
Left ventricular assist device implantation combined with hemiarch replacement for severe aortic atherosclerosis
Highlight box
Surgical highlights
• Hemi replacement with simultaneous left ventricular assist device (LVAD) implantation to avoid outflow graft anastomosis to a severe atherosclerotic aorta.
What is conventional and what is novel/modified?
• Conventionally, the outflow graft of the LVAD can be anastomosed to either the ascending aorta or other arteries, such as the descending aorta.
• Through modification of the conventional approach, the LVAD outflow graft can be sutured to the artificial aortic graft after replacing the diseased ascending aorta entirely.
What is the implication, and what should change now?
• To reduce the risk of stroke following LVAD insertion, a thorough evaluation of the condition of the thoracic aorta is necessary prior to surgery. Concomitant aortic surgery can be considered as an alternative in patients with severe atherosclerosis or calcification in the aorta.
Introduction
Left ventricular assist device (LVAD) implantation has been a widely used treatment for selected patients with advanced heart failure. Rates of LVAD-associated stroke are reported to range from 10% to 30% depending on the follow-up period (1,2).
Concomitant aortic surgeries in patients requiring LVAD implantation are uncommon, and an aortic replacement with simultaneous LVAD implantation to avoid outflow graft anastomosis to a severe atherosclerotic aorta has not been reported. We describe a successful LVAD implantation combined with hemiarch replacement for severe aortic atherosclerosis. We present this article in accordance with the SUPER reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-255/rc).
Preoperative preparations and requirements
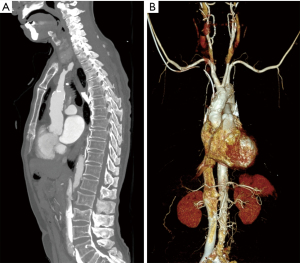
A 72-year-old male patient developed ischemic cardiomyopathy 11 years previously. Recently, the patient presented with symptoms of congestive heart failure classified as New York Heart Association Class IV (3). Echocardiography showed severe left ventricular systolic dysfunction with an ejection fraction of 19% and a dilated left ventricle (70 mm). Computed tomography (CT) revealed severe atherosclerosis of the ascending aorta (Figure 1). There were no preoperative neurological deficits observed, and no abnormalities were observed on brain magnetic resonance imaging. Since we had determined that the patient’s aorta was unclampable, and besides, risky for future embolism, LVAD implantation with hemiarch replacement for the purpose of destination therapy was planned. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Seoul St. Mary’s Hospital (No. KC23ZASI0127) and with the Declaration of Helsinki (as revised in 2013). Written Informed Consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
Under general anesthesia, right axillary access was used for arterial perfusion with an 8-mm vascular graft to avoid cannulation of the ascending aorta. After a median sternotomy, right atrial cannulation was performed for cardiopulmonary bypass (CPB). After initiating CPB, the left ventricular apex was accessed for pump core implantation (HeartMate 3). The device was implanted and then the drive line tunnel was created.
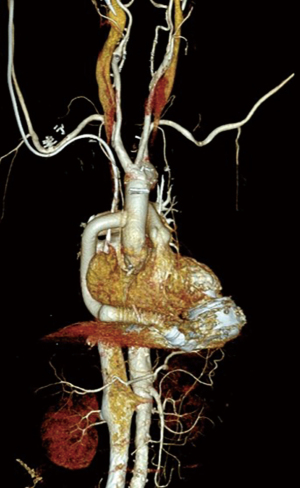
Under moderate hypothermia (28 ℃), the innominate artery was clamped. As resection of the atherosclerotic ascending aorta began, systemic circulatory arrest was also initiated. Bilateral antegrade cerebral perfusion was instituted using an additional perfusion catheter in the left common carotid artery. After cardioplegic solution was administrated, distal aortic anastomosis for the hemiarch replacement was performed immediately proximal to the innominate artery using a single branch 24-mm Hemashield graft (Figure 2A). The proximal prosthetic graft anastomosis was finished during full systemic perfusion with rewarming. The HeartMate 3 outflow graft was anastomosed to the ascending aortic graft in an end-to-side fashion using 4-0 prolene (Figure 2B). After the outflow graft was de-aired, the LVAD was turned on, and weaning from CPB was attempted. CPB time was 112 min. Distal ischemic time was 23 min. Bilateral regional cerebral oxygen saturation values did not drop below 55% during the operation.

Postoperative considerations and tasks
The patient was extubated on postoperative day (POD) 0. There were no abnormal neurological signs. Postoperative CT scan on POD 9 showed a well-placed aortic graft and the implanted LVAD (Figure 3). After being discharged from our hospital on POD 25, the patient has been under outpatient observation for 137 days following the surgery, and no unusual complications have been observed.
Tips and pearls
When performing an anastomosis of the LVAD outflow graft to the ascending aorta, it is important to accurately identify areas of atherosclerosis or calcification in the ascending aorta using imaging techniques such as CT or epiaortic ultrasonography. This information should be taken into consideration when determining the extent and approach of the surgery to prevent stroke.
Discussion
LVAD implantation with simultaneous thoracic aortic replacement has been rarely reported (4,5), and most of the cases used cannulation and cross clamping of the ascending aorta for arterial perfusion. Though a total arch replacement with LVAD support has been reported, the aortic surgery was performed for aortic aneurysm, not for avoiding stroke from atherosclerosis (6).
The effects of LVAD flow on aortic wall have been investigated. Shear stress due to ventricular assist device flow is reported to cause endothelial damage and atherosclerotic change in native vessels (1). Significantly increased medial degeneration and atherosclerotic changes after LVAD support have also been reported (7). Therefore, we determined that replacing the entire diseased ascending aorta would be beneficial not only in terms of short-term results but also in preventing stroke in this patient from a long-term perspective.
There have been several efforts to avoid outflow graft anastomosis at the ascending aorta (8,9). However, most of the methods require femoral arterial cannulation for cardiopulmonary bypass, which can promote cerebral embolism. Outflow graft anastomosis at the axillary artery can cause swelling of the upper extremity and requires cumbersome procedures including tunneling of the outflow graft, and even anastomosis of the outflow graft at the descending aorta did not decrease number of readmissions or hospital length of stay per year for cerebrovascular accidents (10).
There are several points for discussion including limitations of this study. One of the potential drawbacks of this case is the possibility that asymptomatic cerebral infarction was not detected through postoperative brain imaging. We opted for LVAD implantation for destination therapy in this patient. However, had we performed it as a bridge to transplant, we anticipate there could be some potential drawbacks to consider. Specifically, significant adhesions around the replaced ascending aortic graft may need to be removed during subsequent heart transplantation, and there could also be difficulties during not only the arterial cannulation for CPB, but also the aortic anastomosis with or without removal of the artificial graft that was replaced. Therefore, it is important to take these limitations into account when planning for LVAD implantation combined with hemiarch replacement as a bridge to transplant.
We chose for hemiarch replacement to avoid applying an aortic clamp and replace the entire diseased ascending aorta. Depending on the degree of aortic disease, an alternative approach could be to connect the outflow graft selectively to the healthy aortic wall following circulatory arrest after aortotomy without clamping the aorta. Alternatively, depending on the circumstances, it may be feasible to replace just the severely affected part of the aorta, rather than the entire aorta.
Cases of LVAD implantation with concomitant aortic replacement are quite rare. Long-term outcomes of the patients who have undergone combined aortic procedures as in our case are unknown, but we could not leave the calcified ascending aorta because partial clamping and anastomosis to the aorta carried a high risk of stroke and aortic dissection. So far, we have hardly found reports of aortic replacement in LVAD cases like the one we describe, aimed toward avoiding outflow graft anastomosis to a severely atheromatous aorta.
Conclusions
In summary, our experience suggests that LVAD implantation with aortic replacement might be feasible in select patients, especially those at high risk for perioperative stroke from aortic atherosclerosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-255/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-255/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-255/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Seoul St. Mary’s Hospital (No. KC23ZASI0127) and with the Declaration of Helsinki (as revised in 2013). Written Informed Consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Plecash AR, Byrne D, Flexman A, et al. Stroke in Patients with Left Ventricular Assist Devices. Cerebrovasc Dis 2022;51:3-13. [Crossref] [PubMed]
- Gosev I, Wood K, Ayers B, et al. Implantation of a fully magnetically levitated left ventricular assist device using a sternal-sparing surgical technique. J Heart Lung Transplant 2020;39:37-44. [Crossref] [PubMed]
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, Mass: Little, Brown & Co; 1994:253-6.
- Gode S, Erkanlı K, Başgoze S, et al. Left Ventricular Assist Device Implantation Combined with Bentall Procedure. Braz J Cardiovasc Surg 2019;34:233-6. [Crossref] [PubMed]
- Huenges K, Panholzer B, Cremer J, et al. Left Ventricular Assist Device Implantation with Concomitant Aortic Valve and Ascending Aortic Replacement. Case Rep Med 2018;2018:9057351. [Crossref] [PubMed]
- Akiyama M, Hosoyama K, Kumagai K, et al. Continuous flow left ventricular assist device implantation concomitant with aortic arch replacement and aortic valve closure in a patient with end-stage heart failure associated with bicuspid aortic valve. J Artif Organs 2015;18:365-9. [Crossref] [PubMed]
- Segura AM, Gregoric I, Radovancevic R, et al. Morphologic changes in the aortic wall media after support with a continuous-flow left ventricular assist device. J Heart Lung Transplant 2013;32:1096-100. [Crossref] [PubMed]
- El-Sayed Ahmed MM, Aftab M, Singh SK, et al. Left ventricular assist device outflow graft: alternative sites. Ann Cardiothorac Surg 2014;3:541-5. [PubMed]
- Riebandt J, Sandner S, Mahr S, et al. Minimally invasive thoratec Heartmate II implantation in the setting of severe thoracic aortic calcification. Ann Thorac Surg 2013;96:1094-6. [Crossref] [PubMed]
- Dorken Gallastegi A, Hoşcoşkun EB, Kahraman Ü, et al. Long-Term Outcomes in Ventricular Assist Device Outflow Cannula Anastomosis to the Descending Aorta. Ann Thorac Surg 2022;114:1377-85. [Crossref] [PubMed]


