
Open and endovascular repair of thoracoabdominal aortic aneurysm—a narrative review
Introduction
Thoracoabdominal aortic aneurysms (TAAAs) account for approximately 10% of all aortic aneurysms (1). Successful repair of TAAAs requires careful consideration of the individual patient’s clinical and aortic anatomical characteristics. While open repair has traditionally been the standard approach, endovascular repair has evolved over the last two decades to handle complex patient anatomy. Ischemic injury is a known and feared complication of both open and endovascular TAAA repair, and numerous strategies for prevention of renal, visceral, and spinal cord ischemia have been developed and tested. Herein we present a comprehensive narrative review of the epidemiology, classification, and pathophysiology of TAAAs, approaches to repair, outcomes after surgical intervention, and common complications with their strategies for their prevention. We further discuss the most recent American Heart Association (AHA)/American College of Cardiology (ACC) Clinical Practice Guidelines for management of TAAA, changes in the current recommendations compared with prior iterations, and the evidence that forms the basis of these updated recommendations. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1880/rc).
Methods
We searched PubMed for references with the terms “thoracoabdominal aortic aneurysm”, “endovascular thoracoabdominal aortic aneurysm repair”, “open thoracoabdominal aortic aneurysm repair” or their combination in the title or abstract. We also identified relevant articles from the references lists of selected articles. We prioritized randomized trials and publications from the last 15 years but cited other references where historically relevant and necessary. The search strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | November 4th, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | “thoracoabdominal aortic aneurysm”, “endovascular thoracoabdominal aortic aneurysm repair”, “open thoracoabdominal aortic aneurysm repair” |
| Timeframe | 2007–2022 |
| Inclusion criteria | Prioritized English-language and randomized trials, included observational studies |
| Selection process | Junior and senior author agreement |
| Additional considerations | Identification of historically relevant or related articles by agreement between junior and senior author |
Epidemiology and risk factors
TAAAs have an incidence of ten per 100,000 person-years (1) and account for approximately 10% of all aortic aneurysms, with an increasing incidence in the last twenty years attributable to incidental diagnoses secondary to the increased use of cross-sectional imaging (2). The preponderance (60–70%) of TAAAs occur in men (3,4). However, men with TAAAs are less likely than women to suffer a dissection or rupture, as demonstrated in a 2002 retrospective study of 721 TAAA patients (446 male, 275 female) by Davies et al. that found that male sex was associated with a lower risk of dissection or rupture [odds ratio (OR) 0.340, 95% CI: 1.14–0.819; P=0.0162] (3). The annual growth rate is also higher in women than in men (4). A 2017 retrospective study by Cheung et al. of 82 TAAA patients found that sex was significantly associated with greater aneurysm growth on multivariable analysis (4); a 2021 retrospective study of 907 TAAA patients (292 women) found a growth rate of 0.17 cm per year in men compared to 0.25 cm per year in women (P<0.001) (5).
Risk factors for TAAA include hypertension (present in the vast preponderance of patients with TAAAs), atherosclerosis, smoking, and chronic obstructive pulmonary disease (COPD) (6). Hereditary disorders, including syndromes such as Marfan, Loeys-Dietz, and Ehlers-Danlos, account for 15–20% of all TAAAs and put patients at higher risk of aortic dissection (7). Chronic aortic dissection is related either to hypertension or the aforementioned hereditary disorders, and 30–40% of patients with chronic dissection will require TAAA repair (6-8). Other disorders carrying an increased risk of TAAA include familial thoracic aortic disease (7), inflammatory aortitis, autoimmune disorders, traumatic injury, and congenital conditions (coarctation of the aorta, Turner syndrome) (9).
Pathophysiology, natural history, and classification
The normal diameter of the descending thoracic aorta ranges from 2.42–2.98 cm in men and 2.2–2.68 cm in women, with a decrease in size ranging from 0.2–0.5 cm as the aorta traverses the diaphragm and abdomen (10,11). The normal diameter varies by the segment of the aorta in question and by the anatomical characteristics (sex, height, body size) of the individual patient.
The aortic wall is comprised of three layers: intima, media, and adventitia. Degradation of structural proteins in the media leads to aneurysmal degeneration. In particular, loss of collagen and elastin in the medial layer and deposition of proteoglycans contributes to the loss of structural integrity. The vast majority, approximately 70%, of TAAAs are termed degenerative aneurysms, in which atherosclerotic disease is superimposed on, and contributes to, medial degeneration (12-14). A detailed description of the biochemical pathways and proteins contributing to degeneration of the aortic wall is not yet clearly delineated and is beyond the scope of this review.
Hemodynamic stress against the weakened wall of the aorta leads to dilatation of those areas and formation of the aneurysm. Hypertension increases the pressure against the aortic wall and accelerates the process of dilatation. A tear or disruption of the intima, often secondary to excessive hemodynamic force associated with hypertension, leads to the creation and eventual propagation of a dissection. The average growth rate of TAAAs has been reported between 0.10 cm per year to 0.42 cm per year (3,15,16). Extent of dilatation, or aortic diameter, has historically been the best predictor of rupture, dissection, and mortality (3,6,16,17), and forms the basis for current guideline recommendations for surgical intervention. Without surgical intervention, the 5-year survival of TAAA ranges from 10–20% (1,15).
TAAAs may present with compressive symptoms, such as intrascapular pain, chest pain radiating to the back, or abdominal pain. Depending upon the size and location of the aneurysm, other symptoms may include dysphagia secondary to compression of the esophagus, wheezing or coughing secondary to airway compression, or hoarseness secondary to stretching of the recurrent laryngeal nerve (18). In addition, embolization of thrombus associated with the aneurysm wall, or of atheromatous debris, may cause malperfusion of any of a number of end organ targets. Ischemic symptoms with end organ dysfunction, including mesenteric ischemia, renal ischemia, or peripheral limb ischemia, and symptoms of erosion of the aneurysm into abutting structures (i.e., aorto-enteric fistula presenting with gastrointestinal bleeding) should be urgently or emergently addressed (9). However, most TAAAs are asymptomatic until dissection or rupture (9).
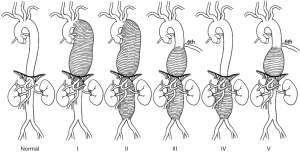
TAAAs involve the descending thoracic aorta and the abdominal aorta, ranging from below the origin of the left subclavian artery and to the bifurcation of the abdominal aorta, and are classified based upon the predicted extent of an open operative repair using the Crawford classification system (13), depicted in Figure 1. Extent I repairs start distal to the origin of the left subclavian artery (proximal to the sixth rib) and extend to the renal arteries. Extent II repairs reach from the left subclavian (proximal to the sixth rib) and extend below the renal arteries, to the aortoiliac bifurcation. Extent III repairs cover from distal to the sixth rib, above the level of the diaphragm and inclusive of the distal thoracic aorta, to the abdominal aorta. Extent IV repairs begin below the level of the diaphragm and extend to the aortoiliac bifurcation, including the visceral aortic segment. A more recent addition to the traditional Crawford classification, Extent V aneurysms arise in the distal descending thoracic aorta, distal to the sixth rib, but extends to include only the visceral segment of the abdominal aorta (19).
Indications for repair
Elective repair of asymptomatic aneurysms is based on the principle that repair should be undertaken when the annual risk of rupture exceeds the surgical mortality and morbidity. Therefore, patients with an unacceptably high surgical risk (i.e., cardiopulmonary or other end organ failure) or patients with shorter life expectancy for other reasons may not be candidates for repair. According to the 2022 AHA/ACC guidelines (9), asymptomatic TAAAs should be recommended for open repair, which is still the standard of care, at a threshold aortic diameter of 6.0 cm [Class of Recommendation (COR) 1, Level of Evidence (LOE) B-NR] or at a threshold of 5.5 cm if the repair is performed by an experienced surgeon working as part of a multidisciplinary aortic team (COR 2A, LOE B-NR); consideration should be given to endovascular repair for patients who are not able to tolerate open surgery (9). The 2022 guidelines also recommend consideration of repair at 5.5 cm or less in patients with high-risk features for rupture, including annual growth of the aneurysm by 0.5 cm or more, symptomatic aneurysms, significant change in the appearance of the aneurysm, saccular aneurysms, or aneurysms with penetrating atherosclerotic ulcers (COR 2A, LOE B-NR). The Society for Vascular Surgery guidelines also recommend repair at lower thresholds in populations with hereditary thoracic aortic disorders (20-22). Repair is recommended at a threshold of 5.5 cm for isolated descending thoracic aneurysms (DTAs) (COR 1, LOE B-NR) (9).
These recommendations are intended to prevent aortic rupture. A retrospective study by Zafar et al. of 907 patients with DTAs and TAAAs (8) found that at an aortic diameter of 6.0 cm, the annual rate of rupture, dissection, and death was 19%. 93% of ruptures occurred at a diameter greater than 5.0 cm, yet 80% of dissections occurred at a diameter less than 5.0 cm. Acute type B dissections occurred at a median aortic diameter of 4.1 cm, suggesting that diameter is a better predictor of rupture than of dissection and that current guidelines are not directed toward prevention of dissection.
The association of both institutional and individual surgeon volume with improved mortality and morbidity at high-volume centers and with high volume surgeons when compared with low-volume centers and surgeons has been reported (23-26). In a 2018 meta-analysis of 30 studies and 9,923 patients undergoing open TAAA repair by Moulakakis et al. (26), they found a statistically significant inverse association between mortality and the volume of cases at each vascular center (t=−2.00; P=0.005) on meta-regression analysis. Rocha et al. (25). found that institutional volume of over 60 cases in a ten-year study period (approximately six cases per year) was associated with a significant lower operative mortality after open TAAA repair compared with all other centers (13.8% versus 36.0%; P<0.01). In a 2003 retrospective study of 1,542 patients undergoing TAAA repair by Cowan et al. (23), high-volume surgeons (performing three to eighteen cases per year, median seven cases per year) had significantly lower operative mortality than their low-volume counterparts (11.0% versus 25.6%; P<0.001); the same was true for high-volume centers (five to 31 cases per year, median twelve cases per year) when compared with low-volume institutions (15.0% versus 27.4%; P<0.001) low surgeon volume (defined as one to two cases per year) was a significant predictor of mortality: OR 2.6; P<0.001), as was low institution volume (one to three cases per year) (OR 2.2; P<0.001). Therefore, surgeon and institutional volume have an important relationship to clinical outcomes and should be part of the decision-making process regarding whether, where, and how to undergo repair.
Outcomes of open repair
Single-lung ventilation with a left thoracoabdominal approach is the traditional approach for open TAAA repair; reconstruction of both the aorta and the vasculature to the viscera and kidneys is performed (27). To decrease the risk of recurrent visceral patch aneurysms, a multibranched graft may be preferred in patients with hereditary thoracic aortic disease or in young patients (18). Reimplantation of the intercostal arteries at the T10–T12 level is performed when anatomically indicated in order to limit the risk of spinal ischemic complications.
Early series of open TAAA repair reported high rates of ischemic complications. A 1993 retrospective cohort study by Svensson et al. of 1,509 TAAA patients undergoing 1,679 open TAAA repairs between 1960 and 1991 found a 30-day mortality of 8%, a spinal cord injury (SCI) incidence of 16%, and renal failure incidence of 18% with an incidence of dialysis requirement of 9% (28). On logistic regression, significant predictors of paraplegia or paraparesis were total aortic clamp time, extent of the repair, aortic rupture, patient age, proximal aortic aneurysm, and history of renal dysfunction (P<0.05).
Results have been mixed in large-scale contemporary series, although overall the reported incidence of both SCI and dialysis requirement have decreased. In 2016, Coselli et al. (12). published the largest series to date of open TAAA repair in 3,309 patients and found an in-hospital mortality of 7.5%, permanent paraplegia incidence of 2.9%, paraparesis incidence of 2.4%, and an incidence of renal failure requiring dialysis of 7.6%. In further studies on this same patient cohort, the most important risk factors for mortality or ischemic injury were age, chronic pre-existing pulmonary or kidney disease, and need for urgent or emergent repair (29-32).
In the patients described by Coselli et al. (12), patients over 80 years old had almost quadruple the in-hospital mortality (26%) than the rest of the cohort (6.9%), and age was significantly associated with mortality and ischemic injury. However, a 2018 single-center study by Girardi et al. (33) of 783 patients (96 octogenarians) undergoing open repair of DTA or TAAA found no difference in operative mortality (octogenarians: 5.6% versus remaining cohort: 5.7%; P=0.852), SCI (2.0% versus 2.0%; P=0.715), renal failure requiring dialysis (5.3% versus 5.2%; P=1.00), or respiratory complications (26.3% versus 24.9%; P=0.651). The authors did note that a greater proportion of octogenarians’ repairs (85.4%) were performed using the clamp-and-sew technique when compared with the remaining patients’ repairs (61.6%; P<0.001), leading to a significantly shorter duration of aortic cross-clamp time (30.7 versus 26.6 minutes; P=0.04), which may have contributed to the better postoperative outcomes in this study.
Preoperative renal and pulmonary failure have been identified as risk factors for mortality and morbidity after open TAAA repair. A 2017 single-center retrospective study of 711 open DTA and TAAA patients included 202 with preoperative renal failure and found that these patients also presented with other significantly worse comorbidities, including smoking, chronic pulmonary disease, peripheral vascular disease, and diabetes, than patients without renal failure (34). The incidence of operative mortality was 14.2% in patients with renal failure versus 2.2% in those without, and five-year survival was significantly lower in patients with renal failure as well (45.0% versus 79.8%; P<0.001). Consistent with findings from prior studies (12,28), preoperative renal failure was found to be a significant predictor of operative mortality (OR: 4.91, 95% CI: 2.01–11.97; P<0.001). Another study by the same group included 149 propensity-matched pairs of patients undergoing TAAA and DTA repair with forced expiratory volume in 1 second (FEV1) either above or below 50%, and found an incidence of major adverse events of 33.1% in patients with FEV1 <50% compared with 19.5% in those with FEV1 >50% (P=0.08). In propensity-matched patients undergoing TAAA, there was a significant difference in operative mortality (FEV1 <50%: 12.2% versus FEV1 >50%: 3.5%; P<0.001). This difference was driven by operative mortality in patients undergoing Extent II repair (29.6% versus 7.0%; P=0.013) (35). A summary of the large TAAA repair studies from the last decade is presented in Table 2.
Table 2
| Approach | Year | Study | Study type | Cohort (n) | Operative mortality, % | Permanent SCI, % | Renal failure, % (HD) | Reintervention, % |
|---|---|---|---|---|---|---|---|---|
| Open | 2012 | Lima et al. (36) | P, single-center | 361 | 7.6% | 7.3% | 9.1% | NR |
| Open | 2005 | Estrera et al. (37) | R, single-center | 1,896 | 15.9% | 7.1% | 16.6% | 4.9%† |
| Open | 2016 | Coselli et al. (12) | R, single-center | 3,309 | 7.5% | 5.4% | 7.6% | Freedom from reintervention: 94.1% |
| Open | 2018 | Girardi et al. (33) | R, single-center | 783 | 5.6% | 2.5% | 5.2% | 2.4%† |
| Open | 2019 | Geisbüsch et al. (38) | R, population | 1,422 | 23.9% | NR | NR | NR |
| Open | 2021 | Rocha et al. (25) | R, population | 361 | 17.4% | 3.6% | 12.5% | 13.5% |
| Endo | 2012 | Guillou et al. (39) | R, single-center | 89 | 10% | 7.8% | 6.7% | 4.2% |
| Endo | 2016 | Eagleton et al. (40) | P, single-center | 354 | 4.8% | 4.0% | 2.8% | Freedom from reintervention: 54% |
| Endo | 2019 | Geisbüsch et al. (38) | R, population | 856 | 10.6% | NR | NR | NR |
| Endo | 2019 | Oderich et al. (41) | R, single-center | 316 | 2.5% | 2% | 1% | 30.3% |
| Endo | 2021 | Rocha et al. (25) | R, population | 303 | 10.8% | 4.3% | 6.9% | 23.9% |
†, reintervention for bleeding only, not all-cause. SCI, spinal cord injury; HD, hemodialysis; P, prospective study design; R, retrospective study design; NR, not reported.
While open repair has well-described perioperative risk, as noted above, its long-term success is also well-described, with low reported rates of visceral patch aneurysms, branch-vessel occlusion, pseudoaneurysm, and, importantly, re-intervention, the last of which distinguishes it from endovascular repair. Coselli et al. reported a freedom from repair failure of 95.3%±0.6% at ten years and 94.1%±0.8% at 15 years in the aforementioned cohort (12). Graft infection represents a serious but rare late complication of TAAA repair, with Coselli et al. reporting just eighteen instances at fifteen-year follow up (12); other series have reported a similarly low incidence of graft infection at long-term follow-up, ranging from 0.42% to 2.32% (42,43).
Outcomes of endovascular repair
Endovascular repair of TAAA was described in 2001 (44), and was initially reserved for elderly or high-risk patients for whom open surgery was medically contraindicated. The approach and devices used have improved over time. Endovascular devices have evolved to include branched, fenestrated, and parallel grafts, leading to wider adoption of this technique (45-47). Parallel grafting uses bridging stents placed in parallel to the aortic graft and into the visceral and renal vessels. Small series on parallel grafting for TAAA have shown relatively good rates of technical success and an acceptably low incidence of adverse outcomes (48,49). However, this technique is limited by both aortic calcification and narrow aortic diameter, as well as flow between stent components that may cause endoleak (18). Fenestrated and branched grafts have replaced parallel grafts given the reduction of these limitations.
In a large single-center retrospective study of 354 patients treated by fenestrated and branched endografts for Extent II or III TAAAs, technical success was 94%, 30-day mortality was 4.8%, with an SCI incidence of 8.8%, a permanent SCI incidence of 4%, and an incidence of renal failure requiring dialysis of 2.8%. Similar to the data regarding open surgery, the factors associated with mortality included age [hazard ratio (HR) 1.031, 95% CI: 1.008–1.054; P=0.008], chronic pulmonary disease (HR 1.507, 95% CI: 1.05–2.1543; P=0.024), Extent II repair (HR 1.739, 95% CI: 1.226–2.467; P=0.002), cerebrovascular disease (HR 1.620, 95% CI: 1.096–2.394; P=0.016), and higher American Society of Anesthesiologists Physical Status Classification score (P=0.001) (40). Primary patency of the renal and visceral vasculature ranged from 92% (right renal artery) to 96% (celiac axis) at 36 months. Freedom from unplanned reintervention was 54% at 36 months (95% CI: 0.47–0.61), with 27 reinterventions to maintain branch vessel patency and 67 reinterventions for endoleak, highlighting need for reintervention as a main difference between endovascular and open repair. A summary of the large TAAA repair studies from the last decade is presented in Table 2.
Branched or fenestrated grafts can be standardized or custom-made to fit the individual patient’s anatomy and aneurysm. This requires additional manufacturing time and obviates their urgent/emergent use (i.e., rupture, rapid aneurysm growth). Standardized grafts include branches for the celiac axis, superior mesenteric arteries, and bilateral renal arteries based on standard anatomy and can be used in 50–80% of patients with TAAAs (18,50,51). A meta-analysis by Konstantinou et al. (52) including seven observational studies and 197 patients undergoing TAAA repair using standardized t-Branch devices found a pooled success rate of 92.75%, with early mortality of 5.8%, permanent paraplegia of 1.3%, and acute renal failure of 18.7%; pooled reintervention rate at a mean follow-up of 15 months (±7 months) was 5.7% (95% CI: 1.70–11.4%). Notably, this study included 32 cases performed for ruptured aneurysm, which may have increased the rate of adverse events but also highlights the utility of standardized devices when compared with custom-made devices. Physician-modified endografts, by their very nature, do not have standardized quality control, and have also been associated with higher rates of adverse events compared with standardized and custom-made devices; their use has declined use of standardized and custom-made devices has increased (41).
Endoleak represents a primary mid- and long-term complication of endovascular TAAA repair, with the reported incidence ranging from 15–66.7% and the reported incidence of re-intervention for endoleak ranged from 3–33% in prior series (39,53,54). Management of endoleak varies depending upon the surgeon, the type of endoleak, and its features; for instance, aneurysm sac growth may suggest the need for intervention, while regression of sac size or endoleak volume may favor observation (39,54). In prior series (39,53,54), type I endoleak was managed with cuff extension for proximal or distal seal, type II endoleak was most common and was managed either with observation or with glue embolization, and type III endoleak was managed with repeat stenting into the visceral or renal arteries to seal the modular joints.
Choosing the best approach: open or endovascular repair?
The open approach has historically been considered the gold standard of TAAA repair. Open repair is suitable for all aortic anatomies and has well-described outcomes, including long-term durability. Endovascular repair may be the only option in patients with severe medical comorbidities (cardiac, pulmonary, and renal) that render open surgery unacceptably high-risk. Endovascular repair has the further benefit of obviating the need for thoracotomy, extracorporeal circulatory support, and aortic cross-clamping. As the endovascular approach has become more common, its indications have grown to include younger and lower-risk patients. Yet despite its increased use, the need for appropriate preoperative aortic anatomy and the higher rate of reinterventions may present barriers to wider adoption.
Some patient groups are poor candidates for endovascular repair, such as those with connective tissue diseases, and should be offered open repair due to the underlying risk of continued and progressive aortic degeneration, as well as the risk of iatrogenic injury with endovascular techniques (2022 AHA/ACC guideline recommendation COR 1, LOE C-LD) (9). Additional aortic length is required for a landing zone in endovascular repair, as is an aortic lumen narrow enough to seal the endograft, thus some aneurysms may not be anatomically suited to endovascular repair. The passing of wires, catheters, and grafts demands adequate peripheral access, therefore poor access sites or severe peripheral vascular disease may render this approach impossible (9). The most recent AHA/ACC guidelines now state that in patients with intact degenerative TAAA and suitable anatomy, endovascular repair with fenestrated or branched grafts may be considered (COR 2B, LOE B-NR) (9).
Open repair remains the recommended approach for ruptured TAAA (COR 1, LOE B-NR), however, most recent guidelines do state that if a patient presenting with rupture is hemodynamically stable, endovascular repair may be considered (9). Gaudino et al. (55) compared the outcomes of 61 pairs of contemporary, propensity-matched patients undergoing open repair of either ruptured or intact DTA or TAAA. After matching, there was no significant difference in operative mortality, SCI, or any other major postoperative complications.
There are no prospective randomized trials directly comparing the two approaches, which limits the quality of the data that might guide clinicians and patients alike. Results of observational studies have been mixed. A 2018 meta-analysis by Rocha et al. included eight observational studies directly comparing the open and endovascular approaches. Two studies used propensity-matching to account for the large baseline heterogeneity between the patient populations undergoing endovascular or open repair (56-58) and found no difference in mortality. Yet pooled analysis of all unmatched and unadjusted studies found a lower mortality [relative risk (RR) 0.63, 95% CI: 0.45–0.87; P<0.01] and lower risk of SCI (RR 0.65, 95% CI: 0.42–1.01; P=0.05) with endovascular repair. A 2020 systematic review and meta-analysis (59) of 71 studies (24 on endovascular TAAA repair and 47 on open TAAA repair) by the same group found that there was no significant difference in operative mortality (endovascular: 7.4% versus open 8.9%; P=0.21), permanent paralysis (endovascular: 5.2% versus open: 4.5%; P=0.39) or long-term dialysis requirement (endovascular: 3.7 % versus open: 3.8%; P=0.93) despite significantly different preoperative patient characteristics.
A recent retrospective database study (38) including 2,607 cases (856 endovascular, 1,422 open, and 354 hybrid) found decreased in-hospital mortality among patients who underwent elective endovascular repair compared with open and hybrid repair (OR 0.35, 95% CI: 0.24–0.51; P=0.001); notably, only 18% of these cases were performed at a low-volume center. Another database study by Rocha et al. (25) of 664 patients undergoing surgical repair for TAAA (303 endovascular, 361 open, 241 propensity-matched patient pairs) found that open repair was associated with a higher incidence of in-hospital mortality (17.4% versus 10.8%; P=0.04) and complications, defined as the composite of SCI, permanent dialysis, or stroke (26.1% versus 17.4%; P=0.02), than endovascular repair, however, there was no difference in mortality at long-term (8 years) follow-up (HR 1.9, 95% CI: 0.78–1.50). However, these reported outcomes (in particular the in-hospital mortality rates) are less favorable than outcomes reported from single-center, high-volume series, once again reflecting the importance of surgeon and center experience. There was no significant difference in the incidence of late adverse events between groups, with the exception of vascular reintervention, which was higher in the endovascular group (HR 2.64; P<0.01). A proposed algorithm for helping clinicians decide on the most appropriate surgical approach for TAAA repair is provided in Figure 2.
Ischemic injury
Strategies used to prevent ischemic injury after TAAA range from staged repair, preservation of the left subclavian and internal iliac arteries, and distal perfusion using left heart or cardiopulmonary bypass, to cold renal perfusion, hypothermia, and cerebral spinal fluid (CSF) drainage, among others (9,14,18). Improvements in the rate of ischemic complications in more recent series are a reflection of utilization of techniques that mitigate the risk of intraoperative ischemia. Current and past recommendations on strategies to prevent ischemic injury are provided in Figure 3.

SCI
SCI is the most dreaded complication of TAAA repair. A 2022 meta-analysis by Gaudino et al. (60) of 169 studies and 22,634 patients undergoing DTA and TAAA repair found a pooled SCI rate of 4.5% (95% CI: 3.8–5.4), 3.5% in DTA repair (95% CI: 1.8–6.7), and 7.6% in TAAA repair (95% CI: 6.2–9.3). Open repair had a permanent SCI rate of 5.7% (95% CI: 4.3–7.5), while endovascular repair had a permanent SCI rate of 3.9% (95% CI: 3.1–4.8; P=0.03); Extent II repair had the highest rate of permanent SCI (15%, 95% CI: 10.0–22.0; P<0.001).
Contemporary studies have reported that spinal cord drainage leads to decreased incidence of SCI, forming the basis for a COR 1, LOE A recommendation from the AHA/ACC guidelines for its use in both open and endovascular repair in patients at high risk for SCI (9). Coselli et al. (61) randomized 145 patients undergoing TAAA repair to CSF drainage for 48 hours postoperatively or no drainage, and found a significant reduction in paraplegia or paraparesis between the two groups (drainage: 2.6% versus no drainage: 13.0%; P=0.03); other studies have reported similar benefits with CSF drainage (62,63). Estrera et al. reported a decreased risk of neurologic deficit after DTA repair with the use of distal aortic perfusion and CSF drainage (OR 0.19; P=0.02) (37); Safi et al. reported similar benefits with the same intervention (63). CSF drainage is now the only technique for SCI prevention to receive a Class 1 AHA/ACC recommendation. In addition, guidelines provide a COR 1, LOE B-NR recommendation for management of delayed SCI by increasing arterial pressure and decreasing intrathecal pressure (with CSF drainage).
Lima et al. demonstrated in a retrospective study of 250 patients undergoing DTA and TAAA repairs (150 with intrathecal papaverine) that those receiving intrathecal papaverine had significantly lower rates of permanent paraplegia (3.6% versus 7.5%; 0=0.01) and paraparesis (1.6% versus 6.3%; P=0.01) than patients who did not (36). Intrathecal papaverine use was supported by a COR 2B, LOE B recommendation in the 2010 guidelines, but does not receive such a recommendation any longer (14,36). The 2010 ACC/AHA guidelines gave a COR 2A, LOE B recommendation for moderate systemic hypothermia for neuroprotection during open repair, based on a 2003 study of 132 patients that found that moderate systemic hypothermia had a lower risk of transient neurologic deficit (6.6%) compared with mild systemic hypothermia (32%, P=0.04); this recommendation is no longer made in the updated guidelines (64). Neuromonitoring with somatosensory and motor evoked potentials has been used by some groups to guide surgical strategy and to identify optimal perfusion pressure for patients at high risk of SCI, yet data have been mixed, and intraoperative neuromonitoring is no longer recommended (9,14,65-67).
Several strategies in spinal cord protection are based on the concept of a ‘collateral network’ of spinal cord perfusion, which posits that spinal cord nutrient flow is derived from an axial network of small arteries in the spinal canal and surrounding tissue that accept inputs from the hypogastric and subclavian arteries in addition to the known supply from the intercostal and lumbar (segmental) vessels (18,68,69). The natural corollary of the concept is that cord nutrient flow from one source can be increased in response to reduction from another source; conversely, nutrient ‘steal’ can also occur (68). This theory supports deliberate hypertension and CSF drainage in the postoperative period as the spinal cord develops its collateral nutrient supply (18). This theory also supports preoperative interventions such as staged aortic repair (70) and minimally invasive segmental artery coil embolization (18,71,72).
Renal failure
Cold renal perfusion has been found to be effective at minimizing renal injury, leading to its widespread use in open repair (73-75). A 2002 study by Köksoy et al. (73) randomized 30 patients undergoing Extent II TAAA repairs to cold crystalloid perfusion or normothermic blood, and found on multivariable analysis that the use of cold crystalloid perfusion was protective against renal dysfunction (OR 0.133; P=0.02); however, a larger 2009 trial by Lemaire et al. randomized 172 patients to cold crystalloid perfusion or cold blood perfusion and found that there was no difference between groups in the incidence of renal failure (3% in each group) (74). Use of cold renal perfusion and blood perfusion is strongly recommended (COR 1, LOE A) (9), while the use of furosemide, mannitol, or dopamine is recommended against by the 2010 AHA/ACC guidelines when used solely for the purpose of renal protection (COR 3, LOE B) (14). In addition, patients who present with compromised visceral or renal perfusion may require further interventions to improve flow to the SMA, celiac axis, or renal arteries (COR 1, LOE B-NR) (9).
Follow-up and surveillance
Postoperative surveillance imaging should be performed annually for patients who have undergone open TAAA repair (COR 2A, LOE B-NR), with a decreasing frequency over time if imaging findings remain stable (9). Given the higher rate of needed reintervention with endovascular repair, these patients should undergo imaging at one and 12 months postoperatively (COR 1, LOE B-NR) (9), with subsequent annual surveillance provided the interval imaging is stable. Younger patients (i.e., patients with hereditary connective tissue disorders) or patients undergoing endovascular repair who may require more frequent imaging may utilize magnetic resonance imaging for surveillance given the risk of repeat exposure to radiation with computed tomography, which is the usual recommended modality (COR 2A, LOE B-NR) (9). Lifelong management of comorbidities with improved diet, smoking cessation, and medical control of hypertension and hyperlipidemia is necessary. Despite these measures, long-term survival after TAAA repair is poor (12,25,76). Coselli et al. (12) reported survival of 36.8% (±1) at 10 years and 18.3% (±0.9%) at fifteen years.
Conclusions
TAAA has had an increasing incidence over the last two decades, rendering a knowledge of its pathophysiology, surveillance, and management increasingly important. Open repair remains the standard of care, and is the preferred method of repair in appropriate risk patients given the comparable perioperative outcomes and reduced risk of reintervention. Advancements in endovascular repair have expanded the patient population to whom this approach may be offered and it is an acceptable option at centers experienced in these techniques. Ischemic injury remains a primary concern of both endovascular and open TAAA repair, and strategies for prevention of spinal and visceral ischemia continue to evolve, with spinal cord drainage and cold renal perfusion having the most evidence to support their use. Continued improvements in technique and innovations in endovascular technologies may further improve outcomes in the future. Irrespective of the approach chosen for TAAA repair, the relationship between surgeon and center experience and clinical outcomes cannot be understated, and significant consideration should be given to the choice of a high-volume center and a surgeon with expertise in TAAA repair, regardless of whether an open or endovascular technique is utilized.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1880/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1880/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1880/coif). LH is partially supported by a T-32 Multidisciplinary Research Training Grant in Cardiovascular Disease from the National Heart, Lung, and Blood Institute (1 T32 HL160520-01A1). CL serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998;280:1926-9. [Crossref] [PubMed]
- Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006;114:2611-8. [Crossref] [PubMed]
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27; discussion 27-8. [Crossref] [PubMed]
- Cheung K, Boodhwani M, Chan KL, et al. Thoracic Aortic Aneurysm Growth: Role of Sex and Aneurysm Etiology. J Am Heart Assoc 2017;6:e003792. [Crossref] [PubMed]
- Chen JF, Zafar MA, Wu J, et al. Increased Virulence of Descending Thoracic and Thoracoabdominal Aortic Aneurysms in Women. Ann Thorac Surg 2021;112:45-52. [Crossref] [PubMed]
- Kim JB, Kim K, Lindsay ME, et al. Risk of rupture or dissection in descending thoracic aortic aneurysm. Circulation 2015;132:1620-9. [Crossref] [PubMed]
- Pinard A, Jones GT, Milewicz DM. Genetics of Thoracic and Abdominal Aortic Diseases. Circ Res 2019;124:588-606. [Crossref] [PubMed]
- Zafar MA, Chen JF, Wu J, et al. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2021;161:498-511.e1. [Crossref] [PubMed]
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334-482. [Crossref] [PubMed]
- Pham MHC, Ballegaard C, de Knegt MC, et al. Normal values of aortic dimensions assessed by multidetector computed tomography in the Copenhagen General Population Study. Eur Heart J Cardiovasc Imaging 2019;20:939-48. [Crossref] [PubMed]
- Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991;13:452-8. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2016;151:1323-37. [Crossref] [PubMed]
- Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389-404. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-129. Erratum in: J Am Coll Cardiol 2013;62:1039-40. [Crossref] [PubMed]
- Cambria RA, Gloviczki P, Stanson AW, et al. Outcome and expansion rate of 57 thoracoabdominal aortic aneurysms managed nonoperatively. Am J Surg 1995;170:213-7. [Crossref] [PubMed]
- Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 1997;113:476-91; discussion 489-91. [Crossref] [PubMed]
- Coady MA, Rizzo JA, Hammond GL, et al. Surgical intervention criteria for thoracic aortic aneurysms: a study of growth rates and complications. Ann Thorac Surg 1999;67:1922-6; discussion 1953-8. [Crossref] [PubMed]
- Ouzounian M, Tadros RO, Svensson LG, et al. Thoracoabdominal Aortic Disease and Repair: JACC Focus Seminar, Part 3. J Am Coll Cardiol 2022;80:845-56. [Crossref] [PubMed]
- Safi HJ, Winnerkvist A, Miller CC 3rd, et al. Effect of extended cross-clamp time during thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 1998;66:1204-9. [Crossref] [PubMed]
- Upchurch GR Jr, Escobar GA, Azizzadeh A, et al. Society for Vascular Surgery clinical practice guidelines of thoracic endovascular aortic repair for descending thoracic aortic aneurysms. J Vasc Surg 2021;73:55S-83S. [Crossref] [PubMed]
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002;123:360-1. [Crossref] [PubMed]
- Zafar MA, Li Y, Rizzo JA, et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg 2018;155:1938-50. [Crossref] [PubMed]
- Cowan JA Jr, Dimick JB, Henke PK, et al. Surgical treatment of intact thoracoabdominal aortic aneurysms in the United States: hospital and surgeon volume-related outcomes. J Vasc Surg 2003;37:1169-74. [Crossref] [PubMed]
- Greenberg RK, Lu Q, Roselli EE, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-17. [Crossref] [PubMed]
- Rocha RV, Lindsay TF, Austin PC, et al. Outcomes after endovascular versus open thoracoabdominal aortic aneurysm repair: A population-based study. J Thorac Cardiovasc Surg 2021;161:516-527.e6. [Crossref] [PubMed]
- Moulakakis KG, Karaolanis G, Antonopoulos CN, et al. Open repair of thoracoabdominal aortic aneurysms in experienced centers. J Vasc Surg 2018;68:634-645.e12. [Crossref] [PubMed]
- Coselli JS, de la Cruz KI, Preventza O, et al. Extent II Thoracoabdominal Aortic Aneurysm Repair: How I Do It. Semin Thorac Cardiovasc Surg 2016;28:221-37. [Crossref] [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68; discussion 368-70. [Crossref] [PubMed]
- Aftab M, Songdechakraiwut T, Green SY, et al. Contemporary outcomes of open thoracoabdominal aortic aneurysm repair in octogenarians. J Thorac Cardiovasc Surg 2015;149:S134-41. [Crossref] [PubMed]
- Chatterjee S, LeMaire SA, Amarasekara HS, et al. Early-Stage Acute Kidney Injury Adversely Affects Thoracoabdominal Aortic Aneurysm Repair Outcomes. Ann Thorac Surg 2019;107:1720-6. [Crossref] [PubMed]
- Chatterjee S, LeMaire SA, Amarasekara HS, et al. Differential presentation in acuity and outcomes based on socioeconomic status in patients who undergo thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg 2022;163:1990-1998.e1. [Crossref] [PubMed]
- Songdechakraiwut T, Aftab M, Chatterjee S, et al. Tracheostomy After Thoracoabdominal Aortic Aneurysm Repair: Risk Factors and Outcomes. Ann Thorac Surg 2019;108:778-84. [Crossref] [PubMed]
- Girardi LN, Lau C, Ohmes LB, et al. Open repair of descending and thoracoabdominal aortic aneurysms in octogenarians. J Vasc Surg 2018;68:1287-1296.e3. [Crossref] [PubMed]
- Girardi LN, Ohmes LB, Lau C, et al. Open repair of descending thoracic and thoracoabdominal aortic aneurysms in patients with preoperative renal failure. Eur J Cardiothorac Surg 2017;51:971-7. [Crossref] [PubMed]
- Girardi LN, Lau C, Munjal M, et al. Impact of preoperative pulmonary function on outcomes after open repair of descending and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2017;153:S22-S29.e2.
- Lima B, Nowicki ER, Blackstone EH, et al. Spinal cord protective strategies during descending and thoracoabdominal aortic aneurysm repair in the modern era: the role of intrathecal papaverine. J Thorac Cardiovasc Surg 2012;143:945-952.e1. [Crossref] [PubMed]
- Estrera AL, Miller CC 3rd, Chen EP, et al. Descending thoracic aortic aneurysm repair: 12-year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann Thorac Surg 2005;80:1290-6; discussion 1296. [Crossref] [PubMed]
- Geisbüsch S, Kuehnl A, Salvermoser M, et al. Editor's Choice - Hospital Incidence, Treatment, and In Hospital Mortality Following Open and Endovascular Surgery for Thoraco-abdominal Aortic Aneurysms in Germany from 2005 to 2014: Secondary Data Analysis of the Nationwide German DRG Microdata. Eur J Vasc Endovasc Surg 2019;57:488-98. [Crossref] [PubMed]
- Guillou M, Bianchini A, Sobocinski J, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2012;56:65-73. [Crossref] [PubMed]
- Eagleton MJ, Follansbee M, Wolski K, et al. Fenestrated and branched endovascular aneurysm repair outcomes for type II and III thoracoabdominal aortic aneurysms. J Vasc Surg 2016;63:930-42. [Crossref] [PubMed]
- Oderich GS, Ribeiro MS, Sandri GA, et al. Evolution from physician-modified to company-manufactured fenestrated-branched endografts to treat pararenal and thoracoabdominal aortic aneurysms. J Vasc Surg 2019;70:31-42.e7. [Crossref] [PubMed]
- Latz CA, Cambria RP, Patel VI, et al. Durability of open surgical repair of type I-III thoracoabdominal aortic aneurysm. J Vasc Surg 2019;70:413-23. [Crossref] [PubMed]
- Latz CA, Patel VI, Cambria RP, et al. Durability of open surgical repair of type IV thoracoabdominal aortic aneurysm. J Vasc Surg 2019;69:661-70. [Crossref] [PubMed]
- Chuter TA, Gordon RL, Reilly LM, et al. An endovascular system for thoracoabdominal aortic aneurysm repair. J Endovasc Ther 2001;8:25-33. [Crossref] [PubMed]
- Anderson JL, Berce M, Hartley DE. Endoluminal aortic grafting with renal and superior mesenteric artery incorporation by graft fenestration. J Endovasc Ther 2001;8:3-15. [Crossref] [PubMed]
- Chuter TA, Rapp JH, Hiramoto JS, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2008;47:6-16. [Crossref] [PubMed]
- Greenberg RK, Lytle B. Endovascular repair of thoracoabdominal aneurysms. Circulation 2008;117:2288-96. [Crossref] [PubMed]
- Schwierz E, Kolvenbach RR, Yoshida R, et al. Experience with the sandwich technique in endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg 2014;59:1562-9. [Crossref] [PubMed]
- Lobato AC, Camacho-Lobato L. Endovascular treatment of complex aortic aneurysms using the sandwich technique. J Endovasc Ther 2012;19:691-706. [Crossref] [PubMed]
- Sweet MP, Hiramoto JS, Park KH, et al. A standardized multi-branched thoracoabdominal stent-graft for endovascular aneurysm repair. J Endovasc Ther 2009;16:359-64. [Crossref] [PubMed]
- Fernandez CC, Sobel JD, Gasper WJ, et al. Standard off-the-shelf versus custom-made multibranched thoracoabdominal aortic stent grafts. J Vasc Surg 2016;63:1208-15. [Crossref] [PubMed]
- Konstantinou N, Antonopoulos CN, Jerkku T, et al. Systematic review and meta-analysis of published studies on endovascular repair of thoracoabdominal aortic aneurysms with the t-Branch off-the-shelf multibranched endograft. J Vasc Surg 2020;72:716-725.e1. [Crossref] [PubMed]
- Roselli EE, Greenberg RK, Pfaff K, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2007;133:1474-82. [Crossref] [PubMed]
- Kitagawa A, Greenberg RK, Eagleton MJ, et al. Fenestrated and branched endovascular aortic repair for chronic type B aortic dissection with thoracoabdominal aneurysms. J Vasc Surg 2013;58:625-34. [Crossref] [PubMed]
- Gaudino M, Lau C, Munjal M, et al. Open repair of ruptured descending thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg 2015;150:814-21. [Crossref] [PubMed]
- Bertoglio L, Cambiaghi T, Ferrer C, et al. Comparison of sacrificed healthy aorta during thoracoabdominal aortic aneurysm repair using off-the-shelf endovascular branched devices and open surgery. J Vasc Surg 2018;67:695-702. [Crossref] [PubMed]
- Ferrer C, Cao P, De Rango P, et al. A propensity-matched comparison for endovascular and open repair of thoracoabdominal aortic aneurysms. J Vasc Surg 2016;63:1201-7. [Crossref] [PubMed]
- Rocha RV, Friedrich JO, Elbatarny M, et al. A systematic review and meta-analysis of early outcomes after endovascular versus open repair of thoracoabdominal aortic aneurysms. J Vasc Surg 2018;68:1936-1945.e5. [Crossref] [PubMed]
- Rocha RV, Lindsay TF, Friedrich JO, et al. Systematic review of contemporary outcomes of endovascular and open thoracoabdominal aortic aneurysm repair. J Vasc Surg 2020;71:1396-1412.e12. [Crossref] [PubMed]
- Gaudino M, Khan FM, Rahouma M, et al. Spinal cord injury after open and endovascular repair of descending thoracic and thoracoabdominal aortic aneurysms: A meta-analysis. J Thorac Cardiovasc Surg 2022;163:552-64. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9. [Crossref] [PubMed]
- Khan SN, Stansby G. Cerebrospinal fluid drainage for thoracic and thoracoabdominal aortic aneurysm surgery. Cochrane Database Syst Rev 2012;10:CD003635. [Crossref] [PubMed]
- Safi HJ, Miller CC 3rd, Huynh TT, et al. Distal aortic perfusion and cerebrospinal fluid drainage for thoracoabdominal and descending thoracic aortic repair: ten years of organ protection. Ann Surg 2003;238:372-80; discussion 380-1. [Crossref] [PubMed]
- Svensson LG, Khitin L, Nadolny EM, et al. Systemic temperature and paralysis after thoracoabdominal and descending aortic operations. Arch Surg 2003;138:175-9; discussion 180. [Crossref] [PubMed]
- Guerit JM, Witdoeckt C, Verhelst R, et al. Sensitivity, specificity, and surgical impact of somatosensory evoked potentials in descending aorta surgery. Ann Thorac Surg 1999;67:1943-6; discussion 1953-8. [Crossref] [PubMed]
- Ogino H, Sasaki H, Minatoya K, et al. Combined use of adamkiewicz artery demonstration and motor-evoked potentials in descending and thoracoabdominal repair. Ann Thorac Surg 2006;82:592-6. [Crossref] [PubMed]
- Schurink GW, Nijenhuis RJ, Backes WH, et al. Assessment of spinal cord circulation and function in endovascular treatment of thoracic aortic aneurysms. Ann Thorac Surg 2007;83:S877-81; discussion S890-2. [Crossref] [PubMed]
- Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg 2007;83:S865-9; discussion S890-2. [Crossref] [PubMed]
- Svensson LG, Hess KR, Coselli JS, et al. Influence of segmental arteries, extent, and atriofemoral bypass on postoperative paraplegia after thoracoabdominal aortic operations. J Vasc Surg 1994;20:255-62. [Crossref] [PubMed]
- Etz CD, Zoli S, Mueller CS, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg 2010;139:1464-72. [Crossref] [PubMed]
- Branzan D, Etz CD, Moche M, et al. Ischaemic preconditioning of the spinal cord to prevent spinal cord ischaemia during endovascular repair of thoracoabdominal aortic aneurysm: first clinical experience. EuroIntervention 2018;14:828-35. [Crossref] [PubMed]
- Petroff D, Czerny M, Kölbel T, et al. Paraplegia prevention in aortic aneurysm repair by thoracoabdominal staging with 'minimally invasive staged segmental artery coil embolisation' (MIS2ACE): trial protocol for a randomised controlled multicentre trial. BMJ Open 2019;9:e025488. [Crossref] [PubMed]
- Köksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg 2002;73:730-8. [Crossref] [PubMed]
- Lemaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9; discussion 19. [Crossref] [PubMed]
- Svensson LG, Coselli JS, Safi HJ, et al. Appraisal of adjuncts to prevent acute renal failure after surgery on the thoracic or thoracoabdominal aorta. J Vasc Surg 1989;10:230-9. [Crossref] [PubMed]
- Tong MZ, Eagleton MJ, Roselli EE, et al. Outcomes of Open Versus Endovascular Repair of Descending Thoracic and Thoracoabdominal Aortic Aneurysms. Ann Thorac Surg 2022;113:1144-52. [Crossref] [PubMed]



