PD on AE was only significant when the PD exposure time exceeded one year, and the RR did not increase any more when the exposure time exceeded two years. This implies that there is a cumulative effect of PD on AE over the first year, and that the effect becomes stable when the exposure time exceeds 1 year. This may also indicate that psychological interventions delivered within a time window of one year may be able to prevent the poor asthma outcomes in asthmatic individuals with co-morbid PD.
We found substantial heterogeneity in the outcome of AE. After adjusting for some confounding factors such as age, sex, and race of the participants, glucocorticoid use, and prior exacerbations, the heterogeneity among the studies was reduced (21,22,25). Substantial heterogeneity also existed in the outcome of ED visits because of asthma, and adjustment for confounding factors such as age, sex, race, years of education, smoking, asthma control, inhaled corticosteroid dose, and medication adherence resulted in a significant statistical difference for the relation between PD and ED visits because of asthma (10,12,20,21). We therefore infer that the heterogeneity among the studies was mainly caused by differences in the baseline characteristics, clinical characteristics, asthma medications, and medication adherence of the study participants, and these factors may have a significant impact on ED visits because of asthma.
It should be recognized that, in Figures 2,3, the studies by Mancuso et al. (22) and Powell et al. (26) had much larger weight than the study by Sumino et al. (20), which included the largest sample size. The weight given to each study in the meta-analysis was determined using the inverse of the variance of the effect estimate (32). As a general rule, if the variance of the effect estimate is the decisive factor for the weight given to a study, studies with a larger sample size will have a larger weight than studies with a smaller sample size, as they typically have smaller variance. However, perhaps because of large variation in the characteristics of the participants in the study by Sumino et al., including asthma severity, asthma control level, and the presence of severe co-morbidities, this study had large variance (95% CI) in the effect estimate even though it had the largest sample size. As a result, a small weight was given to this study in the meta-analysis.
The mechanism underlying the interactions between PD and asthma or asthma outcomes is not well understood. In asthma patients, PD, including depression and anxiety, may reduce self-esteem, decrease internal feelings of control, and ultimately undermine the self-efficacy of asthma control (33). This may explain why patients with PD have reduced asthma-related emotional functioning and response to stimuli and have more difficulty dealing with their disease. This could lead to further activity limitations and non-adherence to medicines, which then results in poor asthma outcomes. Furthermore, in our recently published magnetic resonance imaging and voxel-based morphometry study, we reported structural changes in the right superior temporal gyrus in female asthma patients with depression, and reduced gray matter volume in the right superior temporal gyrus was associated with increased airway hyper-responsiveness (34). This suggests that the right superior temporal gyrus may play a critical role in the link between asthma and depression. This brain region may also play a role in the link between depression and AE. In a study based on a murine model of asthma, Forsythe et al. reported that inflammatory cell numbers in bronchoalveolar lavage fluid were significantly increased after long-term stress stimulations, and these changes in airway inflammation might result from the loss of anti-inflammatory response to endogenous corticosterone (35). In humans, the possible exacerbated chronic inflammatory response induced by repeated stress may translate to increased long-term damage of the airway and gradual deterioration in function through remodeling (35). It is also speculated that depression could directly influence the autonomic nervous system and act on direct respiratory resistance, symptom perception, and AE (36,37). Other studies also report that risk behaviors such as smoking, physical inactivity, obesity, and health-service seeking associated with depression might influence asthma outcomes (38,39). All of these factors may contribute to the association between PD and AE. Goodwin et al. suggested that the association between PD and asthma may reflect the effects of common factors associated with both asthma and PD, rather than a direct causal link (40). Further studies are needed to clarify the specific mechanisms contributing to these associations.
Our analysis indicated that PD was significantly associated with adverse asthma outcomes. We therefore have reason to believe that psychological intervention may be an effective therapeutic measure for asthmatic patients with co-morbid PD. A systematic review did not support the effectiveness of psychological interventions for subjects with asthma (13,14); however, the psychological interventions were varied, did not have a clear theoretical underpinning, and were always used in addition to pharmacological treatments rather than as an alternative (13). Additionally, psychological co-morbidity is difficult to characterize and often not diagnosed (14), and it was often difficult to discern whether the aim of a psychological intervention was for general adjustment to asthma or for a psychological co-morbidity, because no detailed descriptions of the need for the psychological treatment were provided (14). All of these factors made it difficult for the review to make firm conclusions and any results must be viewed with caution.
Several limitations of our study should be addressed. Firstly, the definitions of PD were not identical in the studies included in our analysis. Eight different tools with different time frames were used. Nevertheless, most of the tools have high sensitivity and specificity and good reliability and validity when compared with other criterion standards, and there is evidence that the psychological status of patients with stable asthma remains clinically stable over a 5-year period (41). Anxiety and depression are common co-morbid psychological disorders and it was difficult to identify groups of patients presenting with only anxiety or only depression clearly. Secondly, the definitions of AE were also not consistent in the studies included in our analysis. To address this, we referred to the report by the American Thoracic Society and European Respiratory Society and adopted secondary outcomes to accurately describe AE (3). Thirdly, of the studies included in our analysis, none adjusted for the severity of asthma, only one adjusted for asthma control level (12), and only one adjusted for prior AE (21) despite it being widely recognized that all of these factors are significantly associated with further AE (2). Fourthly, the studies included in our analysis reported different measures of RRs, such as odds ratio, hazard ratio, risk ratio and incidence rate ratio. The data were directly pooled as RR with 95%CI, which is typically reported in similar studies (42,43).
Conclusions
Our meta-analysis based on prospective cohort studies strongly suggests that PD is associated with significantly increased risk of AE presenting as hospitalizations, unscheduled doctor visits, and ED visits because of asthma. Depression had a stronger influence on the outcomes than anxiety. PD only showed a significant association with AE when the PD exposure time exceeded 1 year. These findings support the adverse impact of co-morbid PD on AE and suggest the existence of an asthma psycho-phenotype. Physicians should consider the psychological state of the patient before establishing treatment and intervention regimens. Further studies are needed to clarify the mechanisms underlying the interaction between PD and AE.
Acknowledgements
Funding: This study was partly supported by the National Natural Science Foundation of China (No. 81370122), Ministry of Science and Technology of China (2012BAI05B02), Youth Science Funding of Sichuan University (No. 2011SCU04B17), and Program for New Century Excellent Talents in University (No. NCET-12-0380).
Footnote
Conflicts of Interest: Dr. G Wang received funding from the China AstraZeneca Lung Foundation in 2013. The other authors have no conflicts of interest to declare.
Methods
Details of the search strategy and article screening
Electronic databases were searched using the following key words based on previous studies and MeSH database of PubMed: (asthma OR wheezing) AND (emotion* OR behavior* OR psych* OR mental* OR anxi* OR depress* OR distress* OR panic* OR personality) AND [(exacerbat* OR attack OR episode OR acute) OR (glucocorticoid OR corticosteroid OR cortin OR steroid) OR emergency OR (hospitalization OR admission) OR (unplanned OR unscheduled)] (1,44). All titles and abstracts identified by this search were assessed independently by two reviewers. The full text of each article that potentially met the inclusion criteria was obtained for further assessment. The reference lists of all relevant reviews and potentially relevant articles were also searched. Search results were not restricted to a particular language.
Details of the extracted data and quality assessment
The data extracted from each article included the elementary characteristics of the study (first author, year of publication, study population, sample size, follow-up duration, and follow-up loss), baseline characteristics of the participators (age at recruitment, gender, ethnicity, socioeconomic status, and smoking history), and clinical characteristics (diagnosis, duration, severity, lung function, asthma control status, asthma medications, adherence to treatment, and exacerbations during the previous year) (45,46).
Study quality was assessed and scored independently by two reviewers (LZ and XZ) using the Newcastle-Ottawa Quality Assessment Scale (47). The Newcastle-Ottawa Quality Assessment Scale is a validated tool for assessing the quality of non-randomized studies, including cohort studies. It consists of three parts (study selection, compatibility, and study outcomes) and has a maximum score of nine. Any problems or disagreements between the two reviewers were solved by a third reviewer (GW).
References
- GINA Report. Global strategy for asthma management and prevention. Revised 2015. Available online: http://www.ginasthma.org/
- Lane S, Molina J, Plusa T. An international observational prospective study to determine the cost of asthma exacerbations (COAX). Respir Med 2006;100:434-50. [Crossref] [PubMed]
- Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59-99. [Crossref] [PubMed]
- Lu Y, Mak KK, van Bever HP, Ng TP, et al. Prevalence of anxiety and depressive symptoms in adolescents with asthma: a meta-analysis and meta-regression. Pediatr Allergy Immunol 2012;23:707-15. [Crossref] [PubMed]
- Goldney RD, Ruffin R, Fisher LJ, et al. Asthma symptoms associated with depression and lower quality of life: a population survey. Med J Aust 2003;178:437-41. Erratum in: Med J Aust 2003;179:17. [PubMed]
- Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med 2006;173:953-7. [Crossref] [PubMed]
- Feldman JM, Lehrer PM, Borson S, et al. Health care use and quality of life among patients with asthma and panic disorder. J Asthma 2005;42:179-84. [Crossref] [PubMed]
- Krauskopf KA, Sofianou A, Goel MS, et al. Depressive symptoms, low adherence, and poor asthma outcomes in the elderly. J Asthma 2013;50:260-6. [Crossref] [PubMed]
- Dirks JF, Schraa JC, Brown EL, et al. Psycho-maintenance in asthma: hospitalization rates and financial impact. Br J Med Psychol 1980;53:349-54. [Crossref] [PubMed]
- Eisner MD, Katz PP, Lactao G, et al. Impact of depressive symptoms on adult asthma outcomes. Ann Allergy Asthma Immunol 2005;94:566-74. [Crossref] [PubMed]
- Wisnivesky JP, Leventhal H, Halm EA. Predictors of asthma-related health care utilization and quality of life among inner-city patients with asthma. J Allergy Clin Immunol 2005;116:636-42. Erratum in: J Allergy Clin Immunol 2006;117:1140. [Crossref] [PubMed]
- Ahmedani BK, Peterson EL, Wells KE, et al. Examining the relationship between depression and asthma exacerbations in a prospective follow-up study. Psychosom Med 2013;75:305-10. [Crossref] [PubMed]
- Yorke J, Fleming SL, Shuldham CM. Psychological interventions for adults with asthma. Cochrane Database Syst Rev 2006.CD002982. [PubMed]
- Yorke J, Fleming S, Shuldham C. Psychological interventions for children with asthma. Cochrane Database Syst Rev 2005.CD003272. [PubMed]
- Ritz T, Meuret AE, Trueba AF, et al. Psychosocial factors and behavioral medicine interventions in asthma. J Consult Clin Psychol 2013;81:231-50. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Wang G, Murphy VE, Namazy J, et al. The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2014;27:934-42. [Crossref] [PubMed]
- Fan T, Wang G, Mao B, et al. Prophylactic administration of parenteral steroids for preventing airway complications after extubation in adults: meta-analysis of randomised placebo controlled trials. BMJ 2008;337:a1841. [Crossref] [PubMed]
- Schünemann H, Brożek J, Guyatt G, et al. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. Available online: http://www.gradeworkinggroup.org/publications/JCE_series.htm
- Sumino K, O'Brian K, Bartle B, et al. Coexisting chronic conditions associated with mortality and morbidity in adult patients with asthma. J Asthma 2014;51:306-14. [Crossref] [PubMed]
- Favreau H, Bacon SL, Labrecque M, et al. Prospective impact of panic disorder and panic-anxiety on asthma control, health service use, and quality of life in adult patients with asthma over a 4-year follow-up. Psychosom Med 2014;76:147-55. [Crossref] [PubMed]
- Mancuso CA, Rincon M, McCulloch CE, et al. Self-efficacy, depressive symptoms, and patients' expectations predict outcomes in asthma. Med Care 2001;39:1326-38. [Crossref] [PubMed]
- Wainwright NW, Surtees PG, Wareham NJ, et al. Psychosocial factors and incident asthma hospital admissions in the EPIC-Norfolk cohort study. Allergy 2007;62:554-60. Erratum in: Allergy 2008;63:382. [Crossref] [PubMed]
- Schneider A, Löwe B, Meyer FJ, et al. Depression and panic disorder as predictors of health outcomes for patients with asthma in primary care. Respir Med 2008;102:359-66. [Crossref] [PubMed]
- Wang G, Zhou T, Wang L, et al. Relationship between current psychological symptoms and future risk of asthma outcomes: a 12-month prospective cohort study. J Asthma 2011;48:1041-50. [Crossref] [PubMed]
- Powell H, McCaffery K, Murphy VE, et al. Psychosocial variables are related to future exacerbation risk and perinatal outcomes in pregnant women with asthma. J Asthma 2013;50:383-9. [Crossref] [PubMed]
- McDowell I, Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. 3th ed. New York, NY: Oxford University Press, Inc., 2006.
- Löwe B, Spitzer RL, Gräfe K, et al. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. J Affect Disord 2004;78:131-40. [Crossref] [PubMed]
- Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749-56. [Crossref] [PubMed]
- Goodwin RD, Messineo K, Bregante A, et al. Prevalence of probable mental disorders among pediatric asthma patients in an inner-city clinic. J Asthma 2005;42:643-7. [Crossref] [PubMed]
- Lavoie KL, Bacon SL, Barone S, et al. What is worse for asthma control and quality of life: depressive disorders, anxiety disorders, or both? Chest 2006;130:1039-47. [Crossref] [PubMed]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. (Version 5.1.0).
- Wang G, Wang L, Szczepaniak WS, et al. Psychological status in uncontrolled asthma is not related to airway hyperresponsiveness. J Asthma 2010;47:93-9. [Crossref] [PubMed]
- Wang L, Wang T, Liu S, et al. Cerebral anatomical changes in female asthma patients with and without depression compared to healthy controls and patients with depression. J Asthma 2014;51:927-33. [Crossref] [PubMed]
- Forsythe P, Ebeling C, Gordon JR, et al. Opposing effects of short- and long-term stress on airway inflammation. Am J Respir Crit Care Med 2004;169:220-6. [Crossref] [PubMed]
- Ritz T, Claussen C, Dahme B. Experimentally induced emotions, facial muscle activity, and respiratory resistance in asthmatic and non-asthmatic individuals. Br J Med Psychol 2001;74:167-82. [Crossref] [PubMed]
- Krommydas GC, Gourgoulianis KI, Angelopoulos NV, et al. Depression and pulmonary function in outpatients with asthma. Respir Med 2004;98:220-4. [Crossref] [PubMed]
- Strine TW, Mokdad AH, Balluz LS, et al. Impact of depression and anxiety on quality of life, health behaviors, and asthma control among adults in the United States with asthma, 2006. J Asthma 2008;45:123-33. [Crossref] [PubMed]
- Boudreau M, Bacon SL, Ouellet K, et al. Mediator effect of depressive symptoms on the association between BMI and asthma control in adults. Chest 2014;146:348-54. [Crossref] [PubMed]
- Goodwin RD, Fergusson DM, Horwood LJ. Asthma and depressive and anxiety disorders among young persons in the community. Psychol Med 2004;34:1465-74. [Crossref] [PubMed]
- Oga T, Nishimura K, Tsukino M, et al. Analysis of longitudinal changes in the psychological status of patients with asthma. Respir Med 2007;101:2133-8. [Crossref] [PubMed]
- Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA 2011;305:2448-55. [Crossref] [PubMed]
- Aune D, Saugstad OD, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311:1536-46. [Crossref] [PubMed]
- Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59-99. [Crossref] [PubMed]
- Hermosa JL, Sánchez CB, Rubio MC, et al. Factors associated with the control of severe asthma. J Asthma 2010;47:124-30. [Crossref] [PubMed]
- Yıldız F; ASIT Study Group. Factors influencing asthma control: results of a real-life prospective observational asthma inhaler treatment (ASIT) study. J Asthma Allergy 2013;6:93-101. [Crossref] [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf
- Guideline for the Diagnosis and Management of Asthma National Heart, Lung and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol 1991;88:425-534. [PubMed]
- Blanc PD, Cisternas M, Smith S, et al. Asthma, employment status, and disability among adults treated by pulmonary and allergy specialists. Chest 1996;109:688-96. [Crossref] [PubMed]
- Blanc PD, Jones M, Besson C, et al. Work disability among adults with asthma. Chest 1993;104:1371-7. [Crossref] [PubMed]
- Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol 1992;45:461-72. [Crossref] [PubMed]
- National Institute of Health. Global initiative for asthma-global strategy for asthma management and prevention. 2005. Available online: http://www.ginasthma.com
- GINA Report. Global strategy for asthma management and prevention. Revised 2006. Available online: http://ginasthma.org/
- Shen J, Johnston M, Hays RD. Asthma outcome measures. Expert Rev Pharmacoecon Outcomes Res 2011;11:447-53. [Crossref] [PubMed]
- Hasin D, Samet S, Nunes E, et al. Diagnosis of comorbid psychiatric disorders in substance users assessed with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV. Am J Psychiatry 2006;163:689-96. [Crossref] [PubMed]
- Spitzer RL, Wakefield JC. DSM-IV diagnostic criterion for clinical significance: does it help solve the false positives problem? Am J Psychiatry 1999;156:1856-64. [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. [Crossref] [PubMed]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [Crossref] [PubMed]
- Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. [Crossref] [PubMed]
- Whooley MA, Avins AL, Miranda J, et al. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med 1997;12:439-45. [Crossref] [PubMed]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301-6. [Crossref] [PubMed]
- Loerch B, Szegedi A, Kohnen R, et al. The primary care evaluation of mental disorders (PRIME-MD), German version: a comparison with the CIDI. J Psychiatr Res 2000;34:211-20. [Crossref] [PubMed]



