
Patient-specific stent for hemolytic anemia due to a kinked ascending aortic graft
Highlight box
Surgical highlights
• Placing patient-specific bare-metal stent inside the kinked ascending aortic graft to relieve the mechanical hemolysis.
What is conventional and what is novel/modified?
• Conventionally, the kinked ascending aortic graft can be replaced with a new graft through a redo sternotomy for thoracic aortic surgery.
• This novel approach involves the insertion of a bare metal stent within the kinked aortic graft under local anesthesia to alleviate the degree of kinking.
What is the implication, and what should change now?
• For patients considered high-risk for redo thoracic aortic surgery, an endovascular approach involving the insertion of a bare metal stent into the kinked aortic graft under local anesthesia may be considered to alleviate mechanical hemolysis caused by the kinked graft.
Introduction
Hemolytic anemia after thoracic aorta surgery is rare (1,2). About 20% of cases of hemolytic anemia after aortic surgery are attributed to kinking of the aortic graft (2), and most of these kinked grafts are treated by reoperation. We describe the insertion of a patient-specific bare-metal stent for the treatment of hemolytic anemia in a patient with a kinked aortic graft. We present this article in accordance with the SUPER reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-688/rc).
Preoperative preparations and requirements
A 69-year-old woman with a history of ascending aorta replacement for acute type A aortic dissection 7 years ago was transferred from another hospital for treatment of hemolytic anemia requiring frequent blood transfusions. About a month ago, the Hematology Department of another hospital diagnosed this patient with anemia and started administering frequent transfusions of one unit of packed red blood cells (400 mL) per day before they were transferred to our hospital. Hemolytic anemia was suspected due to low haptoglobin levels and high lactate dehydrogenase (LDH) levels, but a direct Coombs test and other autoimmune studies yielded negative results. The patient was suspected to have mechanical hemolytic anemia resulting from a previous aortic surgery performed at another hospital, and was transferred to our hospital’s cardiovascular surgery department with the expectation of possible surgical or procedural treatment.
Relevant laboratory findings included hemoglobin, 7.2 g/dL (normal range, 12.0–16.0); hematocrit, 22.8% (normal range, 34.0–49.0%); LDH, 1,084 IU/L (normal range, 0–250); reticulocyte count, 4.94% (normal range, 0.2–2.0%); and haptoglobin, 17 mg/dL (normal range, 30–200). Schistocytes in the peripheral blood were grade +3. The ADAMTS13 assay (3) revealed an enzyme activity of 69.3% (normal range, 50–160%) and the patient had normal kidney function, as indicated by a creatinine level of 0.76 mg/dL (normal range, 0.5–0.9), ruling out thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS). Various tests for microbial culture were negative, thereby excluding hemolysis due to infection or other causes. The initial chest computed tomography (CT) showed a sharply kinked prosthetic graft (Figure 1A) and confirmed the diagnosis of microangiopathic hemolytic anemia (MAHA) due to a kinked aortic graft.

The patient was bedridden from 1 year ago with vascular dementia, vertebral compression fracture, and paraplegia from a spinal epidural hematoma as comorbidities. Also, the patient’s Katz frailty index was 0. Furthermore, there is a mortality risk of up to 15% in reoperation after replacement of the thoracic aorta (4). Therefore, we planned an endovascular intervention with local anesthesia rather than a redo surgery. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Seoul Saint Mary Hospital (No. KC23ZASI0002) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s family for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
We decided to use a bare-metal stent oversized by 15%. The size of the previous prosthetic aortic graft was 28 mm, and we chose a 32 mm × 80 mm patient-specific bare-metal stent (S & G BIOTECH, Yongin-si, Gyeonggi-do, Republic of Korea) based on a model of the Hercules vascular stent (a proprietary stent with a diameter of 24 mm and a length of 80 mm). To mitigate the risk of arch vessel occlusion resulting from stent migration, we chose to use a bare metal stent.
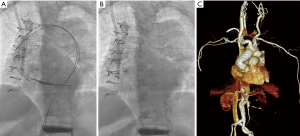
After local anesthesia, diagnostic angiography from the left common femoral artery (CFA) showed the acute angle of the folded graft (Figure 1B). A stiff guidewire was advanced into the left ventricle from the right CFA, and another stiff guidewire was introduced from the left CFA into the ascending aortic graft for additional support (Figure 1C). The stent delivery system was introduced through 16-Fr sheath in the right CFA and the main body was deployed. Several rounds of ballooning in the bare-metal stent widened the kinked area of the aortic graft (Figure 2A,2B). Using a 46-mm Reliant stent graft balloon catheter (Medtronic Inc., 710 Medtronic Parkway N. E. Minneapolis, MN 55432, USA), we performed balloon inflation while observing the shape of the contrast agent inside the balloon, which was initially bent and then straightened, while manually adjusting the pressure. The total procedure time was 124 minutes. The fluoroscopic procedure lasted for 23 minutes, and a total of 120 mL of contrast agent (Iodixanol) was used.
Postoperative considerations and tasks
The chest CT after the intervention suggested a well deployed stent and mild reduction of the angulated graft (Figure 2C). Postoperatively, the patient’s hematocrit gradually increased to 39.5% and the schistocytes decreased to grade +1. Additionally, there was a decrease in LDH value to 334 IU/L and reticulocyte count to 1.68%. The most significant improvement was the reduction in the frequency of packed red blood cell transfusions, which had been frequent prior to surgery, to approximately once every two weeks. Very unfortunately, the patient died 32 days after the procedure with fungal pneumonia.
Tips and pearls
It is crucial to ensure that no damage is inflicted upon the aortic valve when placing the stiff wire in the left ventricle.
To mitigate the risk of arch vessel occlusion due to stent migration, it is advisable to opt for a bare metal stent.
Discussion
The main reason for graft kinking is inappropriate graft length, and the proper length and size of the graft must be carefully determined to prevent kinking of the graft. While several methods for correcting the kinked graft without cardiopulmonary bypass and aortic cross clamp have been described (5,6), these techniques have usually been applied immediately after graft interposition at the first surgery. Most cases of graft-associated hemolytic anemia have been treated by second surgeries to replace the graft (2). So far, we have hardly found reports of endovascular approaches like the one we describe to alleviate fold formation in the inner surface of a kinked graft.
To determine the cause of the patient’s hemolytic anemia, it took a significant amount of time to completely exclude infectious diseases or internal issues like TTP or HUS, so the patient was transferred to our hospital. Additionally, it is believed that the long-term use of steroids for the purpose of treating such misdiagnosed diseases may have eventually caused fungal pneumonia. Hence, diagnosing mechanical hemolytic anemia can be time-consuming since other conditions need to be ruled out, emphasizing the importance of prompt diagnosis and treatment for a patient’s prognosis.
In this case, it took a considerable amount of time for hemolytic anemia to occur after the initial aortic surgery, making it challenging to pinpoint the aortic surgery site as the cause of the hemolytic anemia. However, it is important to note that mechanical hemolytic anemia can manifest not only immediately after cardiovascular surgery, but also after a prolonged period of time. The onset of hemolysis can be delayed for as long as 180 months after surgery (1). Although the precise mechanism of this delayed hemolysis is not fully understood, it can be speculated that graft kinking itself may occur gradually due to progressive growth of the surrounding tissues, or that stenotic portions within the graft worsen over time. In the case of this patient, since CT images from immediately after the previous aorta surgery were not available, it was impossible to determine when the graft kinking became severe.
There are some limitations in this case. The stent placed in the area where the ascending aorta was replaced with a graft did not fully straighten out, possibly due to severe adhesion around the replaced aorta after a long period. Furthermore, there is uncertainty about whether hemolysis was completely resolved since the patient died, and this could not be proven over an extended period. Nonetheless, there is clinical significance in attempting to solve the problem as non-invasively as possible to avoid repeat sternotomy in high-risk surgical patients. It is also believed that some significance can be found in the fact that the patient’s MAHA levels improved and the frequency of blood transfusions decreased.
Prior to the procedure, we attempted to assess the pressure gradient through a preoperative transthoracic echocardiogram. However, evaluating the blood flow within the kinked ascending aortic graft proved challenging due to poor echo window resulting in a failed attempt. Performing a transesophageal echocardiogram would have provided clear diagnostic evidence. However, considering the patient’s comorbidities, we deemed transesophageal echocardiogram, which requires sedation, to carry risks. In fact, even the main procedure we performed, stent insertion, was conducted under local anesthesia, as the use of sedative agents solely for diagnostic purposes posed concerns. Nevertheless, through interdisciplinary discussions, we eliminated other potential medical diagnoses and treatments, ultimately determining that the kinked aortic graft was the underlying cause of hemolysis. Through discussions with the patient and her family, we decided to pursue minimally invasive treatment options rather than riskier surgical interventions. Therefore, we attempted stent insertion under local anesthesia instead of general anesthesia. It was originally intended as an effort to improve the hemolytic condition rather than achieve complete correction. We provided sufficient explanation of the advantages and disadvantages of the procedure to the patient and her family, and obtained their consent.
A significant pressure gradient could not be obtained from the kinked graft during the procedure. It is reported that hemolysis after aortic surgery can be caused not only by the main causes of graft kinking and stenosis, but also by rare occurrences such as a folded elephant trunk or an inverted felt strip (1,2).
It is not always the case that a significant difference in the measured pressure gradient based on the valve is observed when hemolysis occurs in patients with artificial heart valves compared to those without hemolysis (7,8). Hemolysis can occur due to various factors such as turbulence, shear stress, contact with prosthetic material, and compression, leading to damage (9). However, if magnetic resonance imaging had been used to capture changes in turbulent flow before and after the procedure, even better evidence could have been obtained in this case.
We selected the size of the stent to be approximately 15% oversized compared to the existing artificial aortic graft. However, it should be noted that there is no objective evidence supporting this specific choice. It is anticipated that many more cases or clinical trials will be necessary to address the issue of selecting the appropriate size in the future.
Through this experience, we have come to the cautious judgment that prompt treatment should be considered in patients suspected of mechanical hemolysis, even if there is no clear evidence of infection and there is prominent evidence indicating MAHA, as a significant pressure gradient may not be measured.
Conclusions
In summary, our experience suggests that a patient-specific bare-metal stent in a kinked graft causing hemolytic anemia might be feasible in selected patients, especially those at high risk from reoperation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-688/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-688/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-688/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Seoul Saint Mary Hospital (No. KC23ZASI0002) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s family for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davison MA, Norton DM, Popoff AM, et al. Hemolysis following wrap aortoplasty for Type A aortic dissection repair: Case report and review of the literature. Vasc Med 2018;23:400-6. [Crossref] [PubMed]
- Kitahara H, Yoshitake A, Hachiya T, et al. Kinked Graft and Anastomotic Stenosis-Induced Hemolytic Anemia Requiring Reoperation. Ann Vasc Surg 2016;30:308.e1-4. [Crossref] [PubMed]
- Tso ACY, Sum CLL, Ong KH. Reference range for ADAMTS13 antigen, activity and anti-ADAMTS13 antibody in the healthy adult Singapore population. Singapore Med J 2022;63:214-8. [Crossref] [PubMed]
- Estrera AL, Miller CC 3rd, Porat E, et al. Determinants of early and late outcome for reoperations of the proximal aorta. Ann Thorac Surg 2004;78:837-45; discussion 837-45. [Crossref] [PubMed]
- Zattera GF, Actis Dato GM, Del Ponte S, et al. A simple method to correct aortic tube graft kinking without cardiopulmonary by-pass and aortic clamping. Eur J Cardiothorac Surg 2000;18:611-2. [Crossref] [PubMed]
- Nezic D, Milicic M, Boricic M, et al. Modification of an Old Technique to Correct Kinking of Tubular Graft Interposed to Reconstruct Ascending Aorta. Heart Lung Circ 2021;30:e139-41. [Crossref] [PubMed]
- Laflamme J, Puri R, Urena M, et al. Incidence and risk factors of hemolysis after transcatheter aortic valve implantation with a balloon-expandable valve. Am J Cardiol 2015;115:1574-9. [Crossref] [PubMed]
- Suedkamp M, Lercher AJ, Mueller-Riemenschneider F, et al. Hemolysis parameters of St. Jude Medical: Hemodynamic Plus and Regent valves in aortic position. Int J Cardiol 2004;95:89-93. [Crossref] [PubMed]
- Maraj R, Jacobs LE, Ioli A, et al. Evaluation of hemolysis in patients with prosthetic heart valves. Clin Cardiol 1998;21:387-92. [Crossref] [PubMed]


