
Safety and efficacy of two-drug combination in elderly patients with locally advanced non-small cell lung cancer and validation of the Charlson Index as a predictor of survival
Highlight box
Key findings
• This study proposes to use the Charlson comorbidity index to determine the feasibility of chemoradiotherapy of locally advanced non-small cell lung cancer (NSCLC) in elderly patients. This study also shows promising results for the addition of taxane to platinum-based chemoradiotherapy in this setting.
What is known and what is new?
• Common eligibility criteria for chemoradiotherapy in elderly patients with locally advanced NSCLC are performance status 0 to 2, adequate hematological, renal and liver function.
• In our study the Charlson comorbidity index correlated with severe acute toxicities and overall survival.
• Our study also showed good outcomes for the doublet platinum and taxane chemotherapy.
What is the implication, and what should change now?
• The Charlson comorbidity index could be proposed to determine the intensity of treatment of stage III NSCLC in elderly patients.
• Future prospective studies should investigate the use of doublet chemotherapy in comparison with platinum-based chemotherapy.
Introduction
Lung cancer is one of the most common malignancies and the leading cause of cancer-related death (1). Nearly 85% of lung cancer cases are attributable to non-small lung cancer (NSCLC), making it the most common form of lung cancer. Unfortunately, a large proportion of patients are diagnosed at a locally advanced stage. For these patients, a multimodal treatment concept consisting of chemoradiotherapy and durvalumab constitutes the current standard of care (2,3).
With a median age at diagnosis of 70 years, NSCLC is a disease closely associated with the elderly (4). Although there is no commonly accepted definition for elderly patients, an age above 70 years is routinely considered (5-7). As the general population continues to age, the incidence of older patients with NSCLC is expected to continue to rise in the coming decades. However, older patients are currently under-represented in clinical trials.
Due to comorbidities and age-related organ impairment, the efficacy and tolerability reported in large trials cannot be extrapolated to this subpopulation. Common eligible criteria for chemoradiotherapy in elderly patients were described previously (3,5): no previous chemotherapy or radiotherapy, Eastern Cooperative Group (ECOG) performance status (PS) of 0 to 2; lung volume receiving 20 Gy <35%, adequate hematological, renal and liver function. Elderly patients seem to benefit from concomitant chemotherapy, similar to their younger counterparts (5) but the individual balancing of opportunities and risks in this critical cohort poses a major clinical challenge. Although parallel administration of chemotherapy and radiotherapy is considered more effective (5), sequential administration is often preferred for reasons pertaining to tolerability (3). Some patients could benefit from the combined strategy and be stratified thanks to the Charlson comorbidity index (8,9) which has already demonstrated its correlation with survival in NSCLC (10-12) since solid tumor is one of the components of the index. The Charlson comorbidity index is calculated as a function of the severity of 12 different factors/comorbidities. It is positively correlated with the risk of death (9).
In this study, we investigated (I) the correlation between Charlson comorbidity and outcome in elderly patients with locally advanced NSCLC treated with definitive chemoradiotherapy. We also (II) addressed the safety and efficacy of two-drug combination in geriatric patients.
We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-108/rc).
Methods
Patient selection
In this multicenter cohort study, all patients from two comprehensive cancer centers were retrospectively identified for analysis. Inclusion criteria were as follows: histologically confirmed stage IIIA, IIIB or IIIC NSCLC; age >70 years; treatment including radiotherapy and chemotherapy; patients treated between December 2006 and August 2019. Exclusion criteria were as follows: metastatic disease, treatment with chemotherapy only. We could not include patients before 2006 because of the difficulty obtaining comprehensive data before this date.
Patients received chemoradiotherapy in one center (Centre Antoine-Lacassagne) but were referred by two centers (the Centre Antoine-Lacassagne and the teaching hospital CHU of Nice). The study was approved by the Institutional Ethics Board of National Commission on Informatics and Liberty” (CNIL) (registration No. MR 004 – n°F20201128123651) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All families received written information on the study and gave their consent to the anonymous use of patients’ data for research purposes.
Clinical work-up and follow-up
Staging was performed according to the American Joint Committee on Cancer’s eighth TNM classification. As part of staging, a chest-abdominal-pelvis computed-tomography (CT) scan, an 18-FDG PET scan and imaging of the brain (either magnetic resonance imaging or CT scan) were performed. Restaging was performed before radiotherapy according to the same eighth edition of the TNM classification and with the help of a radiologist during a multidisciplinary tumor board. Because the TNM classification has changed between 2006 and 2019, we retrospectively reclassified all patients according to the eighth edition. The Charlson index was used to classify the severity of comorbidities (8). We used the updated version of Quan et al. which depends on the following factors and associated weight (9): heart failure (weight: 2), dementia (weight: 2), chronic pulmonary disease (weight: 1), rheumatologic disease (weight: 1), mild liver disease (weight: 2), diabetes mellitus with chronic complications (weight: 1), hemiplegia/paraplegia (weight: 2), renal disease (weight: 1), any malignancy (weight: 2), moderate or severe liver disease (weight: 4), metastatic solid tumor (weight: 6), acquired immune deficiency syndrome (weight: 4). Charlson index was not noted prospectively in the medical history as a number. It was evaluated retrospectively.
Under radiotherapy, patients were evaluated at least weekly. The follow-up examination was done at months 1, 3, 6, 9 and 12 after completion of radiotherapy and every 4 months thereafter.
Follow-up consisted in a clinical examination, a chest-abdominal CT scan and an 18-FDG PET scan if tumour recurrence was suspected. Tumour response was assessed according to the Response Criteria in Solid Tumours (RECIST) 1.1 criteria (13,14). Toxicities were assessed according to the fifth version of the Common Terminology Criteria for Adverse Events (CTCAE). The toxicity scoring was performed by scrutinizing, retrospectively, the medical histories.
Concurrent chemo-radiotherapy
For patients with a performance status (PS) Eastern Cooperative Group (ECOG) of 0 or 1 without major renal, cardiac, or pulmonary insufficiency, chemotherapy was administered. Two cycles of induction chemotherapy with cisplatin 75 mg/m2 on days 1 and 22, followed by docetaxel 75 mg/m2, were initiated before radiotherapy. In the presence of diabetes or other conditions that put the patient at risk for renal failure or other toxicities, a carboplatin and/or paclitaxel regimen was preferred and administered as follows: paclitaxel 90 to 225 mg/m2, carboplatin area under curve (AUC) 5–6 on days 1 and 22, with dosing chosen according to other concomitant conditions. Concomitant chemoradiotherapy (CRT) started on day 43 and consisted of weekly administration of cisplatin (20 mg/m2) followed by docetaxel (20 mg/m2) for 5 weeks. In case of toxicity risk, a carboplatin and/or paclitaxel regimen was chosen (carboplatin AUC 2 and paclitaxel 45 mg/m2).
Radiotherapy was given on day 43 with concomitant chemotherapy using the 3-dimensional conformal technique. The target dose of 66 Gy was administered using an involved field technique. Treatment was delivered in thirty-three fractions, 5 fractions per week, one fraction per day, using 6 to 18 MV photons. The gross target volume (GTV) represented the initial tumour volume. The clinical target volume (CTV) was defined as the GTV with an additional 3D extension of 1 cm, adjusted for anatomical variations. The planning target volume (PTV) was defined as the CTV with an additional 3-D extension of 1–1.5 cm. The dose constraints for the organ at risk were analogous to QUANTEC (15).
Sequential chemoradiotherapy
The same strategy was applied to sequential chemotherapy as to concurrent CRT, with the exception that induction chemotherapy may comprise more than two cycles (usually four), and concurrent chemotherapy may not be given during radiotherapy.
Other treatments
In the case of strong tumour response and surgical feasibility, surgery was considered for patients approximately 5 weeks after the start of radiotherapy (approximately 46 Gy). For more recent patients, durvalumab (10 mg/kg every 2 weeks for 12 weeks) could be proposed after CRT due to the changes in the standard of care.
Statistical analysis
Median follow-up with a 95% confidence interval (CI) was calculated by reverse Kaplan-Meier method (Schemper Method). Statistical comparisons were performed using Chi-square tests for categorical data and Mann-Whitney U test for continuous variables. Patients with missing data were excluded from the analysis. For analysis of quantitative variables, cut-offs were based on the variable’s median value (albumin, Charlson index, PTV1 volume) or well-known cut-off values (body mass index, left ventricular ejection fraction). Local control (LC), progression free survival (PFS), metastasis free survival (MFS) and overall survival (OS), were estimated via Kaplan-Meier analysis. LC was calculated from the last day of radiotherapy until evidence of local relapse, which was defined as a recurrence within the radiated field. PFS was calculated from the last day of radiotherapy to evidence of progression (excluding death without progression). MFS was calculated from the last day of radiotherapy to evidence of new distant metastasis. OS was calculated from the last day of radiotherapy until death from any cause. Statistical comparisons were performed using Log-rank test. Cox regression multivariate analyses included factors that correlated in univariate analysis. Statistical significance was achieved if P was <0.05. All statistical analyses were two-sided and performed using Statistical Package for the Social Sciences (SPSS) version 21.0.
Results
Characteristics
Between 12/2006 and 08/2019, 58 patients (39 with concomitant CRT and 19 with sequential CRT) were included in this analysis. We initially identified 94 patients, but 36 were excluded (n=10 because of no history of RT and n=26 because of metastatic disease) (Consort Diagram, Figure 1). Patients and treatment characteristics are shown in Tables 1,2, respectively. The median age of the total population was 76.6 years [interquartile range (IQR): 71.6–83.4]. In sum, patients treated with concomitant chemoradiotherapy were slightly younger and had better PS, but the same Charlson index. They were also more likely to be treated with cisplatin-based chemotherapy.
Table 1
| Characteristics | Concomitant chemoradiotherapy (n=39) | Sequential chemoradiotherapy (n=19) | P value |
|---|---|---|---|
| Median age, years (range) | 75.5 (71 to 83.5) | 77.9 (71.1 to 87.3) | 0.02 |
| Median follow-up, months (range) | 28 (10 to 76) | 19 (5 to 52) | 0.01 |
| Gender | |||
| Male | 29 (74.4) | 16 (84.2) | 0.4 |
| Female | 10 (25.6) | 3 (15.8) | |
| Charlson index (range) | 4 (2 to 6) | 4 (2 to 7) | 0.3 |
| Albumin, gr/L | 37 (32 to 43) | 35 (28 to 42) | 0.2 |
| Tobacco use (pack-years) | 50 (20 to 80) | 50 (30 to 100) | 0.6 |
| Performance status | |||
| 0 | 21 (53.8) | 3 (15.8) | 0.006 |
| 1–2 | 18 (46.2) | 16 (84.2) | |
| Body mass index (kg/m2) | 24.8 (20 to 29.7) | 23 (17.8 to 28.7) | 0.2 |
| Left ventricular ejection fraction | 63 (55 to 74) | 60 (25 to 77) | 0.3 |
| Histology | |||
| Squamous | 20 (51.3) | 12 (63.2) | 0.4 |
| Non-squamous | 19 (48.7) | 7 (36.8) | |
Data are shown as median (IQR) or n (%). IQR, interquartile range.
Table 2
| Characteristics | Concomitant chemoradiotherapy | Sequential chemoradiotherapy | P value |
|---|---|---|---|
| Induction chemotherapy | |||
| Carboplatin-paclitaxel | 5 (12.9) | 14 (73.7) | |
| Carboplatin-docetaxel | 13 (33.3) | 4 (21.0) | |
| Cisplatin-docetaxel | 21 (53.8) | 1 (5.3) | |
| Carboplatin based | 18 (46.2) | 18 (94.7) | <0.0001 |
| Cisplatin based | 21 (53.8) | 1 (5.3) | |
| Concomitant chemotherapy | |||
| Carboplatin-pemetrexed | 1 (2.6) | NA | |
| Carboplatin-paclitaxel | 1 (2.6) | ||
| Carboplatin-docetaxel | 20 (51.2) | ||
| Cisplatin-docetaxel | 17 (43.6) | ||
| Median number of chemotherapy cycles | 6 (4 to 8) | 3 (2 to 4) | <0.0001 |
| Radiotherapy | |||
| Median total dose (Gy) | 66 (46 to 66) | 66 (42 to 66) | 0.3 |
| Median dose per fraction (Gy) | 2 (2 to 2) | 2 (2 to 2) | 1 |
| Median number of fractions | 33 (23 to 33) | 33 (21 to 33) | 0.4 |
| Median radiotherapy duration (days) | 50 (35 to 59) | 52 (40 to 57) | 0.6 |
| Median PTV1 size (mL) | 331.7 (120 to 593) | 416 (221 to 601) | 0.08 |
| Median PTV2 size (mL) | 203.8 (115 to 500) | 361.8 (73.4 to 601) | 0.14 |
| Surgery | 3 (7.7) | 0 (0.0) | 0.2 |
| Durvalumab | 1 (2.6) | 1 (5.3) | 0.9 |
Data are shown as median (IQR) or n (%). IQR, interquartile range; NA, not applicable; PTV, planning target volume.
Tumor response and survival
After radiotherapy, the rate of complete response, partial response, stabilized disease and tumor progression were respectively 19%, 70.7%, 5.2% and 3.4% (tumor response could not be evaluated for one patient who died during treatment). These rates were 22.2%/17.9%, 50%/82.1%, 16.7%/0%, 11.1%/0% for sequential and concurrent chemoradiotherapy, respectively (P=0.005, in favor of concurrent treatment).
With a median follow-up of 52 months (IQR: 28–76) there were 44 tumor progressions, 37 distant metastatic progressions, 27 local relapses and 33 deaths, which translated into a 2/4-year PFS, MFS, LC and OS of respectively 35.5%/19.1%, 43.4%/25.6%, 59%/45%, and 66.9%/41.6%. One death occurred during radiotherapy and may be related to treatment toxicity (increase of baseline heart failure).
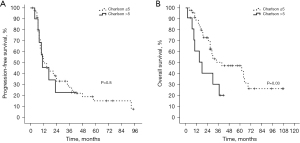
Prognostic factors for PFS and OS are described in Table 3. There was no survival difference between the sequential and concomitant group. There was also no difference in terms of local control (P=0.5) between the sequential and concurrent strategy. Worse performance status (>0) was the only significant prognostic factor for PFS (Log-rank). Female gender and better Charlson index (≤5, Figure 2) were independent prognostic factors of OS (Multivariate Cox Regression). The Charlson index did not correlate with PFS or OS in patients treated with concurrent chemoradiotherapy (P=0.9 and P=0.3, respectively). The Kilmogorov-Smirnov test showed normal and non-normal distribution for age and Charlson index, respectively (P=0.6 and P=0.01, respectively). Figure 3 describes age and Charlson index as a function of treatment type.
Table 3
| Variables | 2-year PFS, % | Univariate analysis (Log-Rank), P value | 2-year OS, % | Univariate analysis (Log-Rank), P value | Multivariate analysis (Cox regression) for OS, HR (95 CI%) | P value |
|---|---|---|---|---|---|---|
| Gender | 0.09 | 0.03 | 2.92 (1.11–7.62) | 0.02 | ||
| Male (n=45) | 27.10 | 63.50 | ||||
| Female (n=13) | 61.50 | 76.90 | ||||
| Performance status | 0.03 | 0.06 | NI | NI | ||
| 0 (n=24) | 43.60 | 86.90 | ||||
| 1–2 (n=34) | 22.40 | 52.20 | ||||
| Stage | 0.5 | 0.9 | NI | NI | ||
| IIIA (n=15) | 33.30 | 52.50 | ||||
| IIIB–IIIC (n=43) | 36.30 | 72.50 | ||||
| Histology | 0.8 | 0.4 | NI | NI | ||
| Non squamous (n=26) | 32.10 | 74.80 | ||||
| Squamous (n=32) | 38.30 | 61.40 | ||||
| Induction chemotherapy | 0.5 | 0.9 | NI | NI | ||
| Carboplatin-based (n=36) | 33.80 | 67.10 | ||||
| Cisplatin-based (n=22) | 38.10 | 66.60 | ||||
| Age | 0.7 | 0.8 | NI | NI | ||
| ≤76 years (n=26) | 36.10 | 62.80 | ||||
| >76 years (n=32) | 34.80 | 70.00 | ||||
| Albumin | 0.5 | 0.3 | NI | NI | ||
| ≤36 gr/L (n=23) | 29.00 | 58.90 | ||||
| >36 gr/L (n=23) | 26.50 | 65.20 | ||||
| Body mass index | 0.3 | 0.4 | NI | NI | ||
| <25 (n=34) | 25.90 | 55.40 | ||||
| ≥25 (n=24) | 39.10 | 82.50 | ||||
| Charlson index | 0.6 | 0.03 | ||||
| <5 (n=47) | 39.50 | 72.80 | 2.12 (1.04–4.34) | 0.03 | ||
| ≥5 (n=11) | 32.30 | 40.40 | ||||
| Concomitant RT | 0.08 | 0.2 | NI | NI | ||
| No (n=19) | 19.50 | 51.70 | ||||
| Yes (n=39) | 36.70 | 73.70 | ||||
| Left ventricular ejection fraction | 0.5 | 0.4 | NI | NI | ||
| ≤60% (n=18) | 20.80 | 73.30 | ||||
| >60% (n=30) | 29.40 | 54.50 | ||||
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; RT, radiotherapy; NI, not included.

Toxicities
The acute toxicities (from the start of chemotherapy to 3 months after radiotherapy) are summarized in Table S1. The most common acute toxicities were anemia (n=56), neutropenia (n=31), pain (n=29), cough (n=31), dyspnea (n=28) and esophagitis (n=40). Acute grade 3–5 toxicities occurred in 34.5% of patients. Charlson index was the only factor correlated with acute toxicity (52.6% if Charlson index >5 vs. 25.6% acute grade 3–5 toxicities, P=0.04). Binary logistic regression was performed including age and Charlson index (correlation in univariate analysis with P<0.1): no factor significantly correlated in multivariate analysis. It is worth noting that the Charlson comorbidity index did not correlate with acute and late grade 3–5 toxicities in the concurrent cohort (P=0.06 and P=0.2, respectively). One patient died during radiotherapy due to heart failure. This patient was 78.3 years old at diagnosis and suffered from chronic heart failure due to ischemic disease. He had a disease stage IIIB, a Charlson index of 6 and a body mass index of 17.8 kg/m2 and received sequential treatment. Heart failure increased during chemotherapy and worsened during radiotherapy after 28 Gy (PTV size of 335 mL).
Late toxicities (at least 3 months after radiotherapy) are listed in Table S2. Figure 4 describes late toxicities as a function of treatment type or Charlson index. The most common toxicities included pain (n=11), cough (n=13), dyspnea (n=17), radiation pneumonitis (n=12) and lung infection (n=10). Thereby, 17.2% of all patients experienced grade 3–5 late toxicities.
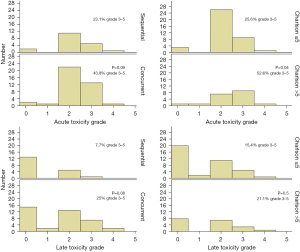
Concurrent treatment was numerically associated with a higher degree of acute and late toxicities (43.8% vs. 23.1% grade 3–5 toxicities, P=0.09; 25% vs. 7.7% grade 3–5 toxicities, P=0.08), but only with a statistical trend. The predictive factors for acute and late toxicities are presented in Table 4. The Charlson index was the only predictive factor for early toxicities.
Table 4
| Variables | Grade 3–5 acute toxicities, % | Chi-square (univariate analysis), P value | Grade 3–5 late toxicities, % | Chi-square (univariate analysis), P value |
|---|---|---|---|---|
| Gender | 0.7 | 0.3 | ||
| Male (n=45) | 33.30 | 20.00 | ||
| Female (n=13) | 38.50 | 7.00 | ||
| Performance status | 0.2 | 0.1 | ||
| 0 (n=24) | 25.00 | 8.30 | ||
| 1–2 (n=34) | 41.20 | 23.50 | ||
| Stage | 0.1 | 0.6 | ||
| IIIA (n=15) | 20.00 | 13.30 | ||
| IIIB–IIIC (n=43) | 39.50 | 18.60 | ||
| Induction chemotherapy | 0.7 | 0.4 | ||
| Carboplatin-based (n=36) | 36.10 | 13.90 | ||
| Cisplatin-based (n=22) | 31.80 | 22.70 | ||
| Age | 0.09 | 0.7 | ||
| ≤76 years (n=26) | 46.20 | 19.20 | ||
| >76 years (n=32) | 25.00 | 15.60 | ||
| Albumin | 0.5 | 0.4 | ||
| ≤36 gr/L (n=23) | 39.10 | 21.70 | ||
| >36 gr/L (n=23) | 30.40 | 13.00 | ||
| Body mass index | 0.3 | 0.9 | ||
| <25 (n=34) | 35.30 | 17.60 | ||
| ≥25 (n=24) | 33.30 | 16.70 | ||
| Charlson index | 0.04 | 0.5 | ||
| ≤5 (n=39) | 25.60 | 15.40 | ||
| >5 (n=19) | 52.60 | 21.10 | ||
| Concomitant RT | 0.09 | 0.08 | ||
| No (n=19) | 23.10 | 7.70 | ||
| Yes (n=39) | 43.80 | 25.00 | ||
| PTV1 volume | 0.6 | 0.4 | ||
| ≤369 mL (n=27) | 33.30 | 22.20 | ||
| >369 mL (n=28) | 39.30 | 14.30 | ||
| Left ventricular ejection fraction | 0.09 | 1.0 | ||
| ≤60% (n=18) | 58.30 | 16.70 | ||
| >60% (n=30) | 27.80 | 16.70 | ||
RT, radiotherapy; PTV, planning target volume.
None of these factors correlated with late toxicity. Concomitant radiotherapy was associated with a numerically higher number of acute and late toxicities (statistical trend).
Discussion
This retrospective, multicenter study demonstrated the efficacy and safety of doublet chemotherapy consisting of a platinum and a taxane in elderly patients irradiated for locally advanced NSCLC, and validated the Charlson index as a feasible assessment score for treatment stratification in this critical cohort.
With a 2-year PFS and OS of 35.5% and 66.9% respectively, our oncological control rates were similar to those reported in the comparable literature (5,16,17).
In a randomized phase III trial of the Japan Clinical Oncology Group (JCOG0301), patients with unresectable stage III NSCLC were randomly assigned to receive chemoradiotherapy (low-dose carboplatin at a dose of 30 mg/m2 intravenously) or radiotherapy alone. The authors demonstrated a significant OS benefit for those in the chemoradiotherapy cohort (HR 0.74, 95% CI: 0.55–0.99, P=0.02) (5). In this study, the 2-year PFS and OS were approximately 20% and 40% in the CRT arm, which appears lower than our experience. Such comparison cannot, however, be rigorously performed because of the retrospective nature of our study and the potential differences in patient characteristics. A possible explanation for our good outcome might be the more intensive two-drug chemotherapy in our study compared to one drug only given in the JCOG0301 study.
Our study showed a trend towards better PFS for concomitant administration; this did not, however, reach statistical significance. On the other hand, we observed a trend toward increased toxicity with the combined regimen; this trend did not, however, reach statistical significance.
Considering the advanced age of the patients and, hence, their limited life expectancy, toxicity assessment is of particular concern. In our study, 18 grade 3 toxicities, 1 grade 4 and 1 grade 5 acute toxicity occurred. The latter was possibly a therapy-related death due to a cardiac defect. Eight grade 3 and 2 grade 4 late toxicities occurred. A meta-analysis including 2,768 young patients and 832 elderly patients demonstrated a higher risk of grade 5 toxicities in the elderly (9% vs. 4%, P<0.01) and a greater risk of treatment discontinuation due to treatment-related toxicities (20% vs. 13%, P<0.01) (18). However, in the JCOG0301 phase III trial, the addition of concomitant chemotherapy did not correlate with an increased risk of late toxicity. They reported a 7% rate of late grade 3/4 toxicities compared to 17.2% in our experience.
Interestingly, the 34.5% acute grade 3–5 toxicity rate observed in our cohort was comparable to the 25–30% rate observed by Antonia et al. (19); likewise, our 17.2% late grade 3–5 toxicity rate as compared with the 8% rate observed by Atagi et al. (5). We can consequently consider that this is a feasible protocol regarding the worst prognosis. Future studies will also have to deal with the effect of adjuvant immune checkpoint inhibitors, which have meanwhile been largely prescribed and are associated with additional toxicities (19-21).
The overall physical and mental status differs considerably among elderly individuals with cancer of the same biological age. Due to this heterogeneous cohort, predictive scores are necessary for treatment selection. Previously, in 171 patients with stage III NSCLC, Firat et al. were able to demonstrate that comorbidity scale could be useful in patient stratification in advanced lung cancer, particularly among the elderly (22).
To determine various dimensions, several scores have been established in clinical practice. One of these scores is the Charlson Comorbidity Index (8,9), where a summation score is calculated based on differently weighted comorbidities. In our study, Charlson index above five was found to predict a higher risk of toxicity. In the JCOG0301 study (5), no factor correlated with an increased risk of toxicities, but the Charlson index was not analyzed.
The small number of patients and retrospective nature of the study weaken our findings, which need further confirmation. It has the merit, however, of being based on a multicenter data collection of a homogeneous underreported cohort.
We could not include patients treated before 2006 because of the lack of comprehensive data and we did not include patients after 2019 in order to have sufficient follow-up.
This population is relatively rare or rarely studied, possibly because general practitioners do not refer very old patients, or seek merely to reflect their wishes. The retrospective nature of our study has biased the accurate collection of toxicities since they may not have been included comprehensively in our database. For the same reason, staging of NSCLC may not have been standardized among patients, thereby introducing a bias in the exact duration until tumour progression.
Conclusions
In conclusion, this study shows good outcomes for doublet platinum and taxane chemotherapy in the management of stage III NSCLC. Using the Charlson index may help to better select patients. More prospective data on this sensitive cohort would be desirable in order more accurately to assess the role of combined therapy and timing.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-108/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-108/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-108/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-108/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Ethics Board of the National Commission on Informatics and Liberty (CNIL) (registration No. MR 004 – n°F20201128123651) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All families received written information on the study and gave their consent to the anonymous use of patients’ data for research purposes.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print. [Crossref] [PubMed]
- Pentheroudakis GESMO Guidelines Committee. Recent eUpdate to the ESMO Clinical Practice Guidelines on early and locally advanced non-small-cell lung cancer (NSCLC). Ann Oncol 2020;31:1265-6. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin 2019;69:452-67. [Crossref] [PubMed]
- Atagi S, Mizusawa J, Ishikura S, et al. Chemoradiotherapy in Elderly Patients With Non-Small-Cell Lung Cancer: Long-Term Follow-Up of a Randomized Trial (JCOG0301). Clin Lung Cancer 2018;19:e619-27. [Crossref] [PubMed]
- Kogure Y, Iwasawa S, Saka H, et al. Efficacy and safety of carboplatin with nab-paclitaxel versus docetaxel in older patients with squamous non-small-cell lung cancer (CAPITAL): a randomised, multicentre, open-label, phase 3 trial. Lancet Healthy Longev 2021;2:e791-800. [Crossref] [PubMed]
- Zaki M, Dominello M, Dyson G, et al. Outcomes of Elderly Patients Who Receive Combined Modality Therapy for Locally Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer 2017;18:e21-6. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676-82. [Crossref] [PubMed]
- Hanazawa H, Matsuo Y, Takeda A, et al. Development and validation of a prognostic model for non-lung cancer death in elderly patients treated with stereotactic body radiotherapy for non-small cell lung cancer. J Radiat Res 2021;rrab093. [Crossref] [PubMed]
- M SG. Impact of Comorbidity Scores on the Overall Survival of Patients With Advanced Non-small Cell Lung Cancer: A Real-World Experience From Eastern India. Cureus 2022;14:e30589. [PubMed]
- Zhao L, Leung LH, Wang J, et al. Association between Charlson comorbidity index score and outcome in patients with stage IIIB-IV non-small cell lung cancer. BMC Pulm Med 2017;17:112. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9. [Crossref] [PubMed]
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940-5. [Crossref] [PubMed]
- Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst 1991;83:417-23. [Crossref] [PubMed]
- Stinchcombe TE, Zhang Y, Vokes EE, et al. Pooled Analysis of Individual Patient Data on Concurrent Chemoradiotherapy for Stage III Non-Small-Cell Lung Cancer in Elderly Patients Compared With Younger Patients Who Participated in US National Cancer Institute Cooperative Group Studies. J Clin Oncol 2017;35:2885-92. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Jain V, Bahia J, Mohebtash M, et al. Cardiovascular Complications Associated With Novel Cancer Immunotherapies. Curr Treat Options Cardiovasc Med 2017;19:36. [Crossref] [PubMed]
- Rahouma M, Karim NA, Baudo M, et al. Cardiotoxicity with immune system targeting drugs: a meta-analysis of anti-PD/PD-L1 immunotherapy randomized clinical trials. Immunotherapy 2019;11:725-35. [Crossref] [PubMed]
- Firat S, Byhardt RW, Gore E. The effects of comorbidity and age on RTOG study enrollment in Stage III non-small cell lung cancer patients who are eligible for RTOG studies. Int J Radiat Oncol Biol Phys 2010;78:1394-9. [Crossref] [PubMed]



