
Comparison of patient-reported outcomes and clinical outcomes between pleurectomy and pleural covering added to thoracoscopic bullectomy for primary spontaneous pneumothorax
Highlight box
Key findings
• In our cohort, parietal pleurectomy and pleural covering showed comparable patient-reported outcomes (PROs) and clinical outcomes in patients with primary spontaneous pneumothorax (PSP).
What is known and what is new?
• Treatment modality and surgical approach affects PROs in patients with PSP.
• Surgical procedures added to thoracoscopic bullectomy were evaluated by PROs in addition to clinical outcomes for PSP treatment.
What is the implication, and what should change now?
• To determine the optimal surgical procedure for PSP, further studies including a more detailed evaluation of PROs are warranted.
Introduction
Although the main component of surgery for primary spontaneous pneumothorax (PSP) is regarded as resection of the bulla/bleb (bullectomy), which causes air leakage resulting from its rupture, an additional procedure inducing pleural adhesion has an important role in reducing postoperative recurrence, and various methods have been attempted. Since an optimal procedure has not yet been established, an additional procedure varies in clinical practice among countries. Parietal pleurectomy (pleurectomy) or abrasion is recommended in the American College of Chest Physicians and British Thoracic Society (BTS) guidelines. However, in Japan, a visceral pleural covering with a medical prosthesis (covering) is most prevalently used, and patients who undergo parietal pleurectomy account for only approximately 1% of all PSP surgical cases (1-3).
At our institution, pleurectomy with bullectomy has been performed as a standard procedure for PSP, and we recently introduced covering along with the change of the chief surgeon. Consequently, the two procedures were performed in a single thoracic surgery department, according to the surgeon’s preference for each case. We considered this situation to be an optimal field for evaluating the two procedures and thus conceived the present study.
There are only two reports regarding the patient-reported outcomes (PROs) [also known and referred as patient-reported quality of life (QOL)] in surgical patients with pneumothorax. One study compared two different surgical approaches, namely video-assisted thoracic surgery (VATS) and anterolateral thoracotomy, in patients with primary and secondary pneumothorax (the total number of patients analyzed was 20) (4). Another study compared patients treated with chest tube drainage and those who underwent VATS bullectomy with pleurectomy (the total number of patients analyzed was 50) (5). In addition, a study investigated whether psychological distress in patients with spontaneous pneumothorax differed by age group, which could be generally considered an observational study of PROs (6). There has been no investigation comparing PRO between additional procedures to bullectomy, including pleurectomy versus covering for PSP. We launched a prospective PRO assessment study of patients who underwent elective thoracic surgery in our department during the same period.
This study aimed to compare PROs prospectively, perioperative outcomes retrospectively, and long-term outcomes cross-sectionally between pleurectomy and covering added to bullectomy in patients with PSP. Based on our clinical experience, we hypothesized that PRO, including postoperative pain, and surgical outcomes, including recurrence rates, would be comparable between the two procedures. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-214/rc).
Methods
Study design and patients
From January 2015 to April 2018, consecutive patients who underwent surgery for PSP at Hitachi General Hospital were enrolled in a prospective PRO assessment study. Secondary pneumothorax was defined according to the BTS guidelines (2). We performed surgery for PSP in 61 patients and excluded 6 patients from the analysis because 2 patients underwent only bullectomy and 4 patients lacked baseline data of the PRO assessment for unknown reasons. Therefore, we analyzed 55 patients. Although we did not set a lower age limit in the protocol, we enrolled one patient who was 14 years old because the patient could understand the content of the questionnaire and the parents gave oral consent with patient assent.
Our surgical indications for PSP were as follows: ipsilateral recurrence after non-surgical treatment or after spontaneous remission, air leakage persisting for ≥5 days, and social indications included patient preference. Additional surgical procedures for bullectomy were not randomized and were determined according to the surgeon’s preference for each case. In addition, patients, surgeons, and the study administrator were not blinded. One patient who was due for a pleurectomy during the informed consent process ultimately underwent covering according to the parent’s preference.
We reviewed each patient’s electronic medical records to obtain the following clinical and perioperative information: age, sex, history of ipsilateral PSP, affected side, smoking history, Eastern Cooperative Oncology Group performance status, preoperative chest drainage, chest computed tomography (CT) findings, C-reactive protein (CRP) levels, surgical indication, operative time, intraoperative blood loss, duration of postoperative chest tube placement, duration of postoperative hospital stay, postoperative adverse events within 30 days, ipsilateral postoperative pneumothorax recurrence, and treatment of recurrence. The following findings were investigated by CT review: type of lesion [no lesion/blebs (air-filled lesion <1 cm)/bullae (air-filled lesion 1 cm)], number of lesions (no lesion/single/multiple), and distribution of lesions (no lesion/unilateral/bilateral). Postoperative recurrence was defined as the reappearance or enlargement of the air space after chest tube removal, confirmed by chest radiography or CT. Since we evaluated the perioperative and long-term outcomes of the two procedures, we subdivided recurrence into perioperative (within 30 days postoperatively) and late (after 30 days postoperatively). Perioperative recurrence was also counted as a pulmonary fistula among postoperative adverse events, according to the Common Terminology Criteria for Adverse Events, version 4.0.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Ibaraki Hospital Headquarters at Hitachi, Ltd. approved this study (No. 2015-3, No. 2019-70) and this study was registered in UMIN Clinical Trials Registry (UMIN000017597). The need for written informed consent was waived because each questionnaire provided the respondent with the opportunity to refuse to answer, and we provided contact information for opting out of the study on our website.
Surgical procedure
The patients underwent one-lung ventilation with a double-lumen intubation tube under epidural and general anesthesia. The 12-mm thoracic ports were placed at the third intercostal midaxillary line, fifth intercostal anterior axillary line, and sixth intercostal posterior axillary line. We used a 10-mm 30-degree thoracoscope. Pulmonary lesions (apical pleural thickening, blebs, and bullae), with or without air leakage, were resected with endoscopic auto-suture devices (Endo GIA Ultra Universal Stapler, Covidien, Norwalk, CT, USA or Echelon 45 flex, Ethicon Endo-Surgery, Cincinnati, OH, USA). When there were multiple lesions or when the lesions located in a broad area for resection, we combined other techniques, such as bulla ligation and/or soft coagulation with a ball electrode. In our pleurectomy procedure, parietal pleurectomy was performed by blunt dissection. The caudal border of the pleurectomy was at the level of the fifth rib ventrally and the sixth rib dorsally. The cranial border was at the level of the first rib, and the pleura of the thoracic apex and mediastinum were spared to prevent nerve and vessel damage (Video 1). In the covering procedure, the edges of the staple line were ligated with a pre-tied absorbable monofilament loop ligature (ENDOLOOP ligature, Ethicon Endo-Surgery), and the apex of the upper lobe of the lung was covered with a polyglycolic acid (PGA) sheet (Neoveil, 10 cm × 10 cm; Gunze Ltd., Kyoto, Japan). In detail, we passed the ligatures of the ENDOLOOP through the Neoveil sheet in advance outside of the thoracic cavity, guided the sheet into the thoracic cavity along the ligatures, placed the sheet over the staple line, and finally ligated the ligatures over the sheet to prevent displacement of the sheet (Video 2). We neither performed parietal pleural abrasion nor used fibrin glue. A 16- or 20-French double lumen chest tube was placed, and a negative suction of 7 cmH2O was applied.
Postoperative care
Postoperative pain was managed with patient-controlled epidural anesthesia and oral analgesics. The chest tube was removed when the amount of drainage decreased to <200 mL per day without air leakage. When a slight air leakage was suspected, we performed a clamp test of the chest drain to confirm no air leakage. According to our clinical pathway, patients were discharged on postoperative day (POD) 5 if there were no adverse events. Patients visited the outpatient clinic 7–10 days after discharge and at approximately 1 month after the surgery to assess their physical condition with a blood test and chest radiography. When findings at the second visit were unremarkable, the outpatient clinic visit was terminated. Patients were instructed to visit the hospital if they had symptoms associated with pneumothorax.
Prospective PRO assessment
We used the Japanese version of the EuroQOL-5 dimensions-5 level (EQ5D) (registration No. 7772) (7). The EQ5D consists of a descriptive system and visual analog scale (VAS). The descriptive system comprised the following five dimensions: mobility, self-care, usual activity, pain/discomfort, and anxiety/depression. Each dimension contained five levels of response options: no problems, slight problems, moderate problems, severe problems, and extreme problems. The VAS records the patient’s self-rated general health status on a vertical scale of 0–100. One hundred represents “the best health status I can imagine”, whereas zero represents “the worst health status I can imagine”. The questionnaires were administered 1 day before surgery (pre) and at 1, 3, and 5 days and 1 month postoperatively (POD1/POD3/POD5, and POM1, respectively). The responsible thoracic surgeon handed the printed questionnaire directly to each patient. The patients returned the completed questionnaire to any hospital staff member.
Cross-sectional long-term survey
We performed a long-term postoperative survey in February 2020 on persisting symptoms associated with surgery, smoking habits, and recurrence via telephone and mail. We created a structured interview form for the telephone survey. For detection of recurrence, we asked the patients whether they had a medical visit for chest discomfort or pain or dyspnea and whether they had been diagnosed with pneumothorax postoperatively. According to the protocol, we tried to reach at least a second-degree family member by telephone when we could not reach the patient. If we could not reach the patient or a family member, we sent the survey form by mail. Telephone surveys were conducted by two thoracic surgeons (K Kobayashi and H Ichimura).
Statistical analysis
All data for continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as numbers and proportions. Significant differences between the groups were assessed using Student’s t-test or Wilcoxon’s rank sum test for continuous variables and the chi-square or Fisher’s exact test for categorical variables. The recurrence-free period was defined as the interval from the date of surgery to the date of recurrence confirmation. The follow-up period was defined as the period from the date of surgery to the date of confirmation of the results of the long-term survey. Patients that could not complete the long-term survey were censored at the date of the last visit, as confirmed by the medical record. The recurrence-free rate after each surgery was calculated using the Kaplan-Meier method, and comparisons between the covering and pleurectomy groups were made using the log-rank test. Differences were considered significant at P values <0.05. PASW Statistics 18 (SPSS, Chicago, IL, USA) was used to perform all analyses.
Results
Patient characteristics
We analyzed 55 patients (covering, 26; pleurectomy, 29). Patients’ demographic and clinical characteristics are presented in Table 1. None of the variables differed between the covering, and pleurectomy groups, except for the distribution of blebs or bullae (P=0.042).
Table 1
| Characteristics | Covering (n=26) | Pleurectomy (n=29) | P value |
|---|---|---|---|
| Age, years | 26.5 [14–49] | 26.4 [15–49] | 0.968 |
| Sex | 0.490 | ||
| Male | 20 (76.9) | 25 (86.2) | |
| Female | 6 (23.1) | 4 (13.8) | |
| History of ipsilateral PSP on admission | 0.825 | ||
| First onset | 10 (38.5) | 12 (41.4) | |
| Recurrence | 16 (61.5) | 17 (58.6) | |
| PSP site | 0.075 | ||
| Right | 9 (34.6) | 17 (58.6) | |
| Left | 17 (65.4) | 12 (41.4) | |
| Smoking history | 0.577 | ||
| Never | 18 (69.2) | 18 (62.1) | |
| Former or current | 8 (30.8) | 11 (37.9) | |
| PS | 0.237 | ||
| 0 | 14 (53.8) | 11 (37.9) | |
| >1 | 12 (46.2) | 18 (62.1) | |
| Preoperative chest tube placement (yes) | 12 (46.2) | 16 (55.2) | 0.504 |
| Presence of blebs or bullae | |||
| No lesion | 4 (15.4) | 0 (0.0) | 0.079 |
| Blebs (<1 cm) | 11 (42.3) | 17 (58.6) | |
| Bullae (>1 cm) | 11 (42.3) | 12 (41.4) | |
| Blebs or bullae | 0.107 | ||
| No lesion | 4 (15.4) | 0 (0.0) | |
| Single | 4 (15.4) | 7 (24.1) | |
| Multiple | 18 (69.2) | 22 (75.9) | |
| Distribution of blebs or bullae | 0.042* | ||
| No lesion | 4 (15.4) | 0 (0.0) | |
| Unilateral | 9 (34.6) | 7 (24.1) | |
| Bilateral | 13 (50.0) | 22 (75.9) | |
| Indications for surgery | 0.978 | ||
| Ipsilateral recurrent | 16 (61.5) | 18 (62.1) | |
| Persistent air leak (>5 days) | 5 (19.2) | 5 (17.2) | |
| Others | 5 (19.2) | 6 (20.7) |
Data are expressed as number (%) or mean [range]. *, P<0.05. PSP, primary spontaneous pneumothorax; PS, performance status.
Perioperative outcomes
The perioperative outcomes are shown in Table 2. In the pleurectomy group, the operative time was significantly longer than that in the covering group (87.1 vs. 68.3 min, P=0.004). The other outcomes (blood loss, postoperative drainage, hospital stay, and adverse events) were not significantly different between the groups.
Table 2
| Variables | Covering (n=26) | Pleurectomy (n=29) | P value |
|---|---|---|---|
| Operative time (min) | 68.3±21.3 | 87.1±24.3 | 0.004* |
| Blood loss (mL) | 0 (0.0) | 0 (0 to 50) | 0.174 |
| Postoperative drainage (days) | 2.2±0.8 | 2.1±0.4 | 0.579 |
| Postoperative hospital stays (days) | 5.2±1.0 | 5.8±4.1 | 0.469 |
| Postoperative adverse events: present | 1 (3.8) | 3 (10.3) | 0.613 |
| Pulmonary fistula | 1 (3.8) | 2 (6.9) | 0.542 |
| Pleural effusion | 0 (0.0) | 1 (3.4) | 0.527 |
Data are expressed as mean ± standard deviation, median (range) or number (%). *, P<0.05.
The trajectory of perioperative CRP levels is shown in Figure 1. Although the pleurectomy group had a significantly lower CRP level at POD4 than the covering group (3.2 vs. 5.5 mg/dL, P=0.003), those at other time points were comparable.

Patient-reported outcomes
The response rate to the questionnaire did not differ between the covering and pleurectomy groups (Pre/POD1/3/5/POM1: covering, 96.2%/76.9%/88.5%/92.3%/92.3%; pleurectomy, 79.3%/75.9%/82.8%/75.9%/86.2%). The EQ5D VAS scores at each time point are shown in Figure 2. The pleurectomy group was significantly better than the covering group on POD1 (36.9 vs. 52.7, P=0.015), but there were no significant differences between the groups at the other time points.

Figure 3 shows the proportion of respondents who reported slight or more problems in each dimension of the EQ5D. Generally, in all dimensions, the proportion of respondents with slight or more problems increased on POD1 and decreased with time in both groups. There were no significant differences at any time point in any dimension. Regarding pain/discomfort, the proportion of patients with slight or greater pain/discomfort was comparable in both groups. More than half of the patients had slight or more pain/discomfort at POM1 (covering: 54.2%, pleurectomy: 56.0%). Additionally, in terms of relationship between psychological distress and age, we analyzed a proportion of respondents who reported slight or more anxiety/depression and two age groups (<40 and ≥40 years). The proportion of anxiety/depression between <40 and ≥40 years at each time point were as follows: Pre, 37.5% vs. 50.0%, P=0.390; POD1, 58.3% vs. 71.4%, P=0.419; POD3, 27.5% vs. 42.9, P=0.342; POD5, 20.5% vs. 14.3%, P=0.583; POM1, 14.6% vs. 25.0%, P=0.389. No significant difference was noted at each time point.

Long-term outcomes
Table 3 shows results of the long-term survey; the average response rate was 94.6% in both groups. The numbers of patients who answered the survey were 25 and 27 in the covering and pleurectomy groups, respectively. Only one patient in the covering group responded through mail; all others were confirmed via telephone by the surgeon. Postoperative median follow-up time was 38 months for all cases, 35 months in the covering group, and 42 months in the pleurectomy group. There were no significant differences in the follow-up period, smoking habit, late recurrence, and persistent symptoms between the groups. All 9 patients with a persistent symptom complained of pain and discomfort of the chest. One patient in covering group also reported dyspnea. In terms of late recurrence, 1 patient in the pleurectomy group visited our outpatient clinic with back pain on POD 52, and the chest X-ray showed modest collapse in the lower lung field that did not require drainage.
Table 3
| Variables | Covering (n=26) | Pleurectomy (n=29) | P value |
|---|---|---|---|
| Response to the long-term survey | 25 (96.2) | 27 (93.1) | 0.542 |
| Postoperative follow-up (months) | 36.7±13.5 | 41.5±13.5 | 0.809 |
| Late recurrence | 0 (0.0) | 1 (3.4) | 0.519 |
| Postoperative smoking habit: yes | 2 (7.7) | 0 (0.0) | 0.226 |
| Persistent symptom: present | 6 (23.1) | 3 (10.3) | 0.182 |
Data are expressed as number (%) or mean ± standard deviation.
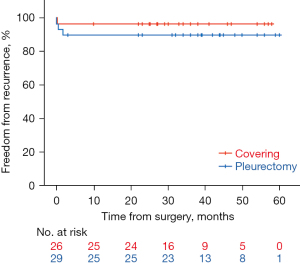
Figure 4 shows Kaplan-Meier curves of postoperative recurrence (perioperative and late) in the covering and pleurectomy groups. Overall, there were 4 recurrent cases (7.3%) among 55 cases (perioperative: 3, late: 1). There was no statistically significant difference in the recurrence rate between the groups (covering: 3.8%, pleurectomy: 10.3%, P=0.364).
Discussion
This is the first study to compare PROs, perioperative outcomes, and long-term outcomes between two different procedures (covering vs. pleurectomy) added to thoracoscopic bullectomy for PSP. Our main finding was that PROs, including pain, appeared to be comparable between the groups. While this was as we hypothesized based on our clinical experiences, it might be unexpected for some thoracic surgeons who anticipate that pleurectomy induces more pain and decreases QOL (8). Moreover, the perioperative and long-term outcomes in the covering and pleurectomy groups were comparable. Our results could serve as a benchmark for the most prevalent procedure in Japan (covering) against the pleurectomy recommended in Western guidelines.
We used the EQ5D as a PRO measure (7). In two previous studies evaluating PRO/QOL in patients with pneumothorax, the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30) and the Short Form 36 were used, respectively (9,10). The EQ5D is a preference-based measure and has been used to measure health utility as the EQ5D index value for the economic evaluation of healthcare outcomes. As we reported previously (11,12), the EQ5D has some advantages for evaluating patients’ QOL in perioperative settings. Namely, it requires only 2 min to answer and has no recall period (<24 h), which enables us to administer the EQ5D every other day during a patient’s hospitalization. Moreover, we reported that the EQ5D VAS was well correlated with the global health status/QOL score of the EORTC QLQ-C30 (13). Therefore, our EQ5D use in the perioperative setting for PSP is acceptable and valid. However, we could not strictly conclude that both procedures resulted in comparable QOL based on our exploratory observational study. When we initiated this study, no data were available for calculating an appropriate sample size to compare the EQ5D VAS scores in patients who had undergone surgery for PSP. Our data will be available for future confirmatory studies using EQ5D.
Regarding pleurectomy, we referred to and modified the technique based on Nathan et al.’s technique (14). For thoracic surgeons skilled in VATS lobectomy to some extent, pleurectomy was not difficult to adapt to and could be performed approximately 30–40 min from skin incision to skin closure. However, for senior doctors training toward board-certified thoracic surgeons, pleurectomy was more difficult to adapt to than covering. In our department, surgery for PSP is often performed by a clinical trainee, which could result in a longer operative time. Although pleurectomy could be an option based on our perioperative and long-term outcomes, concerns about the future influence of pleural adhesions resulting from pleurectomy remain unanswered. However, the same concern applies to covering. Another concern associated with pleurectomy is its effect on pulmonary function. A prospective non-randomized study reported no significant difference in pulmonary function between patients with PSP treated with only a chest tube and VATS bullectomy plus pleurectomy (5). Unfortunately, we did not evaluate the postoperative pulmonary function.
Concerning postoperative pain during the perioperative period, one randomized controlled study (RCT) reported that pleurectomy and pleural abrasion had comparable postoperative pain (15). Another RCT reported that covering resulted in less pain than pleural abrasion (8). Taken together, some may anticipate that covering is the most painless procedure. However, our results contradict the findings derived from recent studies. Herein, the covering group showed a tendency toward prolonged inflammation compared with the pleurectomy group (Figure 1). Since the visceral pleura also has sensory receptors (16) and the inflammation itself was reported as a hyperalgesic factor (17), the inflammation induced by the PGA sheet may enhance chest pain/discomfort. No significant difference in proportion of respondents who reported slight or more anxiety/depression between two age groups (<40 and ≥40 years) in terms of psychological distress, although a study reported that older adult patients with pneumothorax are associated with higher psychological distress (6). Our own preferred technique is the covering method based on the results of this study. The main reason is an ease of adaptation to the technique even for a younger trainee. However, we consider the use of artificial material and cost of the materials are still an issue.
This study has several limitations. The major limitations of this study were that it was a single institutional study with a limited number of patients. Additionally, the perioperative outcomes were evaluated retrospectively. Therefore, our data comparing the two procedures included selection bias. Two thoracic surgeons conducted a long-term cross-sectional survey by telephone, which might have affected the response. In our cohort, the frequency of chronic pain/discomfort was 12.7% (7/55), which might be less than that in recent reports (15,18,19); however, the recurrence rate of the covering group investigated by telephone and mail surveys was 3.8%, which is comparable to our previous data (20).
Conclusions
As for the additional procedures to bullectomy for PSP, pleurectomy and covering appeared to have comparable PROs, including pain and perioperative and long-term outcomes, including the recurrence rate. This study benchmarked the most prevalent option in Japan against pleurectomy, which is rarely performed in Japan. To determine the optimal additional procedure, further studies including a more detailed evaluation of the patient’s experience and functional assessment, are warranted.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-214/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-214/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-214/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-214/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional review board of Ibaraki Hospital Headquarters at Hitachi, Ltd. approved this study (No. 2015-3, No. 2019-70) and this study was registered in UMIN Clinical Trials Registry (UMIN000017597). The need for written informed consent was waived because each questionnaire provided the respondent with the opportunity to refuse to answer, and we provided contact information for opting out of the study on our website.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Shimizu H, Okada M, et al. Thoracic and cardiovascular surgeries in Japan during 2018: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2021;69:179-212. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after surgery for primary or secondary spontaneous pneumothorax: a prospective study comparing different surgical techniques. Interact Cardiovasc Thorac Surg 2008;7:45-9. [Crossref] [PubMed]
- Fung S, Ashmawy H, Schauer A, et al. Does Video-Assisted Thoracoscopic Surgery with Bullectomy and Partial Pleurectomy for Primary Spontaneous Pneumothorax Impair Health-Related Quality of Life and Pulmonary Function? Healthcare (Basel) 2021;9:1463. [Crossref] [PubMed]
- Kim D, Shin HJ, Kim SW, et al. Psychological Problems of Pneumothorax According to Resilience, Stress, and Post-Traumatic Stress. Psychiatry Investig 2017;14:795-800. [Crossref] [PubMed]
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. [Crossref] [PubMed]
- Lee S, Kim HR, Cho S, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. [Crossref] [PubMed]
- Ichimura H, Kobayashi K, Gosho M, et al. Trajectory and profile of quality of life in patients undergoing lung resection for lung cancer during hospitalization according to the EQ-5D. Gen Thorac Cardiovasc Surg 2021;69:1204-13. [Crossref] [PubMed]
- Ichimura H, Kobayashi K, Gosho M, et al. Comparison of Postoperative Quality of Life and Pain with and without a Metal Rib Spreader in Patients Undergoing Lobectomy through Axillary Mini-Thoracotomy for Stage I Lung Cancer. Ann Thorac Cardiovasc Surg 2022;28:129-37. [Crossref] [PubMed]
- Ichimura H, Kobayashi K, Gosho M, et al. Preoperative predictors of restoration in quality of life after surgery for lung cancer. Thorac Cancer 2021;12:835-44. [Crossref] [PubMed]
- Nathan DP, Taylor NE, Low DW, et al. Thoracoscopic total parietal pleurectomy for primary spontaneous pneumothorax. Ann Thorac Surg 2008;85:1825-7. [Crossref] [PubMed]
- Chen JS, Hsu HH, Huang PM, et al. Thoracoscopic pleurodesis for primary spontaneous pneumothorax with high recurrence risk: a prospective randomized trial. Ann Surg 2012;255:440-5. [Crossref] [PubMed]
- Pintelon I, Brouns I, De Proost I, et al. Sensory receptors in the visceral pleura: neurochemical coding and live staining in whole mounts. Am J Respir Cell Mol Biol 2007;36:541-51. [Crossref] [PubMed]
- Schistad EI, Stubhaug A, Furberg AS, et al. C-reactive protein and cold-pressor tolerance in the general population: the Tromsø Study. Pain 2017;158:1280-8. [Crossref] [PubMed]
- Passlick B, Born C, Sienel W, et al. Incidence of chronic pain after minimal-invasive surgery for spontaneous pneumothorax. Eur J Cardiothorac Surg 2001;19:355-8; discussion 358-9. [Crossref] [PubMed]
- Cattoni M, Rotolo N, Mastromarino MG, et al. Chronic chest pain and paresthesia after video-assisted thoracoscopy for primary pneumothorax. J Thorac Dis 2021;13:613-20. [Crossref] [PubMed]
- Ozawa Y, Sakai M, Ichimura H. Covering the staple line with polyglycolic acid sheet versus oxidized regenerated cellulose mesh after thoracoscopic bullectomy for primary spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2018;66:419-24. [Crossref] [PubMed]


