
Tumor volume doubling time as a potential predictor of prognosis in clinical stage I lung squamous cell carcinoma
Highlight box
Key findings
• Tumor volume doubling time (VDT) is a useful prognostic predictor in clinical stage I lung squamous cell carcinoma (SCC).
What is known and what is new?
• The VDT has been reported to be useful in predicting the prognosis NSCLC. This study found that especially in SCC preoperative VDT is a useful predictor of prognosis.
What is the implication, and what should change now?
• Since VDT is a useful prognostic factor in clinical stage I lung SCC, the length of VDT may be a consideration in the selection of the surgical procedure.
Introduction
Lung cancer is one of the most common causes of cancer-related death worldwide (1). The 5-year survival rates of lung cancer are 68–92% with clinical stage I, 53–60% with clinical stage II, 13–36% with clinical stage III, and 0–10% with clinical stage IV, respectively (2).
Among non-small cell lung cancers (NSCLCs), adenocarcinoma accounts for the largest proportion, with various studies and many reported preoperative prognostic factors (3-5). Suzuki et al. found that ground-glass opacity (GGO) on lung computed tomography (CT) predicts a favorable prognosis of adenocarcinoma (4); while Uehara et al. have identified a correlation between adenocarcinoma prognosis and the maximum standardized uptake value (SUVmax) of the tumor (5). Thus, the prognosis of lung adenocarcinomas can be predicted preoperatively with relative ease and accuracy using imaging data.
Prognostic factors have also been analyzed for lung squamous cell carcinoma (SCC). Among patients with lymph node metastasis, Funai et al. found worse prognoses in those with peripheral-type lung SCCs than those with central-type nodules (6). In addition, tumor budding (7), lymphatic invasion (8), and spread through air spaces (STAS) (7,9) have been found to correlate with lung SCC prognosis. Thus, lung SCC prognosis can be predicted by postoperative pathology. However, no effective radiological means of preoperative prognosis prediction has yet been identified for this cancer. In this study, we examined the possibility use of tumor volume doubling time (VDT) for this purpose.
The VDT is known to indicate tumor activity (10), and the VDT has been reported to be useful in predicting the prognosis of lung cancer (11-13) and NSCLC (14).
In lung SCC, there is no preoperative factor to evaluate tumor aggressiveness, so we searched for a suitable predictor and investigated the association between VDT and prognosis. This study aimed to explore radiological preoperative prognostic factors in lung SCC. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-292/rc).
Methods
Ethical statement
This retrospective observational study was conducted on patients who underwent surgery for NSCLC at Yamagata Prefectural Central Hospital. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent for surgical treatment was obtained from all patients, and further individual patient consent was waived owing to the retrospective analysis of this study. The study was approved by the Institutional Review Board of Yamagata Prefectural Central Hospital (IRB No. 4-62) on July 20, 2022.
Data collection
Using our institutional database, which is updated weekly, we identified patients meeting our inclusion criteria for enrollment in this study and gathered the required demographic and clinical data from their medical records. The variables used in this study were age, sex, performance status (PS), smoking status, respiratory function test (forced vital capacity and forced expiratory volume) scores, carcinoembryonic antigen (CEA) levels, cytokeratin 19 fragment (CYFRA) levels, SCC antigen levels, radiological findings on CT, SUVmax on positron emission tomography/CT (PET/CT), surgical procedure, surgery time, blood loss, pathological findings, and postoperative follow-up outcomes (including readmission dates, recurrence dates, recurrence sites, dates of death, and causes of death).
Study design and patients
The inclusion criteria for this study were: (I) clinical stage I lung SCC; (II) underwent lobectomy; and (III) peripherally located tumor (in the outer third of the lung parenchyma). Patients who underwent lobectomies between January 2006 and April 2020 were identified in our medical records. Ninety-five patients were enrolled in this study.
The exclusion criteria were as follows: (I) only one preoperative CT or an interval of <20 days between the first CT and the preoperative CT (in most cases with this short interval between scans, it was because the surgeon judged it necessary to perform the pulmonary resection earlier than planned); (II) the presence of multiple lung cancers; (III) shrunken nodules; (IV) underwent induction therapy; (V) underwent segmentectomy or wedge resections. The latter exclusions were for the following reasons: (I) this demographic included many high-risk patients, including elderly patients and patients with cardiopulmonary complications; as such, this group tends to have poor prognoses; (II) mediastinal lymph node dissections are not routinely performed in this type of surgery and the pathological staging is insufficient. One of the excluded cases is shown in Figure S1. After exclusions, a total of 51 patients were included in our analyses (Figure 1).
Preoperative examination and preoperative (clinical) diagnosis
At their preoperative outpatient visits, all patients underwent physical examination, laboratory test, blood gas analysis, urinalysis, chest X-ray, thin-section CT, 18F-fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT), pulmonary function tests, and electrocardiogram test. The clinical stage of each patient’s cancer was defined by the 8th TNM classification (2). The cases defined by the 7th TNM classification were restaged according to the 8th TNM classification.
The decision to perform either bronchoscopy or a CT-guided needle aspiration biopsy for the diagnosis of pulmonary nodules or mediastinal lymph nodes was made by a cancer treatment board, comprising a thoracic surgeon, a pulmonologist, and a radiologist.
Evaluation using thin-section computed tomography
All the patients underwent preoperative thin-section CT before surgery. Contrast-enhanced thin-section CT was performed in patients without iodine allergies or without severe renal dysfunction. A 320-row Aquilion ONE (Toshiba Medical Systems, Tochigi, Japan) or 64-row Light Speed VCT (GE Healthcare, Tokyo, Japan) scanner was used. The scanning conditions were 120 kV, auto mA, with a 35 cm field of view, slice thickness of 1–1.25 mm, a chest interval of 0.8–1.25 mm, 512×512 pixels, and a scan time of 0.5 s.
The tumor diameter was preoperatively measured in the following CT settings: lung window, window level: −670 to −600 Hounsfield units (HU), window width: 1,500–1,600 HU; mediastinal window, window level: 25–30 HU, window width: 280 HU. One radiologist and at least three surgeons evaluated the radiological tumor findings of the thin-section CT.
Measurement of tumor volume doubling time
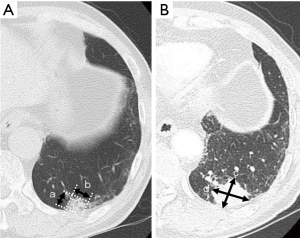
We have previously reported the VDT measurement in detail (15). At first, the long and short diameters of the tumor were measured on the axial image of the thin-section CT. Then, the tumor volume was measured using the following formula:
where X (b or d) denotes the long diameter of the tumor and Y (a or c) denotes the short diameter of the tumor on CT (Figure 2).
VDT was then measured using the Schwartz formula (16):
where T1 denotes the date of the first pulmonary nodule detected; T2, the date of preoperative CT; V1, V at T1; and V2, V at T2.
One doctor calculated and analyzed VDT retrospectively.
Evaluations based on 18F-FDG-PET/CT
PET/CT is performed at other facilities because Yamagata Prefectural Central Hospital has no available equipment; the PET/CT equipment and protocols have also been previously reported (17). All PET/CT images were interpreted by specialized nuclear radiologists.
Surgical procedure
Pulmonary resection was performed using a minimally invasive open surgery approach (18). Patients with clinical T1c or advanced lung cancer underwent lobectomy and mediastinal lymph node dissection, and those with clinical T1b or early lung cancer underwent sublobar resection according to the JCOG0802 protocol (19). Patients who could not tolerate lobectomy underwent sublobar resection, and mediastinal lymph node dissection was not performed on patients who underwent sublobar resection. The mediastinal lymph node dissection is sometimes omitted in elderly patients, patients with serious cardiopulmonary complications, or patients with interstitial lung disease.
Postoperative treatment and follow-up
Regarding non-elderly patients with no severe diseases or complications, those with pathological stage IA3 or IB (8th TNM classification) were recommended to receive uracil–tegafur orally. For patients with completely resected pathological stage II–IIIA cancer, cisplatin-based adjuvant chemotherapy was recommended.
All patients who underwent pulmonary resection were postoperatively followed up: (I) patients with pathological stage I NSCLC underwent blood test of tumor markers and chest CT every 6 months until the second postoperative year as well as blood test every 6 months and chest CT every 6 months alternating with X-ray and CT after the third postoperative year. (II) Patients with pathological stage II or more advanced NSCLC underwent blood test of tumor markers every 3 months and chest CT every 6 months until the third postoperative year as well as blood test every 6 months and alternating X-ray and CT every 6 months from the fourth postoperative year. All patients underwent chest CT annually after the sixth postoperative year.
Types of recurrence
The types of recurrences were classified into the following two categories according to the recurrent lesions when it was first determined as a recurrence postoperatively.
Locoregional recurrence
Locoregional recurrence includes recurrence of the primary tumor, resected margins (parenchymal or bronchial margins), hilar and mediastinal lymph node metastases, intrapulmonary metastases, ipsilateral pleural effusion, and ipsilateral pleura.
Distant recurrence
Distant recurrence includes contralateral intrapulmonary metastases, contralateral pleural effusion, and recurrence of contralateral pleura, brain, bone, adrenal gland, liver, supraclavicular lymph nodes, pericardium, pericardial effusion, and other distant organs.
Histological confirmation of recurrence was not mandatory. In cases where recurrence was diagnosed by biopsy, the date of recurrence was recorded as the date of the biopsy procedure. When no biopsy was performed, recurrence was diagnosed based on the identification of known indicators of recurrence (e.g., enlarged lymph nodes or lesions with high levels of accumulated FDG) on imaging scans, such as CT or PET/CT. In these cases, the recurrence date was recorded as the date on which the imaging was performed.
Statistical analysis
Categorical variables between the two groups were compared with the chi-squared or Fisher’s exact tests. Continuous variables between the two groups were compared with the Mann-Whitney U test.
PS was classified as 0 or 1. Forced expiratory volume (FEV) 1% was classified as greater or less than 70% according to the GOLD classification. Tumor markers were divided into two categories according to abnormal values.
After demonstrating that VDT correlated with survival, the cut-off value of VDT using the Youden index was defined by receiver operating characteristic (ROC) curve analysis on survival. Univariable and multivariable analyses for overall survival (OS) and recurrence-free survival (RFS) were conducted using the Cox proportional-hazards model. OS was defined as the duration between the date of surgery and either the date of death or the date of the final follow-up. RFS was defined as the duration between the date of surgery and the date of locoregional or distant recurrence diagnosis, the date of death, or the date of the final follow-up. The Kaplan-Meier method was used to estimate the OS and RFS curves and to determine the statistical significance of the results.
All analyses were conducted using the JMP® 13 software (SAS Institute Inc., Cary, NC, USA). A P value of <0.05 was considered statistically significant.
Results
The median age of all patients was 73 years (range, 70–79), and detailed patient characteristics are listed in Table 1. All the eligible patients were smokers. The median duration between the date of CT for pulmonary nodule detection and the date of preoperative CT before pulmonary resection was 50 days (range, 20–995). The median VDT was 263 days (range, 91–375).
Table 1
| Variables | Median [IQR] or n [%] |
|---|---|
| Age, years | 73 [70–79] |
| Sex (male/female) | 49 [96]/2 [4] |
| Performance status (0/1) | 49 [96]/2 [4] |
| Smoking status (smoker/non-smoker) | 51 [100]/0 [0] |
| FVC (L) | 3.3 [2.9–3.9] |
| %FVC (%) | 104.6 [93.7–112.9] |
| FEV1 (L) | 2.3 [1.8–2.8] |
| FEV1% (%) | 70.3 [62.9–75.1] |
| CEA (ng/mL) | 2.7 [2.3–4.0] |
| CYFRA (ng/mL) | 1.4 [1.1–2.0] |
| SCC (ng/mL) | 1.6 [1.0–2.3] |
| SUVmax of tumor | 10.1 [6.7–16.5] |
| Tumor diameter on CT (cm) | 2.5 [1.8–3.0] |
| Tumor location (RU/RM/RL/LU/LL) | 15 [29]/2 [4]/19 [37]/9 [18]/6 [12] |
| Clinical stage (IA1/IA2/IA3/IB) | 1 [2]/13 [26]/22 [43]/15 [29] |
| VDT (days) | 263 [91–375] |
| Operative time (minutes) | 154 [140–203] |
| Blood loss (g) | 30 [15–70] |
| Pathological tumor diameter (cm) | 2.2 [1.9–2.6] |
| Pathological T (1/2/3) | 31 [61]/19 [37]/1 [2] |
| Pathological N (0/1/2) | 47 [92]/3 [6]/1 [2] |
| Pathological stage (I/II/III) | 46 [90]/4 [8]/1 [2] |
| Lymphatic invasion (positive/negative) | 2 [4]/49 [96] |
| Vascular invasion (positive/negative) | 11 [22]/40 [78] |
| Pleural invasion (positive/negative) | 11 [22]/40 [78] |
| Adjuvant chemotherapy (yes/no) | 2 [4]/49 [96] |
IQR, interquartile range; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; CEA, carcinoembryonic antigen; CYFRA, cytokeratin 19 fragment; SCC, squamous cell carcinoma antigen; SUVmax, maximum standardized uptake value; CT, computed tomography; RU, right upper-lobe; RM, right middle-lobe; RL, right lower-lobe; LU, left upper-lobe; LL, left lower-lobe; VDT, tumor volume doubling time; T, tumor; N, node.
Table 2 presents the results of our univariable and multivariable analyses of the preoperative clinical and demographic patient variables assessed for their relationships to prognosis, with OS as the prognostic measure. The univariable analysis identified age, performance status (1), CYFRA (>3.5 ng/mL), and VDT as independent prognostic factors for OS. The multivariable analysis identified only VDT as an independent prognostic factor for OS.
Table 2
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Age, years | 1.166 (1.040–1.368) | 0.005 | 1.116 (0.978–1.358) | 0.113 | |
| Sex (male) | – | – | |||
| PS [1] | 9.995 (1.421–46.976) | 0.025 | 2.882 (0.290–26.254) | 0.347 | |
| FEV1% (<70%) | 1.523 (0.499–4.782) | 0.455 | |||
| CEA (>5.0 ng/mL) | 3.010 (0.643–10.825) | 0.146 | |||
| CYFRA (>3.5 ng/mL) | 4.682 (1.018–16.363) | 0.048 | 0.890 (0.109–4.374) | 0.898 | |
| SCC (>2.5 ng/mL) | 1.910 (0.507–6.104) | 0.314 | |||
| SUVmax | 1.013 (0.922–1.105) | 0.770 | |||
| VDT | 0.986 (0.974–0.994) | <0.001 | 0.990 (0.979–0.997) | 0.002 | |
CI, confidence interval; PS, performance status; FEV1, forced expiratory volume in the first second; CEA, carcinoembryonic antigen; CYFRA, cytokeratin 19 fragment; SCC, squamous cell carcinoma antigen; SUVmax, maximum standardized uptake value; VDT, tumor volume doubling time.
Table 3 presents the results of our univariable and multivariable analyses of preoperative clinical and demographic patient variables assessed for their relationships to prognosis, with RFS as the prognostic measure. The univariable analysis identified age, CEA (>5.0 ng/mL), and VDT as independent prognostic factors for RFS. The multivariable analysis identified only VDT as an independent prognostic factor for RFS.
Table 3
| Variables | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Age, years | 1.102 (1.007–1.240) | 0.032 | 1.038 (0.934–1.199) | 0.526 | |
| Sex (male) | – | – | |||
| PS [1] | 5.094 (0.773–19.931) | 0.082 | |||
| FEV1% (<70%) | 1.056 (0.366–2.970) | 0.918 | |||
| CEA (>5.0 ng/mL) | 4.445 (1.326–13.546) | 0.018 | 3.248 (0.894–11.008) | 0.072 | |
| CYFRA (>3.5 ng/mL) | 3.175 (0.709–10.445) | 0.118 | |||
| SCC (>2.5 ng/mL) | 1.468 (0.400–4.422) | 0.531 | |||
| SUVmax | 1.039 (0.959–1.121) | 0.342 | |||
| VDT | 0.985 (0.974–0.993) | <0.001 | 0.989 (0.978–0.995) | <0.001 | |
CI, confidence interval; PS, performance status; FEV1, forced expiratory volume in the first second; CEA, carcinoembryonic antigen; CYFRA, cytokeratin 19 fragment; SCC, squamous cell carcinoma antigen; SUVmax, maximum standardized uptake value; VDT, tumor volume doubling time.
Since VDT was shown to be associated with prognosis, the optimal cut-off values of VDT were identified using ROC curves regarding the death (Figure 3A), or the death and tumor recurrence (Figure 3B). The optimal cut-off value of VDT regarding the death was 150, which had the best sensitivity and specificity (92.3% and 79.0%, respectively). The optimal VDT cut-off value for death and tumor recurrence was also 150, which had the best sensitivity and specificity (93.3% and 83.3%, respectively).
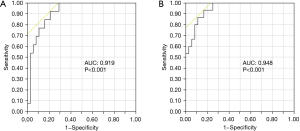
Table S1 presents the relationships between tumor VDT and patient clinical characteristics. There were no significant correlations between VDT and clinical characteristics.
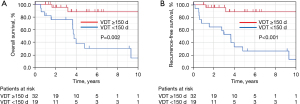
Regarding prognosis, the 5-year OS rates were 88.4% and 30.4% in the group with long (≥150 days) and short (<150 days) VDT, respectively (P=0.002) (Figure 4A). The 5-year RFS rates were 88.8% and 26.5% in the group with long (≥150 days) and short (<150 days) VDT, respectively (P<0.001) (Figure 4B).
We considered that VDT values could be associated with the CT interval. Thus, we analyzed whether there was any correlation between the two variables. Figure S2A plots the relationship between VDT values and the CT interval. The correlation coefficient between the two variables was 0.335 (P=0.016), which was a moderately significant positive relationship. Figure S2B plots the relationship between clinical tumor diameter and CT interval. There was a slight negative relationship between the two variables, with longer CT intervals tending to have higher VDT values but the correlation was not significant (coefficient, −0.021; P=0.886), Longer CT intervals showed a non-significant tendency to smaller tumor diameters.
Discussion
In this study, we analyzed potential preoperative prognostic factors for resected stage I lung SCC and found that preoperative VDT is a significant predictor of prognosis. While several radiological prognostic factors have been identified for lung adenocarcinoma, including GGO (4) and SUVmax (5), few factors have been reported for causing lung SCC. Previous studies have identified several postoperative variables that are correlated with the prognosis of resected lung SCCs, including tumor location (central or peripheral) (6), the presence of lymphatic invasion (8), tumor budding (7), and STAS (7,9). However, no preoperative radiological factors for SCCs have been identified. Tsutani et al. found a correlation between prognosis and the SUVmax of lung adenocarcinomas but not between prognosis and the SUVmax of SCCs (20).
VDT has been reported as a useful tumor activity indicator (10). VDT has also been used in lung cancer screening (21,22). The NELSON study demonstrated that nodules with a VDT of ≥400 days at 3 months were considered nongrowing tumors, nodules with a VDT of ≥600 days at 1 year were considered nongrowing tumors, and nodules with a VDT of <400 days were more likely to be lung cancer (positive predictive value: 63%) (22).
Usuda et al. calculated tumor doubling time using chest X-rays. They set the cut-off value for tumor doubling time at 113 days and noted that nodules with tumor doubling times shorter than 113 days had significantly worse OS (11). Subsequently, imaging technology has developed and thin-section CT is now widely used in clinical practice. As a result, tumor volume can be more easily calculated and tumor doubling time more accurately determined.
In patients with pathological stage I–IIIA resected NSCLC, Miura et al. found significant differences in both OS and RFS between those with VDT >400 days and those with VDT <400 days (14). Their VDT cut-off value of 400 days was based on a study by Veronesi et al. (21). They reported that VDT was useful in differentiating between malignant and benign lung nodules on CT. In their study, adenocarcinoma (including adenocarcinoma in situ and nodules with GGO) accounted for more than 70% of lung cancers. However, there is wide variation in VDT among patients with pathological stage I–IIIA NSCLC. Therefore, rather than setting a uniform cut-off value, research on this topic would be better served by setting different cut-off values for each cancer stage, or by limiting the inclusion criteria to avoid a broad range of VDTs.
Obayashi et al. reviewed the VDTs of each histological subtype of lung cancer and found significantly longer VDTs in lung adenocarcinomas than in lung SCCs (23). Among adenocarcinomas, nodules with GGO had longer VDTs than those without. The reason for the slowing of progression when GGO is present is likely the growth of the GGO in alveolar epithelial replacement patterns, resulting in a relatively slow expansion and longer VDTs.
In addition, Ortega et al. reported that molecular markers such as EGFR and ALK can be used to select target therapy and predict prognosis in lung cancer (24). In lung SCC, compared to adenocarcinoma, the number of useful markers is limited. Although not explored in this study, more detailed analysis of data on molecular markers would be essential in the future.
Setojima et al. investigated the relationship between prognosis and the VDT of the solid components of tumors in clinical stage I-IIA NSCLC (13). They demonstrated that OS and RFS were significant differences in both OS and RFS between those with VDT >215 days and those with VDT <215 days. Their cut-off value of 215 days was established using an ROC curve analysis of tumor recurrence. Since their study included both lung SCC and lung adenocarcinoma, one would expect a longer cut-off value than that required in the present study on lung SCC only, which was 150 days. Therefore, a cut-off value of 150–215 days can be considered reasonable for lung SCCs.
The lack of existing preoperative radiological prognostic factors for lung SCCs makes, our finding that VDT is a prognostic factor in clinical stage I lung SCC cases particularly useful. However, the use of VDT for this purpose is not feasible in cases of complicated obstructive pneumonitis. Since obstructive pneumonitis is a common complication of lung SCC, this is a potential disadvantage of considering VDT as a prognostic factor. A further disadvantage is that VDT cannot be utilized without a preoperative diagnosis of lung SCC.
So why is VDT associated with prognosis in lung SCC? While lung adenocarcinoma often develops in the cells that proliferate during alveolar epithelial replacement (25), the growth pattern of lung SCC can be classified into three different structural patterns known as the alveolar space-filling (ASF) type, the expanding type, and the mixed type (6). The ASF type of SCC tumor grows to fill the alveolar spaces separated by thin septa without destruction of the elastic framework. The expanding type of tumor destroys the surrounding lung parenchyma and the alveolar septum supported by the elastic framework, with clearly outlined lobulation. The mixed type of tumor has characteristics of both the ASF and the expanding types. Funai et al. found a 5-year survival rate among patients with ASF-type tumors of 100% (6). The ASF type represents about 5% of SCC cases. Total 95% of the patients with expanding or mixed-type tumors have worse prognoses than ASF-type patients. There is likely to be a relationship between tumor VDTs and lung SCC growth patterns.
High SUVmax has been demonstrated as a poor prognostic factor in NSCLC by Paesmans in a meta-analysis (26). Among NSCLCs, the degree of FDG accumulation differs between lung adenocarcinoma and SCC. Notably, FDG accumulation is correlated with the degree of glucose transporter 1 (GLUT1) expression, and GLUT1 expression is higher in SCC than in adenocarcinoma and small cell lung cancer with higher FDG accumulation (27). Thus, FDG accumulation was originally higher in SCC. Tsutani et al. reported that SUVmax is a useful prognostic factor in PET/CT in adenocarcinoma but not in SCC (20). Similarly, in the present study, SUVmax was not identified as a significant prognostic factor in lung SCC.
In NSCLC, Nakamura et al. have reported correlations between VDT and gender, smoking status, and the presence of EGFR mutations (28). Tann et al. found VDT to be correlated with SUVmax in stage I lung cancer (29). However, the present study identified no significant relationships between VDT and clinicopathological factors, which might be due to small sample size. However, the lack of correlations with VDT suggests that VDT may reflect characteristics of the tumors themselves.
Limitations
The present study had some limitations. First, as a single-institution retrospective study, the study might be selection bias. Also, the sample size is 51 patients, which is small. Lung SCC is less common than adenocarcinoma, and the proportion of patients with early stage is even smaller. Therefore, it is reasonable that the sample size is small, but it is also necessary to consider integrating data from multiple facilities in order to confirm more reliable results. Second, only one of the authors calculated the tumor VDTs, which might result in potential error possibilities that could not be avoided if they had been evaluated by two or more people. Third, the patients with the worst prognoses were excluded and this may have resulted in a less representative sample. Fourth, despite the prognostic utility of VDT, there are various problems with VDT measurements, such as the inaccuracy of tumor volume measurements taken from axial CT sections. Facilities with easy access to three-dimensional (3D) software should use it as Sone et al. have shown that 3D software can provide more accurate VDT measurements than can be achieved manually on 2D CT (30). Measurements in 3D are accurate, but there is an error in the measurement of VDT in slightly smaller nodules affected by the thickness of the CT slice (31).
Conclusions
The results of this study indicate that VDT is a useful prognostic predictor in clinical stage I lung SCC. In the future, further data are needed to confirm our study results.
Acknowledgments
This study was presented at the Meeting of the World Conference on Lung Cancer in 2022, Vienna, Austria during August 6–9, 2022.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-292/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-292/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-292/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-292/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent for surgical treatment was obtained from all patients, and further individual patient consent was waived owing to the retrospective analysis of this study. The study was approved by the Institutional Review Board of Yamagata Prefectural Central Hospital (IRB No. 4-62) on July 20, 2022.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Batmunkh K, Cho S, Yum S, et al. Prognostic stratification of pathological node-negative lung adenocarcinoma by carcinoembryonic antigen level. Interact Cardiovasc Thorac Surg 2020;30:820-6. [Crossref] [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. "Early" peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [Crossref] [PubMed]
- Uehara H, Tsutani Y, Okumura S, et al. Prognostic role of positron emission tomography and high-resolution computed tomography in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2013;96:1958-65. [Crossref] [PubMed]
- Funai K, Yokose T, Ishii G, et al. Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. Am J Surg Pathol 2003;27:978-84. [Crossref] [PubMed]
- Stögbauer F, Lautizi M, Kriegsmann M, et al. Tumour cell budding and spread through air spaces in squamous cell carcinoma of the lung - Determination and validation of optimal prognostic cut-offs. Lung Cancer 2022;169:1-12. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Mimura T, et al. Prognostic impact of lymphatic invasion for pathological stage I squamous cell carcinoma of the lung. Gen Thorac Cardiovasc Surg 2015;63:153-8. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Endo M, et al. Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer 2018;120:14-21. [Crossref] [PubMed]
- COLLINS VP. LOEFFLER RK, TIVEY H. Observations on growth rates of human tumors. Am J Roentgenol Radium Ther Nucl Med 1956;76:988-1000. [PubMed]
- Usuda K, Saito Y, Sagawa M, et al. Tumor doubling time and prognostic assessment of patients with primary lung cancer. Cancer 1994;74:2239-44. [Crossref] [PubMed]
- Arai T, Kuroishi T, Saito Y, et al. Tumor doubling time and prognosis in lung cancer patients: evaluation from chest films and clinical follow-up study. Japanese Lung Cancer Screening Research Group. Jpn J Clin Oncol 1994;24:199-204. [PubMed]
- Setojima Y, Shimada Y, Tanaka T, et al. Prognostic impact of solid-part tumour volume doubling time in patients with radiological part-solid or solid lung cancer. Eur J Cardiothorac Surg 2020;57:763-70. [PubMed]
- Miura K, Hamanaka K, Koizumi T, et al. Solid component tumor doubling time is a prognostic factor in non-small cell lung cancer patients. J Cardiothorac Surg 2019;14:57. [Crossref] [PubMed]
- Nakahashi K, Shiono S, Nakatsuka M, et al. Prediction of lymph node metastasis of clinical stage IA non-small cell lung cancer based on the tumor volume doubling time. Surg Today 2022;52:1063-71. [Crossref] [PubMed]
- SCHWARTZ M.. A biomathematical approach to clinical tumor growth. Cancer 1961;14:1272-94. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. The prognostic value of positron emission tomography/computed tomography in pulmonary metastasectomy. J Thorac Dis 2018;10:1738-46. [Crossref] [PubMed]
- Asamura H. Minimally invasive open surgery approach for the surgical resection of thoracic malignancies. Thorac Surg Clin 2008;18:269-73. vi. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Misumi K, et al. Difference in prognostic significance of maximum standardized uptake value on [18F]-fluoro-2-deoxyglucose positron emission tomography between adenocarcinoma and squamous cell carcinoma of the lung. Jpn J Clin Oncol 2011;41:890-6. [Crossref] [PubMed]
- Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157:776-84. [Crossref] [PubMed]
- Xu DM, van der Zaag-Loonen HJ, Oudkerk M, et al. Smooth or attached solid indeterminate nodules detected at baseline CT screening in the NELSON study: cancer risk during 1 year of follow-up. Radiology 2009;250:264-72. [Crossref] [PubMed]
- Obayashi K, Shimizu K, Nakazawa S, et al. The impact of histology and ground-glass opacity component on volume doubling time in primary lung cancer. J Thorac Dis 2018;10:5428-34. [Crossref] [PubMed]
- Ortega MA, Navarro F, Pekarek L, et al. Exploring histopathological and serum biomarkers in lung adenocarcinoma: Clinical applications and translational opportunities Int J Oncol 2022;61:154. (Review). [Crossref] [PubMed]
- Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669-92. [Crossref] [PubMed]
- Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol 2010;5:612-9. [Crossref] [PubMed]
- Schuurbiers OC, Meijer TW, Kaanders JH, et al. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. J Thorac Oncol 2014;9:1485-93. [Crossref] [PubMed]
- Nakamura R, Inage Y, Tobita R, et al. Epidermal growth factor receptor mutations: effect on volume doubling time of non-small-cell lung cancer patients. J Thorac Oncol 2014;9:1340-4. [Crossref] [PubMed]
- Tann M, Sandrasegaran K, Winer-Muram HT, et al. Can FDG-PET be used to predict growth of stage I lung cancer? Clin Radiol 2008;63:856-63. [Crossref] [PubMed]
- Sone S, Hanaoka T, Ogata H, et al. Small peripheral lung carcinomas with five-year post-surgical follow-up: assessment by semi-automated volumetric measurement of tumour size, CT value and growth rate on TSCT. Eur Radiol 2012;22:104-19. [Crossref] [PubMed]
- Nietert PJ, Ravenel JG, Leue WM, et al. Imprecision in automated volume measurements of pulmonary nodules and its effect on the level of uncertainty in volume doubling time estimation. Chest 2009;135:1580-7. [Crossref] [PubMed]



