
Risk factors for the recurrence of primary spontaneous pneumothorax after video-assisted thoracoscopic surgery in patients younger than 40 years
Highlight box
Key findings
• Among patients with primary spontaneous pneumothorax who underwent video-assisted thoracoscopic bullectomy, age younger than 20 years at surgery, history of contralateral pneumothorax, and no staple line reinforcement were significant prognostic factors for postoperative recurrence.
What is known and what is new?
• Young age and a history of ipsilateral pneumothorax are risk factors for postoperative recurrence. Reinforcing the staple line with a polyglycolic acid sheet and fibrin glue effectively prevents recurrence.
• A history of contralateral pneumothorax may also be a prognostic factor, and using autologous blood instead of fibrin glue may effectively prevent postoperative recurrence.
What is the implication, and what should change now?
• Patient explanations and postoperative observations should be carefully performed. It should be recognized that recurrence rates may be higher for younger patients and those with a history of pneumothorax. Autologous blood may enhance staple line reinforcement and prevent recurrence.
Introduction
Primary spontaneous pneumothorax (PSP) is common in general hospitals. It is more common in young, tall, thin male individuals without underlying lung disease, and it rare occurs in persons older than 40 years (1). Surgical treatment was performed for recurrent pneumothorax or prolonged air leakage. In recent years, bullectomy using video-assisted thoracoscopic surgery (VATS) has become the standard procedure for pneumothorax surgery because it is less invasive and more cosmetically effective than open thoracotomy (2,3). However, its recurrence rate is approximately 4–11%, which is higher than that of open thoracotomy, and the recurrence rate after bullectomy under open thoracotomy is approximately 1% (4,5). Pleurectomy, mechanical or chemical pleurodesis, and staple line reinforcement prevent postoperative recurrence after VATS bullectomy for PSP (6). As a guideline, staple line coverage with an absorbable mesh was introduced in the European Respiratory Society statement about PSP (7), which described that the covering technique appears to be effective for reducing recurrent PSP; however, it suggested that this should be confirmed by further studies. Polyglycolic acid (PGA) sheets are often used as a reinforcing material against staple lines, and the following methods of use have been reported: (I) PGA sheets alone; (II) PGA sheets with fibrin glue; and (III) PGA sheets with autologous blood (8). Since 2014, PGA sheets have been applied as reinforcement against staple lines by spraying 10 mL of autologous blood to fix the sheets at our institution. In the present study, we investigated the outcomes of patients who underwent VATS bullectomy for PSP to evaluate the effectiveness of surgery for preventing PSP recurrence. Furthermore, we reviewed the clinical features to identify the prognostic factors related to recurrence after surgery. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-257/rc) (9).
Methods
This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This retrospective study was approved by the Institutional Review Board of Yokohama Municipal Citizen’s Hospital (No. 23-02-01), and the requirement for individual consent for this retrospective analysis was waived. Patients 40 years of age or younger without underlying pulmonary disease who underwent surgery for PSP for the first time at Yokohama Municipal Citizen’s Hospital between January 2008 and November 2022 were included in this study. The following clinical features were retrospectively collected from medical records: age at surgery; sex; smoking history; body mass index (BMI); history of both ipsilateral and contralateral PSP; surgical procedure; and follow-up period. During surgery, the bullae and blebs were resected using a stapling device. In some patients, staple line coverage was performed with a PGA sheet, and 10 mL of autologous blood taken from a radial arterial line was sprayed. Patients typically attended routine follow-up appointments 2 weeks and 4 to 8 weeks after surgery. Recurrence was defined as a diagnosis of ipsilateral pneumothorax at a medical institution after the initial appointment. Further additional follow-up was conducted by phone or mail for all patients.
Statistical analysis
All statistical analyses were performed using SPSS software (version 26.0; IBM Corporation, Armonk, NY, USA). Categorical variables of the two groups were evaluated using the chi-square test. Recurrence-free survival (RFS) was estimated using the Kaplan-Meier method, and differences between survival curves were analyzed using the log-rank test. The Cox proportional hazards regression model was used to evaluate the potential prognostic factors for recurrence after VATS bullectomy and to calculate hazard ratios. Statistical significance was set at P<0.05.
Results
During this study, VATS bullectomy for pneumothorax was performed for 249 patients. The study cohort included 207 patients who underwent VATS bullectomy for PSP. Twenty-seven patients with postoperative recurrence, six patients with emphysema, four patients with Marfan syndrome, three patients with catamenial pneumothorax, one patient with aspergillus infection, and one patient with pneumocystis infection were excluded. The median follow-up period was 31.3 months (range, 1.0–143.2). Among 207 patients, 27 (13.0%) experienced postoperative recurrence of ipsilateral PSP. The median time between surgery and recurrence was 14.2 months (range, 0.7–104.2). The clinical features and differences between the recurrent and nonrecurrent groups are shown in Table 1. There were no significant differences in sex, smoking history, BMI, history of ipsilateral pneumothorax, laterality, hemothorax, bullae or blebs at the apex of the lung, and number of bulla or bleb resections. Compared with the nonrecurrence group, more patients in the recurrence group were younger than 20 years, had a history of contralateral pneumothorax, and had no staple line reinforcement with a PGA sheet and autologous blood. The univariate analysis indicated that age younger than 20 years, history of contralateral pneumothorax, and no staple line reinforcement were significant prognostic factors for poor RFS (Table 2). The Kaplan-Meier curves of RFS after VATS bullectomy for these factors are shown in Figure 1. The results of the multivariate analysis of RFS after VATS bullectomy are shown in Table 3. The multivariate analysis using the Cox regression analysis revealed that age younger than 20 years [P=0.039; hazard ratio (HR) =2.337; 95% confidence interval (CI), 3.283–17.287), history of contralateral pneumothorax (P<0.001; HR =7.533; 95% CI, 1.486–12.336), and no staple line reinforcement (P=0.007; HR =4.282; 95% CI, 1.043–5.236) were risk factors for recurrence after VATS bullectomy for PSP.
Table 1
| Variables | Recurrence (n=27) | Non-recurrence (n=180) | P value |
|---|---|---|---|
| Age at operation (years) | 0.005 | ||
| <20 | 17 | 63 | |
| ≥20 | 10 | 117 | |
| Sex | 0.560 | ||
| Male | 25 | 160 | |
| Female | 2 | 20 | |
| Smoking history | 0.224 | ||
| Yes | 4 | 46 | |
| No | 23 | 134 | |
| Body mass index (kg/m2) | 0.683 | ||
| <18.5 | 11 | 66 | |
| ≥18.5 | 16 | 114 | |
| History of ipsilateral pneumothorax | 0.176 | ||
| Yes | 18 | 95 | |
| No | 9 | 85 | |
| History of contralateral pneumothorax | 0.001 | ||
| Yes | 10 | 23 | |
| No | 17 | 157 | |
| Operation side | 0.610 | ||
| Right | 10 | 76 | |
| Left | 17 | 104 | |
| Hemopneumothorax | 0.497 | ||
| Yes | 1 | 13 | |
| No | 26 | 167 | |
| Bulla or bleb location | 0.570 | ||
| Apical area | 25 | 168 | |
| Other area | 2 | 12 | |
| Number of resections | 0.630 | ||
| 1 | 21 | 147 | |
| ≥2 | 6 | 33 | |
| Stapling line reinforcement | 0.002 | ||
| Yes | 5 | 91 | |
| No | 22 | 89 |
Table 2
| Variables | 1-year RFS (%) | 3-year RFS (%) | P value |
|---|---|---|---|
| Age at operation (years) | 0.014 | ||
| <20 | 90.4 | 82.5 | |
| ≥20 | 95.6 | 88.9 | |
| Sex | 0.537 | ||
| Male | 86.8 | 56.7 | |
| Female | 100.0 | 91.7 | |
| Smoking history | 0.214 | ||
| Yes | 93.3 | 90.2 | |
| No | 93.5 | 84.8 | |
| Body mass index (kg/m2) | 0.894 | ||
| <18.5 | 94.2 | 88.5 | |
| ≥18.5 | 93.0 | 84.8 | |
| History of ipsilateral pneumothorax | 0.122 | ||
| Yes | 91.9 | 81.4 | |
| No | 95.3 | 91.6 | |
| History of contralateral pneumothorax | <0.001 | ||
| Yes | 86.8 | 56.7 | |
| No | 94.7 | 91.1 | |
| Operation side | 0.666 | ||
| Right | 93.5 | 85.9 | |
| Left | 93.4 | 86.6 | |
| Hemopneumothorax | 0.439 | ||
| Yes | 100.0 | 92.9 | |
| No | 93.5 | 85.7 | |
| Bulla or bleb location | 0.933 | ||
| Apical area | 93.5 | 85.8 | |
| Other area | 92.9 | 79.6 | |
| Number of resections | 0.792 | ||
| 1 | 94.6 | 87.3 | |
| ≥2 | 88.6 | 81.7 | |
| Staple line reinforcement | 0.034 | ||
| Yes | 96.6 | 92.8 | |
| No | 90.6 | 82.1 |
RFS, recurrence-free survival.
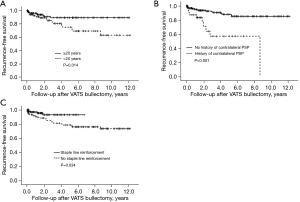
Table 3
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age at operation (years) | |||
| ≥20 | |||
| <20 | 2.337 | 3.283–17.287 | 0.039 |
| History of contralateral pneumothorax | |||
| No | |||
| Yes | 7.533 | 1.486–12.336 | <0.001 |
| Staple line reinforcement | |||
| Yes | |||
| No | 4.282 | 1.043–5.236 | 0.007 |
HR, hazard ratio; CI, confidence interval.
Discussion
The postoperative recurrence rate of VATS bullectomy for PSP at our institution was 13.0%, which is somewhat higher than the recurrence rates reported by previous publications (4,5). However, the recurrence rate of cases with staple line reinforcement was 5.2%, which is comparable to the recurrence rate after thoracoscopic surgery with staple line reinforcement with a PGA sheet reported by previous works (2.6–13.8%) (10-14). Furthermore, we demonstrated that age younger than 20 years, a history of contralateral pneumothorax, and no staple line reinforcement with a PGA sheet and autologous blood were significant prognostic factors for unfavorable outcomes.
Although age younger than 20 years is considered a risk factor for postoperative recurrence of PSP, it has been reported that postoperative recurrence may be more common in younger patients. Risk factors have been reported as 17, 20, and 23 years of age and younger (13,15,16). It has been shown that patients with PSP at a young age, when physical development is rapid, gain weight normally but grow taller quickly, resulting in an elongated body shape (17). The rapid increase in the vertical dimensions of the thorax compared to the horizontal dimensions during this period is thought to have some effect on the intrathoracic pressure at the apex of the lungs and promote bulla formation (18), which may be a cause of postoperative PSP recurrence in young patients.
We also showed that a preoperative history of contralateral pneumothorax might be a risk factor for recurrence after VATS bullectomy. A history of ipsilateral pneumothorax is a possible risk factor (13,16); however, there are no reports of a history of contralateral pneumothorax as a risk factor. Although there is no clear reason for this, we know empirically that patients with PSP often have bilaterally symmetrical lung bulla on computed tomography. A history of pneumothorax may reflect body type or pleural fragility that predisposes the bulla to form or fail.
Pleurectomy and mechanical or chemical pleurodesis are simple and effective methods that have been performed to prevent postoperative PSP recurrence after VATS bullectomy for PSP (6). However, postoperative pain, postoperative bleeding, disruption of normal pleural physiology, and the potential for severe intrapleural adhesions may be disadvantages for younger patients (6,19,20). As an alternative to these preventive methods, staple line coverage has been performed to reinforce the visceral pleura. The use of staple line reinforcement with a PGA sheet for preventing the postoperative recurrence of PSP has been reviewed by many studies performed in Asian countries, and it has been found to be effective (8,10,12-14). According to the 2014 annual report of the Japanese Association for Thoracic Surgery, of 12,673 patients who underwent VATS bullectomy, the visceral pleura was reinforced with absorbable sheets in 6,432 (50.7%) (5). Due to its softness, the PGA sheet is associated with difficulties during thoracoscopic surgery when applying it to fit the staple line after bullectomy properly. Therefore, many centers use fibrin glue when the PGA sheets are fitted to the staple line. However, it is difficult to completely inactivate and remove viruses, such as human parvovirus B19, during the current manufacturing process for fractionated plasma products, and there is a risk of infection (21). Therefore, we used autologous blood instead of fibrin glue to fix PGA sheets. The effectiveness of staple line reinforcement using PGA sheets with autologous blood for preventing the postoperative recurrence of PSP has been demonstrated only by studies performed in Japan, and the study populations were limited (22,23). In this study, the method using PGA sheets with autologous blood had a recurrence rate comparable to that of prophylaxis using PGA sheets and fibrin glue (10-14). Autologous blood may be superior to fibrin glue in terms of simplicity, infection, and cost-effectiveness. However, as shown by using autologous blood during pleurodesis for prolonged pneumothorax and postoperative air leaks (24,25), spraying as little as 10 mL of autologous blood may cause pleurodesis via pleural irritation and inflammation due to clot formation and subsequent blood fibrinogen activity. In addition, PGA sheets induce severe inflammation and adhesions between the parietal and visceral pleura, which may adversely affect future thoracic surgery (26). In this study, five patients in the group with staple line reinforcement using a PGA sheet and autologous blood experienced recurrence; however, because all were minor pneumothorax occurrences that did not require surgery, it was not possible to determine the actual extent of adhesions that had occurred.
This study had several limitations. It was conducted at a single institution and retrospective. Although this study excluded patients with secondary spontaneous pneumothorax other than PSP based on the medical records and CT images, the bulla was not located at the apical area in 14 patients; therefore, spontaneous pneumothorax caused by genetic predisposition may not have been completely excluded (27). Furthermore, the study population was relatively small, and only 27 patients experienced recurrence. Therefore, selection and information bias may have existed, and the study might have been underpowered to perform statistical analyses. A multicenter prospective study is needed to confirm the results of this study.
Conclusions
Among patients with PSP younger than 40 years of age who underwent VATS bullectomy, age younger than 20 years at surgery and a history of contralateral pneumothorax were considered risk factors for postoperative recurrence. In addition, reinforcing the staple line with a PGA sheet and spraying of autologous blood may effectively prevent postoperative recurrence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-257/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-257/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-257/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-257/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. [Crossref] [PubMed]
- Vohra HA, Adamson L, Weeden DF. Does video-assisted thoracoscopic pleurectomy result in better outcomes than open pleurectomy for primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2008;7:673-7. [Crossref] [PubMed]
- Lin Z, Zhang Z, Wang Q, et al. A systematic review and meta-analysis of video-assisted thoracoscopic surgery treating spontaneous pneumothorax. J Thorac Dis 2021;13:3093-104. [Crossref] [PubMed]
- Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet 2007;370:329-35. [Crossref] [PubMed]
- Goto T, Kadota Y, Mori T, et al. Video-assisted thoracic surgery for pneumothorax: republication of a systematic review and a proposal by the guideline committee of the Japanese association for chest surgery 2014. Gen Thorac Cardiovasc Surg 2015;63:8-13. [Crossref] [PubMed]
- Porcel JM, Lee P. Thoracoscopy for Spontaneous Pneumothorax. J Clin Med 2021;10:3835. [Crossref] [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- Kadomatsu Y, Fukui T, Mori S, et al. Polyglycolic acid sheet covering to prevent recurrence after surgery for spontaneous pneumothorax: a meta-analysis. Sci Rep 2021;11:3392. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [Crossref] [PubMed]
- Sakamoto K, Takei H, Nishii T, et al. Staple line coverage with absorbable mesh after thoracoscopic bullectomy for spontaneous pneumothorax. Surg Endosc 2004;18:478-81. [Crossref] [PubMed]
- Lee S, Kim HR, Cho S, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Mao Y, Zhang Z, Zeng W, et al. A clinical study of efficacy of polyglycolic acid patch in surgery for pneumothorax:a systematic review and meta-analysis. J Cardiothorac Surg 2020;15:117. [Crossref] [PubMed]
- Asano H, Ohtsuka T, Noda Y, et al. Risk factors for recurrence of primary spontaneous pneumothorax after thoracoscopic surgery. J Thorac Dis 2019;11:1940-4. [Crossref] [PubMed]
- Kim KS. Polyglycolic acid sheet with fibrin glue technique without pleural abrasion in uniportal VATS for primary spontaneous pneumothorax. J Thorac Dis 2020;12:690-5. [Crossref] [PubMed]
- Choi SY, Kim YH, Jo KH, et al. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax in children. Pediatr Surg Int 2013;29:505-9. [Crossref] [PubMed]
- Nakayama T, Takahashi Y, Uehara H, et al. Outcome and risk factors of recurrence after thoracoscopic bullectomy in young adults with primary spontaneous pneumothorax. Surg Today 2017;47:859-64. [Crossref] [PubMed]
- Fujino S, Inoue S, Tezuka N, et al. Physical development of surgically treated patients with primary spontaneous pneumothorax. Chest 1999;116:899-902. [Crossref] [PubMed]
- West JB. Distribution of mechanical stress in the lung, a possible factor in localisation of pulmonary disease. Lancet 1971;1:839-41. [Crossref] [PubMed]
- Maier A, Anegg U, Renner H, et al. Four-year experience with pleural abrasion using a rotating brush during video-assisted thoracoscopy. Surg Endosc 2000;14:75-8. [Crossref] [PubMed]
- Zijl JA, Sinninghe Damste HE, Smits PJ. Video-assisted thoracoscopic introduction of talc in the treatment of recurrent spontaneous pneumothorax. Eur J Surg 2000;166:283-5. [Crossref] [PubMed]
- Kawamura M, Sawafuji M, Watanabe M, et al. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg 2002;73:1098-100. [Crossref] [PubMed]
- Ichinari H, Mine K, Taneda Y, et al. Effects of covering visceral pleura with polyglycolic acid sheet and autologous blood after thoracoscopic bullectomy for spontaneous pneumothorax. Jpn J Chest Surg 2007;21:111-4. [Crossref]
- Ishida H, Nitanda H, Sakaguchi H, et al. Visceral pleura covered with a polyglycolic acid sheet and autologous blood after thoracoscopic bullectomy for spontaneous pneumothorax. Jpn J Chest Surg 2007;21:645-9. [Crossref]
- Robinson CL. Autologous blood for pleurodesis in recurrent and chronic spontaneous pneumothorax. Can J Surg 1987;30:428-9. [PubMed]
- Manley K, Coonar A, Wells F, et al. Blood patch for persistent air leak: a review of the current literature. Curr Opin Pulm Med 2012;18:333-8. [Crossref] [PubMed]
- Nakanishi K. An apical symphysial technique using a wide absorbable mesh placed on the apex for primary spontaneous pneumothorax. Surg Endosc 2009;23:2515-21. [Crossref] [PubMed]
- Boone PM, Scott RM, Marciniak SJ, et al. The Genetics of Pneumothorax. Am J Respir Crit Care Med 2019;199:1344-57. [Crossref] [PubMed]


