
Comparison of active versus passive robotic-endoscope-holder-assisted unisurgeon uniportal thoracoscopic surgery in terms of surgical efficacy and patient safety
Highlight box
Key findings
• Robotic-arm-assisted unisurgeon uniportal wedge resection have been shown to be safe and feasible with an expert hand.
What is known and what is new?
• Compared with conventional human-assisted uniportal video-assisted thoracoscopic surgeries, the use of robotic-arm-assisted unisurgeon uniportal surgeries does not result in a longer operation time or worse short-term outcomes.
• At present, passive robotic arm has a higher application value than active robotic arms in terms of their use.
What is the implication, and what should change now?
• With further improvements, robotic-assisted endoscope holders may be able to replace some human assistants in certain procedures, especially in environments where human resources are scarce.
Introduction
Technological advancements and innovation in surgical methods have substantially enhanced the convenience and outcomes of thoracic surgeries (1-3). Some of these advancements include three-dimensional field views, quad-high-definition monitor resolutions, thin staplers, and robotic arms (4-7). The concept of unisurgeon surgery performed using a robotic camera holder has emerged recently (8,9). For this purpose, various robotic arms have been used, such as in passive and active platforms. Passive platforms are usually a type of frame attached to an operating table and can be adjusted manually. These platforms have evolved from primitive pneumatic endoscope holders to modern computer-controlled electric motors, improving ease of operation (5,6). By contrast, active platforms (e.g., AESOP and ViKY) allow for voice control of the movement of the robotic camera holder (10). These efficient platforms have also promoted the development of other active robotic arms, such as the MTG-H110. The MTG-H110 is new pedal-controlled robotic endoscope holder that offers stable endoscopic vision and 6 directions of control for the camera movement. By using both hands and feet during the operation, the surgeon could perform the operation with full use of his two hands without interruption (11). It is expected to not only reduce the need for human assistance but also conduct minimally invasive surgeries. In the present study, we compared active and passive robotic-endoscope-holder-assisted unisurgeon uniportal surgeries with human-assisted uniportal video-assisted thoracoscopic surgeries (VATSs). We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-19/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Chang Gung Memorial Hospital, Taoyuan (No. 202002019B0) and individual consent for this retrospective analysis was waived.
Active robotic endoscope holder
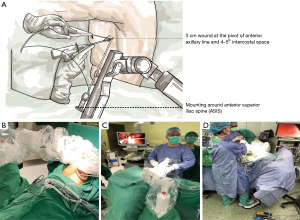
MTG-100 (HIWIN, Taichung) is a new-generation active robotic camera holder that is constructed using the design concept of the remote center of motion to minimize wound size and friction around the wound. Surgeons may adjust the direction of the endoscope in six directions—zoom in/out, upward/downward, and right/left—using a foot pedal or on-board buttons (Figure 1, Video 1). The robotic arm can be mounted on the side bar of an operation table on the side opposite to that of the operator.
Passive robotic endoscope holder
ENDOFIXexo (AKTORmed, Barbing, Germany) is a passive robotic endoscope holder with computer-controlled electric motors. It is a newly verified model used in neurosurgery, otorhinolaryngical surgery, and thoracic surgery. ENDOFIXexo has a total of six computer-controlled joints that can be adjusted manually through an ergonomic control button on the upper side of the endoscope fixation site. This holder can be easily attached to the rail of an operation table.
Robotic arm setting and surgical method
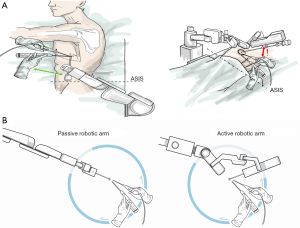
Figure 1A,1B depicts the configurations of the robotic arm during operation. Active and passive robotic endoscope holders shared the same configuration. The robotic-arm-fixing site could be adjusted slightly depending on lesion site. Its position could be fixed above or below the anterior superior iliac spine (ASIS; anatomic landmark; Figure 1A,1B). For example, the robotic arm could be fixed below the ASIS line to resect an upper-lobe lung tumor. By contrast, it could be placed above the ASIS line to resect a lower-lobe lung tumor. But this is a general rule for setting up this type of robotic arm. In actual surgery, moderate adjustments may still be made according to the height and size of the patient. A 3-cm wound was created at the pivot of the anterior axillary line and the fourth or fifth intercostal space. A plastic wound protector was used to ensure clear visibility of the surgical field when the endoscope entered the thoracic cavity. To perform the surgery smoothly and safely, the number of the surgical staff in the operating room was the same as that of human-assisted uniport VATS for pulmonary resection: 1 surgeon, 1 assistant, 1 scrub nurse, and 1 circulating nurse. The assistant could participate in the surgery if the surgeon required any help to tract the lung parenchyma away from the vital structure, such as pulmonary vein, artery, or aorta, or if the robotic endoscope holder failed to ensure adequate surgical vision. The assistant recorded the frequency of help needed and the reason why the surgery could not be performed by a single surgeon. Any help received from a human assistant indicated the failure of the unisurgeon uniportal VATS. We enrolled patients with lung lesion <5 cm, age >18 years old, and without coagulopathy in our analysis and those enrolled patients were all followed up at least for 6 months after surgery. This was a retrospective cohort study, mainly through the sequential use of human assistants, passive robotic arms, and active pedal-control robotic endoscope holder in different periods to evaluate the feasibility and limitations of uniport unisurgeon surgery.
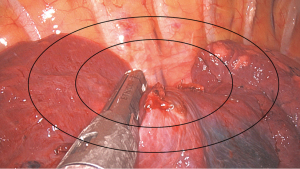
To objectively assess the effects of switching to robotic-endoscope-holder-assisted uniportal VATS, the period between the entry of a patient in the operating room and wound closure was divided into four stages: anesthesia induction, preparation, operation, and sign out; the durations of all the aforementioned stages were recorded. In addition, various surgical and safety parameters, such as drainage tube duration, postoperative hospital stay, rehabilitation performance, possible postoperative complications, were assessed. The number of staplers used during the surgery and images of the surgery were recorded simultaneously to compare surgical field quality among three groups: MA group, MTH-100-assisted uniportal VATS; EA group, ENDOFIXexo-assisted uniportal VATS; and HA group, human-assisted uniportal VATS. Surgical images were captured when the stapler passed through the target lesion and began to cut it. As per our surgical protocol, the images of a surgery and the number of staplers used during the surgery are recorded for insurance claim. These data were used to compare the groups in terms of the quality of surgical images. Surgical images were divided into three zones by two ellipses corresponding to 50% and 80% of the surgical field in length and width, respectively (Figure 2). The zones were defined as central, intermediate, and marginal zones when the target lesion and endostaple were located inside the 50% ellipse, between the 50% and 80% ellipses, and outside the 80% ellipse, respectively.
Statistical analyses
This was a retrospective study. Descriptive statistics were used to summarize cohort characteristics with median and range (min, max) values for continuous variables and frequencies and percentages for categorical variables. For multiple comparisons of continuous variables, statistical evaluation of three groups was performed by Kruskal-Wallis test, whereas categorical data were compared using the Pearson chi-squared test or the Fisher’s exact test, as appropriate. A Bonferroni adjustment was used to adjust for multiple group comparisons. All analyses were performed with SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was determined by a two-tailed P value <0.05.
Results
Those patients who were eligible and underwent uniportal VATS for lung lesion between January 2018 and November 2020 were enrolled for analysis. A total of 228 patients meet the inclusion criteria. Of them, 15, 57, and 156 patients underwent active robotic arm-assisted, passive robotic-assisted, and human-assisted uniport VATS, respectively. All enrolled patients were followed up for 6 months after surgery at least. Table 1 summarizes the demographics of the patients. The EA, MA, and HA groups did not vary in terms of age, sex, body mass index (BMI), smoking status, lesion location, or lesion diameter. Furthermore, the groups did not differ in terms of preoperative preparation time. When the operation time for each surgical approach was considered, the MA group was found to have the shortest operation time in wedge resection.
Table 1
| Variables | Entire cohort | P value | Post-hoc P value | ||||
|---|---|---|---|---|---|---|---|
| HA group (n=156) [1] | EA group (n=57) [2] | MA group (n=15) [3] | [1] vs. [2] | [1] vs. [3] | [2] vs. [3] | ||
| Age (years) | 62 [18, 92] | 63 [19, 93] | 65 [44, 83] | 0.570 | |||
| Gender | 0.591 | ||||||
| Male | 84 (53.8) | 30 (52.6) | 6 (40.0) | ||||
| Female | 72 (46.2) | 27 (47.4) | 9 (60.0) | ||||
| Smoking history | 0.135 | ||||||
| Yes | 58 (37.2) | 17 (29.8) | 2 (13.3) | ||||
| No | 98 (62.8) | 40 (70.2) | 13 (86.7) | ||||
| ACS history | 0.339 | ||||||
| Yes | 8 (5.1) | 4 (7.0) | 2 (13.3) | ||||
| No | 148 (94.9) | 53 (93.0) | 13 (86.7) | ||||
| COPD | 0.661 | ||||||
| Yes | 7 (4.5) | 3 (5.3) | 1 (6.7) | ||||
| No | 149 (95.5) | 54 (94.7) | 14 (93.3) | ||||
| Renal disease | 0.502 | ||||||
| Yes | 5 (3.2) | 1 (1.8) | 1 (6.7) | ||||
| No | 151 (96.8) | 56 (98.2) | 14 (93.3) | ||||
| Body mass index (kg/m2) | 24.5 [15.1, 39.0] | 24.2 [16.0, 36.3] | 23.9 [19.0, 31.7] | 0.935 | |||
| FEV1 (L) | 2.3 [1.1, 4.2] | 2.3 [1.0, 4.3] | 2.2 [1.5, 3.8] | 0.484 | |||
| Preparation time (min) | 15 [5, 23] | 15 [6, 21] | 16 [7, 21] | 0.470 | |||
| Operative time (min) | 135 [38, 310] | 122 [60, 463] | 83 [44, 218] | 0.002 | 1.000 | 0.001 | 0.015 |
| Wedge | 106 [38, 174] | 105 [60, 171] | 81.5 [44, 120] | 0.099 | |||
| Anatomic resection | 156 [59, 310] | 145 [82, 463] | 88 [60, 218] | 0.120 | |||
| Blood loss (mL) | 30 [10, 50] | 30 [10, 50] | 20 [10, 30] | 0.534 | |||
| Chest tube duration (h) | 49 [8, 242] | 48 [23, 195] | 43 [11, 146] | 0.392 | |||
| Post-OP stay (h) | 70 [20, 258] | 72 [24, 203] | 71 [11, 169] | 0.397 | |||
| Operation type | 0.030 | 1.000 | 0.026 | 0.155 | |||
| Wedge | 51 (32.7) | 22 (38.6) | 10 (66.7) | ||||
| Anatomic resection | 105 (67.3) | 35 (61.4) | 5 (33.3) | ||||
| Diagnosis | 0.220 | ||||||
| Malignancy | 114 (73.1) | 43 (75.4) | 8 (53.3) | ||||
| Benign | 42 (26.9) | 14 (24.6) | 7 (46.7) | ||||
| Lesion location | 0.940 | ||||||
| RUL | 37 (23.7) | 14 (24.6) | 5 (33.3) | ||||
| RML | 19 (12.2) | 5 (8.8) | 2 (13.3) | ||||
| RLL | 36 (23.1) | 10 (17.5) | 2 (13.3) | ||||
| LUL | 38 (24.4) | 16 (28.1) | 3 (20.0) | ||||
| LLL | 26 (16.7) | 12 (21.1) | 3 (20.0) | ||||
| Post-OP complication | 0.775 | ||||||
| Yes | 4 (2.6) | 2 (3.5) | 0 (0.0) | ||||
| No | 152 (97.4) | 55 (96.5) | 15 (100.0) | ||||
| Triflow number | |||||||
| Pre-OP | 3 [0, 3] | 3 [0, 3] | 3 [0, 3] | 0.929 | |||
| Post-OP day 1 | 2 [0, 3] | 2 [0, 3] | 1 [0, 3] | 0.120 | |||
| Post-OP day 3 | 3 [0, 3] | 3 [1, 3] | 3 [1, 3] | 0.061 | |||
| Surgeon demand | |||||||
| Wedge | <0.001 | <0.001 | <0.001 | – | |||
| 1 | 0 (0.0) | 22 (100.0) | 10 (100.0) | ||||
| 2 | 51 (100.0) | 0 (0.0) | 0 (0.0) | ||||
| Segmentectomy | <0.001 | <0.001 | <0.001 | 0.006 | |||
| 1 | 0 (0.0) | 20 (95.2) | 2 (40.0) | ||||
| 2 | 44 (100.0) | 1 (4.8) | 3 (60.0) | ||||
| Lobectomy | <0.001 | ||||||
| 1 | 0 (0.0) | 9 (64.3) | – | ||||
| 2 | 61 (100.0) | 5 (35.7) | – | ||||
The data are shown as median [min, max] or n (%). ACS, acute coronary syndrome; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; OP, operation; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
With regard to the feasibility of unisurgeon uniportal surgery, all unisurgeon uniportal wedge resections in the EA and MA groups could be performed successfully without any help from a human assistant. With regard to anatomic resection, the success rate of unisurgeon uniportal segmentectomy was higher in the EA group than in the MA group (95% vs. 40%, respectively). Because the success rate in the MA group was <50% of that in the EA group, active robotic endoscope holder was not used in lobectomy. The reasons for the high rate of failure in the MA group are presented in the discussion section.
Intraoperative images were also captured for comparison (Table 2). Endoscopic image quality could be considered good if the target lesion is at the center of an intraoperative image. Therefore, the images were evaluated with a focus on whether the stapler was at the central area of the image when the stapler passed through a blood vessel, the bronchus, and the lung parenchyma. In wedge resection, the EA, MA, and HA groups had intraoperative images of similar quality. However, in anatomic resection, the image quality was considerably higher in the HA group than in the other groups; in particular, the number of times the stapler was in the marginal zone during the surgery was the lowest in the HA group.
Table 2
| Variables | Entire cohort | P value | Post-hoc P value | ||||
|---|---|---|---|---|---|---|---|
| HA group (n=156) [1] |
EA group (n=57) [2] |
MA group (n=15) [3] |
[1] vs. [2] | [1] vs. [3] | [2] vs. [3] | ||
| No staple | 7 [3, 19] | 7 [3, 17] | 6 [3, 17] | 0.562 | |||
| Wedge | 5 [3, 9] | 4.5 [3, 7] | 5 [3, 17] | 0.245 | |||
| Anatomic resection | 8 [3, 19] | 9 [5, 17] | 10 [7, 12] | 0.709 | |||
| Central | 6 [3, 16] | 5 [3, 15] | 5 [3, 14] | 0.010 | 0.034 | 0.117 | 1.000 |
| Wedge | 5 [3, 9] | 4 [3, 6] | 4.5 [3, 14] | 0.078 | |||
| Anatomic resection | 7 [3, 16] | 7 [4, 15] | 6 [4, 7] | 0.112 | |||
| Intermediate | 1 [0, 4] | 1 [0, 4] | 1 [0, 2] | 0.228 | |||
| Wedge | 0 [0, 2] | 0 [0, 1] | 0 [0, 2] | 0.489 | |||
| Anatomic resection | 1 [0, 4] | 2 [0, 4] | 2 [2, 2] | 0.014 | 0.051 | 0.156 | 1.000 |
| Marginal | 0 [0, 1] | 0 [0, 3] | 0 [0, 3] | <0.001 | <0.001 | 0.011 | 1.000 |
| Wedge | 0 [0, 1] | 0 [0, 1] | 0 [0, 1] | 0.073 | |||
| Anatomic resection | 0 [0, 1] | 1 [0, 3] | 2 [0, 3] | <0.001 | <0.001 | 0.001 | 0.268 |
Continuous variables are presented in median [min, max].
Concerning short-term intraoperative complications, no severe bleeding occurred in any the three groups. Regarding the rate of 30-day postoperative complications in the EA group, 1 patient (1.75%) exhibited prolonged air leak and 1 (1.75%) had pneumonia; the rate of complication was 3.5%. By contrast, no postoperative complications were noted in the MA group. However, in the HA group, 2 patients (1.28%) exhibited prolonged air leak and 2 others (1.28%) had atelectasis. Thus, the three groups did not vary markedly in terms postoperative complications.
Discussion
The use of a robotic arm to assist in surgery has emerged as a recent trend, and preliminary results have been obtained from its implementation in orthopedic and laparoscopic and otolaryngol surgeries (11-14). Accuracy, precision, and low fatigue levels in surgeons and surgical teams, health care manpower reducing are some of the advantages of robotic-arm-assisted surgery. Public health crisis and work hour regulation highlight the importance of the resilience of health care resources (15-17). For more flexible use of health care manpower, in the field of thoracic surgery, Okada et al. first used a robotic arm in lung resection (6). After 13 years, the first unisurgeon uniportal VATS was performed (8). However, related reports are rare and have lacked comprehensive analyses. Our preliminary results suggest that wedge resection is the most suitable approach for unisurgeon uniportal VATS with the help of passive robotic endoscope holder (9). Although the passive robotic endoscope manipulator may offer stable surgical images even at a tricky angle, the surgeon still need to temporarily adjust the position of the scope. Thus, the use of a pedal-controlled robotic endoscope holder was initially regarded as a solution to the aforementioned problem (11,18).
After performing consecutive surgeries for a total of 57 patients, we started using pedal-control endoscope holder in unisurgeon uniportal VATS. Wedge resection required no conversion to human-assisted surgery. Unexpectedly, anatomic resection was more preferable in the EA group than in the MA group. The rate of conversion was 60% (3/5) in the MA group. Because of the inconvenience associated with the use of a pedal-controlled robotic arm in segmentectomy, we stopped using it in uniportal unisurgeon lobectomy. The following are a few problems associated the use of this endoscope holder in unisurgeon uniportal VATS. First, the distance between the buckle and the robotic arm is too large to allow the 30° Hopkins-style endoscope to be smoothly mounted on the robotic arm, which requires more work space than that required for the passive platform; this further increases the frequency of collision between surgical instruments and the endoscope holder (Figure 3A). On the basis of our externally recorded video and the surgeon’s estimation, the passive robotic holder appeared to occupy only 45° to 90° of the total work space for operation, whereas active robotic holder might have occupied 100° to 135° of the work space, which might have increased the frequency of the aforementioned collision and reduced the suitable angle for the endostapler to pass through the relevant blood vessel (Figure 3B). Performing anatomic resection is possible unless the surgical wound is enlarged or the number of wounds is increased. Second, the terminal robotic arm joint can be moved only in the same plane, thus adding to the difficulty of obtaining a panoramic view during surgery. This substantially increases the failure rate of unisurgeon uniportal VATS in anatomic resection. By contrast, wedge resection can be successfully performed unidirectionally; the directions of the instrument and the endoscope are almost parallel. This reduces the collision between surgical instruments and the endoscope. Thus, at present, wedge resection is a promising approach for replacing manpower with a robotic endoscope holder. Staff requirements may be more flexible for a simple procedure than for a relatively complex procedure. In Taiwan and some other area, increasing concerns have been reported regarding the regulation of residents’ work hours (19); the decline in the number of residency applications to surgical departments has resulted in a shortage of efficient staff (20-22). Hence, the introduction of robotic arms to replace manpower is a direction worth considering.
In addition to comparing various perioperative parameters, we compared the quality surgical images among the three groups (Table 2). By calculating the number of times the stapler was in the central, intermediate, and marginal zones in the images, we concluded that the qualities of the intercepted surgical images of the three groups were similar in wedge resection. However, in segmentectomy, the quality of the surgical images of the HA was better than that of the other groups. Because no patient underwent active-arm-assisted lobectomy, we only compared the EA group with the HA group. The image quality of the HA group was better than that of the EA group. This indicates that the flexibility of the two robotic arms is not as good as that of humans in uniportal surgery. The two platforms explored in our study still have room for improvement.
With the use of a control bottom in the passive robotic platform, the position and the angle of the endoscope can be adjusted intuitively until the operator is satisfied. The intuitive and easy-to-use features allow surgeon to hardly change the original single-port surgical technique. The only fly in the ointment was that the surgery was occasionally interrupted temporarily when the surgeon wanted to change the surgical field. By contrast, the surgeon was impressed by the experience of being able to actively control the endoscope without any interruption during the surgery. Nonetheless, this requires high coordination among the surgeon’s hands, feet, and eyes. On balance, the active platform is less efficient than the passive platform.
Before using the robotic arm, we also anticipated what preparations should be made in the event of a major accident, such as a major vessel injury. Both robotic endoscope holder platforms are equipped with a simple dismantling mechanism. Even in the event of major bleeding, surgeon could compress gauze over the bleeding point first. After circulating nurse dismantled the robotic arms and called assistants for help, the following treatment principles were the same as we described in our previous report (23). Fortunately, no intraoperative massive bleeding occurred during the surgeries reviewed in the present study.
Regarding the training for unisurgeon uniportal surgery, the trainer surgeon may experience a low level of fatigue if using a robotic endoscope holder, which would increase their focus on imparting the required skills to the trainees. In the future, we would like to explore the learning curve further by including high numbers of surgery cases and trainee surgeons.
Our study has some limitations. First, it was a single-center retrospective study. The follow up of such application depends on more surgical teams to verify it. Second, in spite of that the robotic endoscope holder-assisted and human-assisted surgeries were performed by the same surgical team. The nature of retrospective data makes it difficult to really provide a high-quality and robust evidence to distinguish the advantages and disadvantages of robotic arm assistance and human assistance uniportal surgery. Nevertheless, this compromise must be made to ensure patient safety and adopt new surgical methods. Subsequent follow-up for surgical outcomes and the training of new surgeons are warranted. Third, the small number of active robotic endoscope holders might have affected the robustness of our findings. However, this resulted from the inherent design-related limitations of active robotic arms. The use of these holders in anatomic resection was difficult. Notably, robotic arms that occupy large portions of the work space in front of surgeons are difficult to use in uniportal VATS. This finding might provide some design hint to the robotic endoscopic holder, especially for those which designed for uniportal VATS. Finally, although the performance of the active robotic endoscope holder in unisurgeon uniportal surgery was not as good as expected, in-depth discussion on unisurgeon multiport VATS is necessary. The performance of these two platforms may vary across different surgical methods, which necessitates further studies.
Conclusions
In simple wedge resection, either active or passive platforms may replace human assistants with an expert hand. However, for more complex uniportal VATS procedures, although the passive robotic arm has a disadvantage related to the temporary interruption of the surgery for adjusting the endoscope angle, it reduces the frequency of collision between the endoscope and VATS instruments and increases the dexterity of the surgeon in operating the instrument.
Acknowledgments
Funding: Statistical assistance was provided by the Clinical Trial Center, Chang Gung Memorial Hospital, Taoyuan, and funded by the Ministry of Health and Welfare of Taiwan (No. MOHW107-TDU-B-212-123005).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-19/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-19/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-19/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-19/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Chang Gung Memorial Hospital, Taoyuan (No. 202002019B0) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valo JK, Kytö V, Sipilä J, et al. Thoracoscopic surgery for lung cancer is associated with improved survival and shortened admission length: a nationwide propensity-matched study. Eur J Cardiothorac Surg 2020;57:100-6. [Crossref] [PubMed]
- Veluswamy RR, Whittaker Brown SA, Mhango G, et al. Comparative Effectiveness of Robotic-Assisted Surgery for Resectable Lung Cancer in Older Patients. Chest 2020;157:1313-21. [Crossref] [PubMed]
- Chaari Z, Montagne F, Sarsam M, et al. Midterm survival of imaging-assisted robotic lung segmentectomy for non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2022;34:1016-23. [Crossref] [PubMed]
- Yang CL, Wang W, Mo LL, et al. Short-Term Outcome of Three-Dimensional Versus Two-Dimensional Video-Assisted Thoracic Surgery for Benign Pulmonary Diseases. Ann Thorac Surg 2016;101:1297-302. [Crossref] [PubMed]
- Ng CS, Pickens A, Siegel JM, et al. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devices†. Eur J Cardiothorac Surg 2016;49:i73-8. [PubMed]
- Okada S, Tanaba Y, Sugawara H, et al. Thoracoscopic major lung resection for primary lung cancer by a single surgeon with a voice-controlled robot and an instrument retraction system. J Thorac Cardiovasc Surg 2000;120:414-5. [Crossref] [PubMed]
- Kunisaki C, Hatori S, Imada T, et al. Video-assisted thoracoscopic esophagectomy with a voice-controlled robot: the AESOP system. Surg Laparosc Endosc Percutan Tech 2004;14:323-7. [Crossref] [PubMed]
- Gonzalez-Rivas D. Unisurgeon' uniportal video-assisted thoracoscopic surgery lobectomy. J Vis Surg 2017;3:163. [Crossref] [PubMed]
- Wu CF, Wu CY, Chao YK, et al. Comparative early results of a robotics-assisted endoscope holder in single port thoracoscopic surgery in the era of COVID-19. Surg Endosc 2022;36:5501-9. [Crossref] [PubMed]
- Takahashi M, Takahashi M, Nishinari N, et al. Clinical evaluation of complete solo surgery with the "ViKY(®)" robotic laparoscope manipulator. Surg Endosc 2017;31:981-6. [Crossref] [PubMed]
- Chan JY, Leung I, Navarro-Alarcon D, et al. Foot-controlled robotic-enabled endoscope holder for endoscopic sinus surgery: A cadaveric feasibility study. Laryngoscope 2016;126:566-9. [Crossref] [PubMed]
- Zhang J, Ndou WS, Ng N, et al. Robotic-arm assisted total knee arthroplasty is associated with improved accuracy and patient reported outcomes: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 2022;30:2677-95. [Crossref] [PubMed]
- Khan MM, Parab SR. Endoscopic cartilage tympanoplasty: A two-handed technique using an endoscope holder. Laryngoscope 2016;126:1893-8. [Crossref] [PubMed]
- Ohmura Y, Suzuki H, Kotani K, et al. Comparative effectiveness of human scope assistant versus robotic scope holder in laparoscopic resection for colorectal cancer. Surg Endosc 2019;33:2206-16. [Crossref] [PubMed]
- Thoracic Surgery Outcomes Research Network, Inc.. COVID-19 Guidance for Triage of Operations for Thoracic Malignancies: A Consensus Statement From Thoracic Surgery Outcomes Research Network. Ann Thorac Surg 2020;110:692-6. [Crossref] [PubMed]
- Spooner J, Lawen T, Ory J. Triaging urological surgeries to cope with the coronavirus-19 pandemic. Curr Opin Urol 2022;32:131-40. [Crossref] [PubMed]
- Cerwenka H, Bacher H, Werkgartner G, et al. Working conditions and trainee shortage in operative disciplines--is our profession ready for the next decade? Langenbecks Arch Surg 2009;394:179-83. [Crossref] [PubMed]
- Zhong F, Li P, Shi J, et al. Foot-Controlled Robot-Enabled EnDOscope Manipulator (FREEDOM) for Sinus Surgery: Design, Control, and Evaluation. IEEE Trans Biomed Eng 2020;67:1530-41. [Crossref] [PubMed]
- Weaver MD, Landrigan CP, Sullivan JP, et al. The Association Between Resident Physician Work-Hour Regulations and Physician Safety and Health. Am J Med 2020;133:e343-54. [Crossref] [PubMed]
- Zagales I, Bourne M, Sutherland M, et al. Regional Population-Based Workforce Shortages in General Surgery by Practicing Surgeon and Resident Trainee. Am Surg 2021;87:855-63. [Crossref] [PubMed]
- Vanderby SA, Carter MW, Latham T, et al. Modeling the cardiac surgery workforce in Canada. Ann Thorac Surg 2010;90:467-73. [Crossref] [PubMed]
- Moffatt-Bruce S, Crestanello J, Way DP, et al. Providing cardiothoracic services in 2035: Signs of trouble ahead. J Thorac Cardiovasc Surg 2018;155:824-9. [Crossref] [PubMed]
- Wu CF, de la Mercedes T, Fernandez R, et al. Management of intra-operative major bleeding during single-port video-assisted thoracoscopic anatomic resection: two-center experience. Surg Endosc 2019;33:1880-9. [Crossref] [PubMed]


