
Use of high flow nasal cannula oxygen therapy for patients infected with SARS-CoV-2 outside intensive care setting
Highlight box
Key findings
• Use of high flow nasal cannula therapy is feasible outside intensive care setting
• Monitoring of SpO2:FiO2 ratio is more practical and at specific therapeutic time points can predict 28-day survival under this setting
What is known and what is new?
• High flow nasal cannula has been used in patients with hypoxemic respiratory failure since the last decade. Majority of the studies has been performed in intensive care setting.
• At the time of infection outbreak when intensive care service is in short supply, the use of high flow nasal cannula therapy outside the intensive care setting for patients with acute hypoxemic respiratory failure should be considered.
What is the implication, and what should change now?
• Serial monitoring of SpO2:FiO2 ratio can be helpful to decide on therapy continuation in outbreak situations when medical resources are limited.
Introduction
In November 2021, a new variant of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), named B1.1.529, was reported to the World Health Organization (WHO), which was subsequently named Omicron variant of SARS-CoV-2. The Omicron variant was found to be more infectious than the original SARS-CoV-2 virus (1). Since 31 Dec 2021, there had been an upsurge in the number of coronavirus disease 2019 (COVID-19) infected in Hong Kong (HK). This wave of infection peaked on 4 Mar 2022. As of 29 May 2022, there were more than one million positive cases reported (2,3).
The very high transmissibility of the Omicron variant resulted in a sudden surge of healthcare demand in HK (4). Due to the low rate of vaccination among the elderly, more than 340,000 patients infected with the Omicron variant were >60 years old (2). They were at a much higher risk of severe infection including respiratory failure, especially for those with multiple comorbidities (3,5).
High flow nasal cannula (HFNC) therapy has been shown to improve oxygenation in patients with acute hypoxemic respiratory failure (AHRF) in the FLORALI Study (6), and it has been used in this group of patients since the last decade (7). HFNC has been shown to reduce intubation in patients with severe COVID-19 infection (8). WHO proposed the use of HFNC therapy as a treatment of mild acute respiratory distress syndrome (ARDS) in COVID-19 infected patients (9). The majority of the studies have been performed in the intensive care unit (ICU) settings. Studies investigating the efficacy of HFNC in improving oxygen saturation in patients with respiratory failure outside the ICU setting were limited (10,11).
During this wave of COVID-19 infection, HK faced a crisis situation. Many patients with COVID-19 associated AHRF were managed in general wards due to inadequate ICU beds. Many of these patients were frail with multiple comorbidities. Unlike practice in other countries (12), HFNC therapy had been rarely used outside the ICU setting in HK prior to 2022. To deal with the crisis, the use of HFNC therapy was implemented in the general wards in two hospitals under the authors’ care. HFNC was given to patients who required >4 L/min O2 via nasal cannula to maintain SpO2 ≥92% (13).
The primary objective of this study is to assess the 28-day mortality of this group of patients. The secondary objective is to explore any predictors of non-survival to help clinical decision-making in future crisis. We present this article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1507/rc).
Methods
This retrospective cohort study was conducted in 6 COVID-19 general medical wards of the Princess Margaret Hospital (PMH), and 4 COVID-19 general wards of the North Lantau Hospital (NLTH) in HK. All patients admitted during the period of 17 March to 30 April 2022 with confirmed SARS-CoV-2 infection, who developed AHRF requiring HFNC support, were recruited. Those patients fulfilling these inclusion criteria but who used HFNC treatment for indications other than AHRF or were subsequently admitted to ICU were excluded.
The following data were retrieved from the clinical records for each recruited patient: demographic (age, sex, comorbidity), clinical (care plan decision, length of hospital stay, 28-day outcome), monitoring [baseline and serial oxygen saturation SpO2/inspiratory oxygen ratio FiO2 ratio (SF ratio) after HFNC therapy], and drug treatment.
Objective scores in describing patients’ underlying illnesses and the severity of their COVID-19 infection were used. The Charlson Comorbidity Index (CCI) was calculated according to the comorbidities of patients. The Clinical Frailty Scale (CFS) was used to describe frailty of the patients. The modified chest X-ray (CXR) scoring system was used for objective comparison of CXR changes due to COVID-19 infection (14). The Comorbidity-Age-Lymphocyte count-Lactate dehydrogenase (CALL) score for prediction for progression risk in patients with COVID-19 pneumonia was calculated, which included data on defined comorbidities, age >60 years old, lymphocyte count <1×109/L and lactate dehydrogenase (LDH) level >250 U/L (15). A CALL score of 4–6 points indicates <10% risk of progression of COVID-19 pneumonia while the risk was 10–40% and >50% for a CALL score 7–9 points and 10–13 points, respectively.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee of the Kowloon West Cluster of the HK Hospital Authority (HA) [Reference No. KW/EX-22-046(172-02)], and individual consent for this retrospective analysis was waived.
Clinical setting and HFNC setup
The 6 COVID-19 general wards of PMH were in the Infectious Disease Centre (IDC) with standard airborne infection isolation room facility. The 4 COVID-19 wards of NLTH were converted from general wards; each equipped with high efficiency particulate air (HEPA) filters and strengthened ventilation. The HFNC device (Optiflow system) delivered up to 60 L/min of humidified gas admixture at 31, 34 or 37 °C. FiO2 was delivered in the range of 0.21–1.00 via a low resistance nasal cannula. HFNC was initiated by the in-charge physician if a patient required >4 L/min O2 via nasal cannula to maintain SpO2 ≥92%. A dedicated team of physicians with critical care training background then took over the management of the patient in the general wards as long as HFNC support was needed. The default settings of HFNC were 40 L/min flow and FiO2 40%. Settings were adjusted according to SpO2 and the respiratory status of patients. The patients were given anti-viral and anti-inflammatory drugs based on the prevailing guideline on the clinical management of adult patients with COVID-19 HK (13).
Statistical analysis
The Statistical Package for Social Sciences (Window version 22.0; SPSS Inc., Chicago, IL, USA) was used for analysis. Descriptive statistics were used to summarize patient demographics data. The Student’s t-test was used to compare continuous variables between the two groups, while the Chi square test or Fisher’s Exact test were used to compare categorical variables. A P value of less than 0.05 was considered statistically significant.
Results
During the study period, 460 SARS-CoV-2 infected patients were admitted to the IDC of PMH and to NLTH. Sixty-five patients required HFNC support in general wards. Nine were transferred to ICU for continuation of respiratory support. Fifty-six patients continued HFNC support in general wards. Seven patients who required HFNC for ventilator weaning or for humidification of airway were excluded. Forty-nine patients were treated for COVID-19 related respiratory failure and were included in the cohort study (Figure 1). There were no complications reported for the use of HFNC during the study period. The demographics, clinical characteristics, treatment and outcomes of the study population are summarized in Table 1. The mean age was 77.5. Thirty-four patients were male and 15 patients were female. Ten patients (20.4%) had received 2 doses of SARS-CoV-2 vaccine, and 24 patients (49%) did not receive any SARS-CoV-2 vaccination. Thirty-three patients (67.3%) received anti-viral treatment while 16 patients (32.7%) did not receive any anti-viral treatment due to delayed presentation. Forty-eight patients (98%) received dexamethasone treatment. Twenty-two patients (44.9%) had positive cytobacteriological growth in sputum culture.
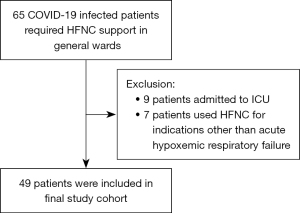
Table 1
| Characteristics, treatments and laboratory results | Numbers (N=49) | 28-day mortality | P value | |
|---|---|---|---|---|
| No (N=26) | Yes (N=23) | |||
| Age (years), mean ± SD | 77.5±11.9 | 75.9±12.1 | 79.2±11.6 | 0.343a |
| Sex (M:F) | 34:15 | 17:9 | 17:6 | 0.518b |
| Source | 0.130b | |||
| Home | 31 | 19 | 12 | |
| Old aged home | 18 | 7 | 11 | |
| Vaccination | 0.469b | |||
| No | 24 | 14 | 10 | |
| Yes | 25 | 12 | 13 | |
| Completed vaccination | 0.026 b | |||
| No | 42 | 25 | 17 | |
| Yes | 7 | 1 | 6 | |
| COVID drug | 0.357b | |||
| No | 16 | 10 | 6 | |
| Yes | 33 | 16 | 17 | |
| Remdesivir | 0.539b | |||
| No | 32 | 18 | 14 | |
| Yes | 17 | 8 | 9 | |
| Molnupivir | 0.947b | |||
| No | 36 | 19 | 17 | |
| Yes | 13 | 7 | 6 | |
| Paxlovid | 0.626b | |||
| No | 46 | 24 | 22 | |
| Yes | 3 | 2 | 1 | |
| Dexamethasone | 1.000c | |||
| No | 1 | 1 | 0 | |
| Yes | 48 | 25 | 23 | |
| Baricitinib | 0.868b | |||
| No | 40 | 21 | 19 | |
| Yes | 9 | 5 | 4 | |
| Tocilizumab | 0.480b | |||
| No | 46 | 25 | 21 | |
| Yes | 3 | 1 | 2 | |
| Low molecular weight heparin | 0.124b | |||
| No | 14 | 5 | 9 | |
| Yes | 35 | 21 | 14 | |
| Vasopressor | 0.189b | |||
| No | 40 | 23 | 17 | |
| Yes | 9 | 3 | 6 | |
| Abnormal troponin I* | 0.321b | |||
| No | 14 | 9 | 5 | |
| Yes | 33 | 16 | 17 | |
| D-dimer* | 0.622b | |||
| <1,000 | 12 | 7 | 5 | |
| >1,000 | 32 | 16 | 16 | |
| C-reactive protein* | 0.343b | |||
| <100 | 21 | 13 | 8 | |
| >100 | 27 | 13 | 14 | |
| Procalcitonin* | 0.238b | |||
| <0.5 | 18 | 13 | 5 | |
| >0.5 | 17 | 9 | 8 | |
| Positive sputum culture* | 0.154b | |||
| No | 26 | 16 | 10 | |
| Yes | 22 | 9 | 13 | |
a, Student’s t-test; b, Chi-square test; c, Fisher’s Exact test; *, detailed descriptions of missing date: 2 missing data of troponin I, 5 missing data of D-dimer, 1 missing data of C-reactive protein, 14 missing data of procalcitonin and 1 missing data of sputum culture. Listwise deletion for missing data is adopted in data analysis. SD, standard deviation; M, male; F, female.
The mean COVID-19 threshold cycle (CT) value of quantitative reverse transcriptase polymerase reaction of the cohort was 22.32. The mean CCI was 5.55. The mean CFS frailty score was 5.27. The mean CALL score was 11.3. The mean CXR score was 9.16. Thirty-six patients (73.5%) had a “Do-Not-Attempt-Cardiopulmonary-Resuscitation (DNACPR)” order, 34 patients (69.4%) had a “do not intubate (DNI)” order. The mean CFS was 5.42 for those patients with a DNACPR order.
Twenty-six patients (53.1%) survived at the 28-day after initiation of HFNC support. Twenty-three patients (46.9%) survived their index hospital admission. For the 36 patients with DNACPR order, 28-day mortality was 61.1% (22 patients) while 11 (30.6%) patients survived the index hospital admission.
The mean SF ratios for 28-day survivors and non-survivors were 175.89 and 136.70 at 2 hours, 189.01 and 134.32 at 24 hours, 228.43 and 157.86 at 48 hours, and 243.51 and 143.70 at 72 hours after initiation of HFNC respectively. Survivors had a statistically higher SF ratio at 2, 24, 48 and 72 hours as compared to non-survivors (P<0.05). Comparing with the SF ratio at the time of HFNC therapy initiation, if there was no significant improvement in SF ratio at 48 or 72 hours, the probability of 28-day mortality was higher (Figure 2).

Receiver operating characteristic (ROC) curve of SF ratio at 48 and 72 hours, which showed the greatest difference between survivors and non-survivors, were plotted respectively to identify threshold value to predict mortality. At 48 hours, an SF ratio of <160 had 92% sensitivity and 75% specificity in predicting mortality. The accuracy of using SF ratio at 48 hours to predict mortality was 79%. At 72 hours, an SF ratio of <191 had 83% sensitivity and 79% specificity in predicting mortality. The accuracy of using SF ratio at 72 hours to predict mortality is 86% (Figure 3).

There was no statistically significant difference in the CALL score, CXR score, CCI between survivors and non-survivors at 28-day.
Discussion
We assessed the effectiveness of HFNC therapy for use in COVID-19 related AHRF outside the ICU setting in two acute hospitals in HK. van Steenkiste et al. performed a retrospective cohort study on the hospital survival of 32 COVID-19 infected patients supported with HFNC therapy in general wards in a large non-academic hospital in the Netherlands (16). The overall CFS was 4 and 25% of patients survived at hospital discharge. Out of the 49 patients in our cohort, the mean CFS was 5.27 and 28-day survival rate was 53.1%. van Steenkiste et al. concluded that HFNC in general wards could be a potential rescue therapy for respiratory failure in vulnerable COVID-19 infected patients. Result of our study shows similar findings and supported the use of HFNC therapy in general ward.
Issa et al. studied the use of HFNC for patents with COVID-19 outside ICU (17). Among the 41 patients included, the mortality rate was only 30%. In this cohort, 20 patients received HFNC therapy as a step-down measure from ICU and mortality was 9.5%. Mortality in the step-up group was 29% and more than half of the group was admitted to ICU. We had excluded patients using HFNC as a step-down measure in our cohort and patients who were subsequently admitted to ICU. As a result, it would not be appropriate to compare the mortality of our cohort with the study performed by Issa et al.
Wang et al. studied a cohort of 27 COVID-19 infected patients with severe acute respiratory failure (18). Among 17 patients who had received HFNC therapy, 11 patients (64%) with an PaO2/FiO2 (PF) ratio ≤200 mmHg at the time of HFNC initiation, required escalation in respiratory support while none of the 6 patients with PF ratio >200 mmHg required escalation in respiratory support. The mean SF ratio at the time of initiation of HFNC was <200 mmHg in our cohort, which would have been predicted by Wang’s study to require escalation in respiratory support. Our cohort still managed to have 53.1% 28-day survival, which further suggested the clinical utility of HFNC therapy in this setting.
Ratio of oxygen saturation (ROX) index, defined as SF ratio to respiratory rate (RR) ratio, has been advocated as a monitoring tool for the detection of HFNC failure (19). However, Badawy et al. showed that RR was not recorded accurately by hospital personnel (20). There was rightward skew and a ‘spot’ estimate with values of 18 and 20 breaths per minute was frequently recorded. Since the SF ratio had also been found to be useful in monitoring patients on HFNC support (21) and the RR parameter was not documented for many of our patients accurately, the ROX index was not used in our study.
There was a statistically significant correlation between lower SF ratio at 2, 24, 48 and 72 hours after starting HFNC and 28-day mortality of COVID-19 infected patients. SF ratio <160 at 48 hours and SF ratio <191 at 72 hours of HFNC initiation were most predictive of mortality in our cohort. Applying these clinical indicators may help to identify patients who are unlikely to benefit from continuing respiratory support, without jeopardizing those who may potentially benefit. This is well illustrated by the notable 28-day mortality rate of 30.6% in the group of patients with DNACPR order in our cohort who might have been excluded from this treatment otherwise. This result is comparable with the result of the study performed by Peters et al. (22). However, our study is not designed to evaluate whether those patients may survive without HFNC support.
When there is no improvement in SF ratio at 48 or 72 hours as compared to the baseline ratio, the life-sustaining treatment should be considered futile. At this point, the health care team should consider discussing and reviewing the care plan with the patient, family or guardian work out a well-defined set of therapeutic goals and end points, which will include withdrawal of treatment (23). A time-limited trial, which usually lasts for a few days, can be used to assess the response to the treatment. If at the end of this trial, no progress is made towards the agreed therapeutic goals, futility is established, and resolution can then be jointly reached to withdraw the life-sustaining treatment (24). This approach may avoid unnecessarily prolonged use of HFNC support and possibly other life-sustaining therapy in medically futile patients, especially in the resource limited outbreak setting.
Limitations
Our study presents several limitations. Due to the retrospective nature of the study, there might be issues of patient selection at initiation of HFNC support. When the HFNC machines were available, the number of COVID-19 AHRF patients started to decrease. As a result, the number of enrolled patients was limited. Furthermore, there was no control group in our study and thus effect of HFNC on 28-day mortality could not be fully ascertained. It would not be possible to examine the effect of organ dysfunction other than respiratory failure on mortality. Not all patients receiving HFNC support were connected to a physiological monitor with measurement of RR or had their arterial blood gas checked. We were not able to obtain accurate RR data and PaO2 results. As a result, ROX index could not be calculated and captured in our study. SpO2 measured by pulse oximeter might not be reliable when patient became very ill which might affect the SpO2:FiO2 ratio.
Conclusions
Use of HFNC oxygen therapy outside the ICU setting for COVID-19 related AHRF is feasible and useful. If standard ICU management is not available, HFNC outside the ICU setting can be considered. Our study showed a statistically significant correlation between lower SF ratio at 2, 24, 48 and 72 hours after starting HFNC and increased 28-day mortality of COVID-19 infected patients. Monitoring with SF ratio thus helps to identify patients who may not benefit from prolonged HFNC support, especially in patients with DNACPR order. Such information can guide clinical decision-making for medical resource allocation in outbreak situations.
Acknowledgments
We thank the doctors and nurses of the Department of Medicine & Geriatrics and Infectious Diseases Team of Princess Margaret Hospital and North Lantau Hospital, for taking care of the patients during the fifth wave of the COVID-19 pandemic.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1507/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1507/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1507/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1507/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee of the Kowloon West Cluster of the HK Hospital Authority (HA) [Reference No. KW/EX-22-046(172-02)], and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Enhancing response to Omicron SARS-CoV-2 variant: technical brief and priority actions for member states. Update 2022;6:21.
- Centre for Health Protection, Hong Kong. Accessed on 31 May 2022. Available online: https://chp.gov.hk/files/pdf/local_situation_covid19_en.pdf
- Smith DJ, Hakim AJ, Leung GM, et al. COVID-19 Mortality and Vaccine Coverage - Hong Kong Special Administrative Region, China, January 6, 2022-March 21, 2022. MMWR Morb Mortal Wkly Rep 2022;71:545-8. [Crossref] [PubMed]
- Cheng VC, Wong SC, Au AK, et al. Explosive outbreak of SARS-CoV-2 Omicron variant is associated with vertical transmission in high-rise residential buildings in Hong Kong. Build Environ 2022;221:109323. [Crossref] [PubMed]
- Zhu J, Wei Z, Suryavanshi M, et al. Characteristics and outcomes of hospitalised adults with COVID-19 in a Global Health Research Network: a cohort study. BMJ Open 2021;11:e051588. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]
- Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA 2021;326:2161-71. Erratum in: JAMA 2022;327:1093. [Crossref] [PubMed]
- Ni YN, Luo J, Yu H, et al. Can high-flow nasal cannula reduce the rate of endotracheal intubation in adult patients with acute respiratory failure compared with conventional oxygen therapy and noninvasive positive pressure ventilation?: A systematic review and meta-analysis. Chest 2017;151:764-75. [Crossref] [PubMed]
- World Health Organization. Living guidance for clinical management of COVID-19. 23 Nov 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf?sequence=1&isAllowed=y
- Zemach S, Helviz Y, Shitrit M, et al. The Use of High-Flow Nasal Cannula Oxygen Outside the ICU. Respir Care 2019;64:1333-42. [Crossref] [PubMed]
- Pirret AM, Takerei SF, Matheson CL, et al. Nasal high flow oxygen therapy in the ward setting: A prospective observational study. Intensive Crit Care Nurs 2017;42:127-34. [Crossref] [PubMed]
- Guy T, Créac'hcadec A, Ricordel C, et al. High-flow nasal oxygen: a safe, efficient treatment for COVID-19 patients not in an ICU. Eur Respir J 2020;56:2001154. [Crossref] [PubMed]
- Hong Kong Hospital Authority Central Committee on Infectious Diseases and Emergency Response. Interim Recommendation on Clinical Management of Adult Cases with Coronavirus Disease 2022;2019:5.
- Setiawati R, Widyoningroem A, Handarini T, et al. Modified Chest X-Ray Scoring System in Evaluating Severity of COVID-19 Patient in Dr. Soetomo General Hospital Surabaya, Indonesia. Int J Gen Med 2021;14:2407-12. [Crossref] [PubMed]
- Ji D, Zhang D, Xu J, et al. Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin Infect Dis 2020;71:1393-9. [Crossref] [PubMed]
- van Steenkiste J, van Herwerden MC, Weller D, et al. High-flow Nasal Cannula therapy: A feasible treatment for vulnerable elderly COVID-19 patients in the wards. Heart Lung 2021;50:654-9. [Crossref] [PubMed]
- Issa I, Söderberg M. High-flow nasal oxygen (HFNO) for patients with Covid-19 outside intensive care units. Respir Med 2021;187:106554. [Crossref] [PubMed]
- Wang K, Zhao W, Li J, et al. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care 2020;10:37. [Crossref] [PubMed]
- Roca O, Messika J, Caralt B, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: The utility of the ROX index. J Crit Care 2016;35:200-5. [Crossref] [PubMed]
- Badawy J, Nguyen OK, Clark C, et al. Is everyone really breathing 20 times a minute? Assessing epidemiology and variation in recorded respiratory rate in hospitalised adults. BMJ Qual Saf 2017;26:832-6. [Crossref] [PubMed]
- Kim JH, Baek AR, Lee SI, et al. ROX index and SpO2/FiO2 ratio for predicting high-flow nasal cannula failure in hypoxemic COVID-19 patients: A multicenter retrospective study. PLoS One 2022;17:e0268431. [Crossref] [PubMed]
- Peters SG, Holets SR, Gay PC. High-flow nasal cannula therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care 2013;58:597-600. [Crossref] [PubMed]
- Hospital Authority Head Office Operations Circular No. 8/2020. Review of Hospital Authority Clinical Ethics Committee (HACEC). Guidelines related to end-of-life decision-making. 2 July 2020.
- Singer PA, Barker G, Bowman KW, et al. Hospital policy on appropriate use of life-sustaining treatment. University of Toronto Joint Centre for Bioethics/Critical Care Medicine Program Task Force. Crit Care Med 2001;29:187-91. [Crossref] [PubMed]


