
Impact of ground glass opacity in a three-dimensional analysis for pathological findings and prognosis in stage IA pure solid lung cancer
Highlight box
Key findings
• The C/T volume ratio determined by a 3D analysis detects GGO and reflects the pathological findings, and further prognostic stratification is possible in early 2D pure solid lung cancer.
What is known and what is new?
• It has been reported that the C/T volume ratio is useful as a preoperative pathological predictor of non-invasive cancer in peripheral small lung cancer, and that the presence or absence of GGO is a prognostic factor. It was newly found that there are many cases in which GGO is detected by the C/T volume ratio even if judged to be pure solid, and that pathological and prognostic stratification is possible.
What is the implication, and what should change now?
• It was found that mechanical assessment in 3D contributed to prognostic stratification better than gross assessment in 2D. In the future, after performing stratification by 3D analysis, the optimal surgical procedure for peripheral small lung cancer should be examined.
Introduction
The consolidation to tumor ratio (C/T ratio) is a preoperative evaluation item that is highly correlated with pathological non-invasive lung cancer (defined as negative for lymph node metastasis, lymphatic and vascular invasion) (1). There are also reports that the C/T ratio can predict pathological N1–2 in clinical N0 cases (2,3). Consensus was obtained by considering reduced surgery for early-stage lung cancer based on this value, and multiple large-scale prospective studies have been conducted in recent years (4-6). Furthermore, with the increase in the use of thin-section computed tomography (CT) due to advances in imaging technology (7), multiple studies in recent years have reported that the prognosis is superior in two-dimensional (2D) CT image evaluations with even a very small ground glass opacity (GGO) component in comparison to pure solid findings (8-11). Therefore, the presence of the GGO component has received increased attention as a reliable prognostic factor in early non-small cell lung cancer (NSCLC) (8,10).
Conventionally, a GGO component observed in adenocarcinoma on CT images is pathologically associated with lepidic growth, which proliferates and spreads by alveolar epithelial replacement while maintaining the existing alveolar structure in the early stage of cancer development. It is considered to be a characteristic of early-stage lung adenocarcinoma, and is associated with a good prognosis (12-14). However, even lesions that are judged to be pure solid on 2D CT images are pathologically accompanied by lepidic growth, and there is a certain percentage of patterns in which lepidic growth is predominant. In other words, there may be a group with a good prognosis among pure solids lesions, which are considered to be associated with a poor prognosis in early-stage lung cancer. We previously reported the usefulness of the consolidation volume/tumor volume ratio (C/T volume ratio), which is the C/T ratio of the lesion volume, determined using a Synapse Vincent three-dimensional (3D) analysis workstation as a preoperative pathological non-invasive lung cancer predictor (15). Similarly, there it is also reported that the volume-based C/T ratio is useful for predicting postoperative upstaging (16). Therefore, in small lung cancer judged to be completely free of GGO on 2D CT (pure solid, C/T ratio =1), the C/T volume ratio calculated by a 3D analysis was significantly different from the above-mentioned imaging findings and pathological findings. We examined whether it could correct the discrepancy and whether it could contribute to prognostic stratification. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-341/rc).
Methods
Study population
Among 1,065 patients with clinical stage IA NSCLC with a maximum tumor diameter of 3 cm who underwent complete resection at our hospital between January 2010 and March 2021, there were 556 patients who were judged to be without GGO on 2D CT. After excluding cases with missing positron emission tomography (PET) data, simultaneous multiple lung cancer, preoperative chemoradiotherapy, or a centrally located tumor (if the tumor is centrally located, the vessels will be close and there is a high possibility of misrecognition by the 3D workstation), and same intralobar pneumonia cases 370 patients were included in the present study. The 8th edition of the American Joint Commission on Cancer TNM classification was used for clinical staging (17,18). With regard to clinical nodal assessment, clinical N0 was defined as non-enlarged lymph nodes (short axis <10 mm) on thin-section CT. Endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal lymph node staging was performed preoperatively to confirm the node-negative status in cases that showed swollen lymph nodes on thin-section CT or uptake on PET. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board (IRB) of Seirei Mikatahara General Hospital approved the study protocol and publication of data (No. 22-64, Approval date: February 24, 2023). Informed consent was obtained from each patient before examination and the contents of this study were disclosed at our hospital. The medical records of each patient were retrospectively reviewed under a waiver of authorization approved by our IRB.
Two-dimensional image evaluation
Tumor evaluation by 2D CT was performed under lung field conditions (window level: −650 to −700 HU; window depth: 1,400–1,500 HU) using thin-section CT imaged at intervals of <2 mm. In addition, the evaluation of lymph nodes was performed under mediastinal conditions (window level: 30–60 HU; window depth: 300–400 HU). All evaluations by 2D CT were performed by a staff of multiple respiratory surgeons (4 thoracic surgeons with sufficient clinical experience and 2 qualified general surgeons but not thoracic surgeons). All staff members were aware that the lesions were likely primary lung cancers, but were unaware of the detailed pathological findings, including histology. All lesions that were the target of this study had all CT sections checked by the above staff, and were judged to be completely free of GGO.
GGO detection method using 3D analysis workstation
All preoperative 2D thin-section CT images were imported into a 3D analysis workstation Synapse Vincent (Fuji Photo Film Co., Ltd., Japan). Using the “Lung Analysis” application in the workstation, the tumor was recognized by specifying the lesion on CT, and the C/T volume ratio was calculated mechanically. Initially, we planned to define C/T volume ratio =1 as 3D pure solid, but GGO can be detected even in squamous cell carcinoma, which theoretically cannot be accompanied by GGO because it does not have a pathological lepidic component. It was judged that there was some degree of mechanical error, and a C/T volume ratio of 0.9 was adopted as the cutoff value. Therefore, in this study, the 3D solid group was defined by a C/T volume ratio of ≥0.9, and the 3D GGO group was defined by a C/T volume ratio of <0.9.
Statistical analysis
The purpose of this study was to investigate the clinical and pathological significance of the presence of GGO identified by a 3D analysis in clinical stage IA radiologically pure solid NSCLC. The primary endpoint was Overall survival (OS), and the secondary endpoint was recurrence-free survival (RFS) and pathological findings. We used Fisher’s exact test for categorical variables and an unpaired t-test for continuous variables to compare the two groups. OS or RFS was estimated using the Kaplan-Meier method and compared between different groups using the log-rank test. In addition, a Cox proportional hazards model was used to identify factors that affect the prognosis. Univariate and multivariate analyses including all covariates were performed using Cox proportional hazards models and adjusted for confounding variables for survival and recurrence. For continuous variables, values higher than the median were classified as the high group. In addition, propensity score matching was used to control for confounding factors and reduce prognostic imbalance caused by a selection bias. Patient background variables adjusted by propensity score matching were sex, age, Brinkman index, clinical stage, and surgical procedure. Patients with equivalent propensity scores were selected using a one-to-one greedy matching algorithm using nearest neighbors without replacement, with a caliper width equal to 0.2 standard deviations of the propensity score logit. Follow-up was performed monthly until 3 months, every 3 months until 2 years, and every 6 months until 5 years after surgery. After 5 years postoperatively, follow-up was continued every six months or 1 year as needed. There was no loss to follow-up in this study, and withdrawal was treated as a discontinued case with respect to survival outcome. P values of <0.05 were considered statistically significant, and all statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
Figure 1 shows a flow chart of this study, and Table 1 shows the patient background. The median follow-up time was 62.2 months. A total of 370 cases were included, 73.2% were male, and the median age was 69 years. The clinical stage was stage IA1 in 6.2% of cases, IA2 in 54.4%, and IA3 in 39.4%. The surgical procedures included lobectomy in 76.5% of cases, segmentectomy in 14.8%, and wedge resection in 8.7%. At our facility, we basically choose lobectomy for 2D pure solid early lung cancer. Segmentectomy may be performed as aggressive reduction surgery based on SUVmax, tumor growth rate, tumor marker values, etc., or as passive reduction surgery considering pulmonary function and complications. Regarding wedge resection, all cases are carried out as passive reduction surgery. The pathological stage was 0–IA3 in 51.9% of cases and IB–IIIB in 48.1%. Adenocarcinoma was the most common histologic type, accounting for 70% of cases, while squamous cell carcinoma accounted for 25.9% of cases. Pathological findings included lymphatic invasion in 42.7% of cases, vascular invasion in 58.1%, lymph node metastasis in 18.1%, lepidic in 39.7%, and lepidic predominant in 10.3%. Since the target period of this study was from 2010, many cases were not evaluated for micropapillary and spread through air spaces (STAS), so they were excluded from the survey items of this time. Two hundred twenty-eight cases (61.6%) in the 3D solid group and 142 cases (38.4%) in the 3D GGO group were classified and compared. Regarding patient background, age, Brinkman index, maximum standardized uptake value (SUVmax), and carcinoembryonic antigen (CEA) were significantly higher in the 3D solid group, the tumor size tended to be larger in the 3D solid group, resulting in less IA1 and more IA3 at the clinical stage. As pathological findings, pathological stage IB–IIIB was significantly more common in the 3D solid group. Squamous cell carcinoma was also more common. The rates of lymphatic invasion, vascular invasion, and lymph node metastasis were also significantly higher in the 3D solid group. In contrast, the rates of lepidic and lepidic predominant pathological findings were significantly higher in the 3D GGO group.
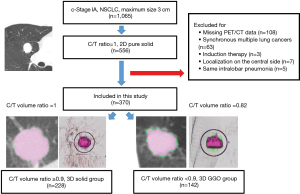
Table 1
| Characteristic | Total (N=370) | 3D solid (N=228) | 3D GGO (N=142) | P value |
|---|---|---|---|---|
| Sex, n (%) | 0.07 | |||
| Male | 271 (73.2) | 175 (76.8) | 96 (67.6) | |
| Female | 99 (26.8) | 53 (23.2) | 46 (32.4) | |
| Median age, years | 69 | 69 | 67 | 0.019 |
| Median Brinkman index | 690 | 750 | 618 | 0.037 |
| Median SUVmax | 4.95 | 7.1 | 4.03 | <0.001 |
| Median CEA, ng/mL | 3.2 | 6.87 | 4.04 | 0.005 |
| cStage (ver. 8), n (%) | 0.002 | |||
| IA1 | 23 (6.2) | 8 (3.5) | 15 (10.6) | |
| IA2 | 201 (54.4) | 117 (51.3) | 84 (59.2) | |
| IA3 | 146 (39.4) | 103 (45.2) | 43 (30.3) | |
| Surgery, n (%) | 0.183 | |||
| Lobectomy | 283 (76.5) | 181 (79.4) | 102 (71.8) | |
| Segmentectomy | 55 (14.8) | 28 (12.3) | 27 (19.0) | |
| Wedge resection | 32 (8.7) | 19 (8.3) | 13 (9.2) | |
| pStage (ver. 8), n (%) | <0.001 | |||
| 0–IA3 | 192 (51.9) | 97 (42.5) | 95 (66.9) | |
| 0 | 4 (1.0) | 1 (0.4) | 3 (2.1) | |
| IA1 | 36 (9.7) | 8 (3.5) | 28 (19.7) | |
| IA2 | 99 (26.7) | 52 (22.8) | 47 (33.1) | |
| IA3 | 53 (14.3) | 36 (15.8) | 17 (12.0) | |
| IB–IIIB | 178 (48.1) | 131 (57.5) | 47 (33.1) | |
| IB | 95 (25.6) | 70 (30.7) | 25 (17.6) | |
| IIA | 2 (0.5) | 1 (0.4) | 1 (0.7) | |
| IIB | 47 (12.7) | 34 (14.9) | 13 (9.2) | |
| IIIA | 28 (7.5) | 22 (9.6) | 6 (4.2) | |
| IIIB | 6 (1.5) | 4 (1.7) | 2 (1.4) | |
| Histology, n (%) | 0.001 | |||
| Adenocarcinoma | 259 (70.0) | 143 (62.7) | 116 (81.7) | |
| Squamous cell carcinoma | 96 (25.9) | 75 (32.9) | 21 (14.8) | |
| Others | 15 (4.1) | 10 (4.4) | 5 (3.5) | |
| Pathological findings, n (%) | ||||
| Ly (+) | 158 (42.7) | 119 (52.2) | 39 (27.5) | <0.001 |
| V (+) | 215 (58.1) | 154 (67.5) | 61 (43.0) | <0.001 |
| LN meta (+) | 67 (18.1) | 51 (22.4) | 16 (11.2) | 0.044 |
| Lepidic (+) | 147 (39.7) | 63 (27.6) | 84 (59.2) | <0.001 |
| Lepidic predominant (+) | 38 (10.3) | 12 (5.3) | 26 (18.3) | <0.001 |
3D, three-dimensional; GGO, ground glass opacity; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; Ly, lymphatic invasion; V, vascular invasion; LN meta, lymph node metastasis.
The Kaplan-Meier curves for OS and RFS are shown in Figure 2. In comparison to the 3D solid group and the 3D GGO group, the 5-year OS rate was 74.1% and 87.8%, respectively, while the 5-year RFS rate was 65.6% and 84.9%. 3D GGO was associated with a significantly better prognosis for both OS and RFS. Univariate and multivariate analyses using a Cox proportional hazards model for OS also showed that age, squamous cell carcinoma, lymph node metastasis, and 3D solid were independent poor prognostic factors. Lepidic and lepidic predominant pathological findings tended to be associated with a better prognosis, but did not show statistical significance in a univariate analysis (Table 2). Similarly, univariate and multivariate analyses using a Cox proportional hazards model for RFS revealed that age, lymphatic invasion, and lymph node metastasis as well as 3D solid, were also independent poor prognostic factors. Similarly, in RFS, lepidic predominant pathology tended to be associated with a favorable prognosis, but there was no significant difference in univariate (Table 3).
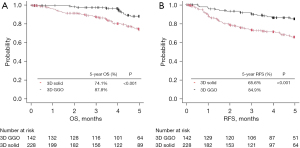
Table 2
| Factor | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Male | 1.827 | 1.002–3.329 | 0.049 | 1.667 | 0.894–3.107 | 0.107 | |
| Age high | 2.879 | 1.717–4.827 | <0.001 | 2.543 | 1.488–4.348 | <0.001 | |
| Brinkman index high | 1.305 | 0.82–2.077 | 0.26 | ||||
| 3D solid | 2.782 | 1.576–4.91 | <0.001 | 1.981 | 1.107–3.544 | 0.021 | |
| SUVmax high | 1.475 | 0.929–2.34 | 0.098 | ||||
| Squamous cell carcinoma | 2.772 | 1.747–4.397 | <0.001 | 2.351 | 1.433–3.858 | <0.001 | |
| Ly | 1.694 | 1.069–2.686 | 0.024 | 1.266 | 0.785–2.042 | 0.332 | |
| V | 1.037 | 0.65–1.655 | 0.877 | ||||
| LN meta | 1.851 | 1.096–3.125 | 0.021 | 2.535 | 1.447–4.441 | 0.001 | |
| Lepidic | 0.75 | 0.458–1.229 | 0.253 | ||||
| Lepidic predominant | 0.747 | 0.323–1.725 | 0.494 | ||||
3D, three-dimensional; SUVmax, maximum standardized uptake value; Ly, lymphatic invasion; V, vascular invasion; LN meta, lymph node metastasis; HR, hazard ratio; CI, confidence interval.
Table 3
| Factor | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Male | 0.783 | 0.5–1.228 | 0.287 | ||||
| Age high | 1.607 | 1.044–2.473 | 0.031 | 2.015 | 1.29–3.147 | 0.002 | |
| Brinkman index high | 0.721 | 0.472–1.101 | 0.129 | ||||
| 3D pure solid | 2.605 | 1.581–4.291 | <0.001 | 1.815 | 1.094–3.012 | 0.021 | |
| SUVmax high | 2.379 | 1.54–3.676 | <0.001 | 1.374 | 0.862–2.19 | 0.181 | |
| Squamous cell carcinoma | 0.98 | 0.595–1.615 | 0.938 | ||||
| Ly | 3.734 | 2.383–5.852 | <0.001 | 2.548 | 1.595–4.072 | <0.001 | |
| V | 2.449 | 1.511–3.969 | <0.001 | 1.299 | 0.768–2.197 | 0.328 | |
| LN meta | 4.961 | 3.245–7.585 | <0.001 | 3.989 | 2.531–6.286 | <0.001 | |
| Lepidic | 1.286 | 0.844–1.958 | 0.24 | ||||
| Lepidic predominant | 0.457 | 0.185–1.129 | 0.08 | ||||
3D, three-dimensional; SUVmax, maximum standardized uptake value; Ly, lymphatic invasion; V, vascular invasion; LN meta, lymph node metastasis; HR, hazard ratio; CI, confidence interval.
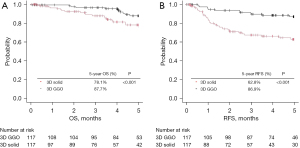
In addition, propensity score matching was performed to control for confounding factors and reduce prognostic imbalance caused by selection bias. Patient background factors and the number of cases other than the pathological stage and pathological findings were arranged (Table 4). As a result, there were 117 cases in both the 3D solid and 3D GGO groups, and a comparison between the two groups was performed again. As before propensity score matching, pathological stage IB–IIIB, lymphatic invasion, vascular invasion, and lymph node metastasis were significantly more frequent in the 3D solid group, and lepidic and lepidic predominant pathological findings were significantly more frequent in the 3D GGO group. In addition, Figure 3 shows the Kaplan-Meier curves of the two groups for OS and RFS after propensity score matching. Similar to the analysis before propensity score matching, 3D GGO was associated with a significantly better prognosis (5-year OS: 78.1% vs. 87.7%, P=0.001; 5-year RFS: 62.8% vs. 86.9%, P<0.001). Univariate and multivariate analyzes for OS and RFS also showed that 3D solid was an independent poor prognostic factor as before propensity score matching (Tables S1,S2).
Table 4
| Characteristic | Total (N=234) | 3D solid (N=117) | 3D GGO (N=117) | P value |
|---|---|---|---|---|
| Sex | 1 | |||
| Male | 162 (69.2) | 81 (69.2) | 81 (69.2) | |
| Female | 72 (30.8) | 36 (30.8) | 36 (30.8) | |
| Age, years | 69 | 67 | 67 | 0.989 |
| Brinkman index | 620 | 694 | 637 | 0.468 |
| SUVmax | 5.6 | 5.72 | 4.3 | 0.124 |
| CEA, ng/mL | 4.2 | 4.19 | 4.11 | 0.452 |
| cStage (ver. 8) | 1 | |||
| IA1 | 12 (5.1) | 6 (5.1) | 6 (5.1) | |
| IA2 | 138 (59.0) | 69 (59.0) | 69 (59.0) | |
| IA3 | 84 (35.9) | 42 (35.9) | 42 (35.9) | |
| Surgery | 1 | |||
| Lobectomy | 182 (77.8) | 91 (77.8) | 91 (77.8) | |
| Segmentectomy | 32 (13.7) | 16 (13.7) | 16 (13.7) | |
| Wedge resection | 20 (8.5) | 10 (8.5) | 10 (8.5) | |
| pStage (ver. 8) | 0.001 | |||
| 0–IA3 | 125 (53.4) | 49 (41.9) | 76 (65.0) | |
| 0 | 3 (1.3) | 1 (0.9) | 2 (1.7) | |
| IA1 | 19 (8.1) | 3 (2.6) | 16 (13.7) | |
| IA2 | 69 (29.5) | 28 (23.9) | 41 (35.0) | |
| IA3 | 34 (14.5) | 17 (14.5) | 17 (14.5) | |
| IB–IVA | 109 (46.6) | 68 (58.1) | 41 (35.0) | |
| IB | 54 (23.1) | 32 (27.4) | 22 (18.8) | |
| IIA | 1 (0.4) | 0 (0.0) | 1 (0.9) | |
| IIB | 32 (13.7) | 20 (17.1) | 12 (10.3) | |
| IIIA | 18 (7.7) | 14 (12.0) | 4 (3.4) | |
| IIIB | 4 (1.7) | 2 (1.8) | 2 (1.7) | |
| Histology | 0.076 | |||
| Adenocarcinoma | 172 (73.5) | 79 (67.5) | 93 (79.5) | |
| Squamous cell carcinoma | 53 (22.6) | 34 (29.1) | 19 (16.2) | |
| Others | 9 (3.8) | 4 (3.4) | 5 (4.3) | |
| Pathological findings | ||||
| Ly (+) | 100 (42.7) | 66 (56.4) | 34 (29.1) | <0.001 |
| V (+) | 136 (58.1) | 82 (70.1) | 54 (46.2) | <0.001 |
| LN meta (+) | 43 (18.4) | 30 (25.6) | 13 (11.1) | 0.006 |
| Lepidic (+) | 97 (41.4) | 33 (28.2) | 64 (54.7) | <0.001 |
| Lepidic predominant (+) | 23 (9.8) | 4 (3.4) | 19 (16.2) | 0.002 |
Data are shown as the median or number (percentage). 3D, three-dimensional; GGO, ground glass opacity; SUVmax, maximum standardized uptake value; CEA, carcinoembryonic antigen; Ly, lymphatic invasion; V, vascular invasion; LN meta, lymph node metastasis.
Discussion
This study shows that the C/T volume ratio calculated using the 3D analysis workstation can predict the presence of GGO more accurately than the 2D image. GGO was present in 3D even if it was purely solid in 2D, and it was shown that GGO lesions in 3D had a good prognosis. Previous studies suggest that the maximum size of the solid part is better than the whole tumor size in conventional CT to predict its invasiveness and prognosis in part solid nodules (19-21). The maximum size of the solid part is incorporated as the clinical T-factor for lung cancer in the 8th edition of the International Union for Control of Cancer TNM classification. In recent years, with the spread of 3D analysis workstations, there are reports that the 3D parameters calculated by such workstations can more accurately evaluate pathological invasiveness and lymph node metastasis (22). We previously compared various parameters calculated by a 3D analysis with other factors, including the C/T ratio, and showed a better correlation of the C/T volume ratio in early-stage lung cancer with pathological non-invasive cancer. We reported on its usefulness (15).
Lung cancer is classified as pure solid or part solid based on CT findings, and solid lung cancer is said to have a worse prognosis than part solid because of its rapid growth and rapid metastasis (23-25). According to our analysis in the present study, even in 2D pure solid cases, a good prognosis was observed in the 3D GGO group when it was judged, based on the C/T volume ratio determined by a 3D analysis, to contain GGO. Therefore, our findings—which were based on 3D analysis—were in line with previous studies reporting that the prognosis of part solid with GGO is better than that of pure solid without GGO (8-11). We speculate that this is due to the fact that tumors can have distorted shapes in 3D rather than the pure round or oval shapes observed in 2D. Although 3D analysis workstations are gradually becoming popular, they are still not available at all facilities, and it may be difficult to use the C/T volume ratio calculated from them as a universal poor prognostic factor at the present time. Based on the results of recent large-scale prospective studies, it is expected that the number of segmentectomy procedures performed for early-stage lung cancer will increase in the future. However, because of the high local recurrence rate, it is considered important to ensure an accurate surgical margin using a 3D analysis workstation when performing segmentectomy. Along with this, it is assumed that the spread of 3D analysis workstations will continue in the future. Therefore, we believe that the versatility of the C/T volume ratio calculated by 3D analyses will increase in the future.
There are many cases in which there is a large difference between the C/T ratio and the C/T volume ratio. Therefore, these are similar but different indicators, and we believe that the C/T volume ratio can more accurately evaluate pathological findings and the prognosis. As a result of using the C/T ratio, the Japan Clinical Oncology Group (JCOG)0802/West Japan Oncology Group (WJOG)4607L demonstrated the superiority of segmentectomy in terms of the prognosis, in comparison to lobectomy in solid predominantly early lung cancer of ≤2 cm (4). However, it remains possible that different results could be obtained if the pathologic findings and prognosis are better screened using the C/T volume ratio. In other words, there may be a certain number of cases in which lobectomy, which is the conventional standard surgery, should be performed instead of aggressive segmentectomy, even for early-stage lung cancer.
In addition, there are reports that the SUVmax and its visual evaluation obtained from 18F-fluorodeoxyglucose-PET can reflect the prognosis and malignancy as a prognostic stratification factor other than the C/T ratio in early-stage lung cancer (26,27). In our analysis in the present study, SUVmax was significantly higher in the 3D pure solid group. However, in univariate and multivariate analyses for OS and RFS, although a high SUVmax tended to be associated with a poor prognosis, no significant difference was found. Regarding the value of SUVmax, it is said that there is a large error between facilities, measuring instruments, and measurers, and it is possible that this analysis did not show a significant difference due to this. Interesting results may be obtained by visual evaluation using the Deauville score, which was reported by Kagimoto et al. (26). The results of JCOG0802/WJOG4607L are of great significance to thoracic surgeons, and there is no objection to expanding the indications for segmentectomy. However, we believe that the expanded range of indications should be studied and examined from various angles in the future.
The present study was associated with some limitations. First, it was a retrospective study conducted in a single center. Second, the correlation with the C/T volume ratio measured by other 3D analysis workstations with similar functions is unknown. However, among the existing 3D analysis workstations used for surgical medicine, the Synapse Vincent system that we used is said to have the highest market share, and the system was as versatile as possible. Third, potential measurement error by Synapse Vincent should be considered. This workstation sometimes recognizes blood vessels running inside the tumor as solid components, and also recognizes the area around the tumor that seems to be an air-filled defective site as GGO. An accurate cut-off value for the C/T volume ratio that includes such errors is an issue for future study. Fourth, we were unable to evaluate the micropapillary and STAS statuses, which are pathological poor prognostic factors. If these items were added to the Cox proportional hazards analysis, it is possible that the analysis would obtain different results. Fifth, this study that as it is an observational study, we could not eliminate the potential effect of the higher clinical stage in 3D solid group on the poorer outcome in this group. Further prospective clinical trial is recommended to overcome this limitation.
Conclusions
In conclusion, from the results of this study, tumors judged to be pure solid by a 2D analysis could be further classified, based on a 3D analysis, into a solid group with a poorer prognosis and a GGO group with a relatively favorable prognosis. We found that the C/T volume ratio determined using 3D analysis could accurately reflect the pathological findings. Moreover, it was found to be an independent prognostic factor. In the future, we expect that various studies using the C/T volume ratio will be conducted at other facilities and multiple institutions, and it will be necessary to confirm the validity of reduction surgery for patients with 3D solid findings, which are associated with a truly poor prognosis in early lung cancer.
Acknowledgments
We thank Japan Medical Communication for editing the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-341/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-341/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-341/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-341/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Review Board (IRB) of Seirei Mikatahara General Hospital approved the study protocol and publication of data (No. 22-64, Approval date: February 24, 2023). Informed consent was obtained from each patient before examination and the contents of this study were disclosed at our hospital. The medical records of each patient were retrospectively reviewed under a waiver of authorization approved by our IRB.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Chen YC, Lin YH, Chien HC, et al. Preoperative consolidation-to-tumor ratio is effective in the prediction of lymph node metastasis in patients with pulmonary ground-glass component nodules. Thorac Cancer 2021;12:1203-9. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Consolidation/Tumor Ratio on Chest Computed Tomography as Predictor of Postoperative Nodal Upstaging in Clinical T1N0 Lung Cancer. World J Surg 2018;42:2872-8. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Suzuki K, Wakabayashi M, Moriya Y, et al. A Nonrandomized Confirmatory Phase III Study of Sublobar Surgical Resection for Peripheral Ground Glass Opacity Dominant Lung Cancer Defined with Thoracic Thin-section Computed Tomography (JCOG0804/WJOG4507L). J Clin Oncol 2017;35:abstr 8561.
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Fukui M, et al. Prognostic Impact of Very Small Ground-Glass Opacity Component in Stage IA Solid Predominant Non-small Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Berry MF, Gao R, Kunder CA, et al. Presence of Even a Small Ground-Glass Component in Lung Adenocarcinoma Predicts Better Survival. Clin Lung Cancer 2018;19:e47-51. [Crossref] [PubMed]
- Aokage K, Miyoshi T, Ishii G, et al. Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:533-42. [Crossref] [PubMed]
- Kim H, Goo JM, Kim YT, et al. Validation of the Eighth Edition Clinical T Categorization System for Clinical Stage IA, Resected Lung Adenocarcinomas: Prognostic Implications of the Ground-Glass Opacity Component. J Thorac Oncol 2020;15:580-8.
- Kuriyama K, Tateishi R, Doi O, et al. CT-pathologic correlation in small peripheral lung cancers. AJR Am J Roentgenol 1987;149:1139-43. [Crossref] [PubMed]
- Koizumi N, Akita S, Sakai K, et al. Cloudy nodule on HRCT: a new clinico-radiologic entity of pulmonary adenocarcinoma. Radiat Med 1995;13:273-8. [PubMed]
- Kuriyama K, Seto M, Kasugai T, et al. Ground-glass opacity on thin-section CT: value in differentiating subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol 1999;173:465-9. [Crossref] [PubMed]
- Tsuchida H, Tanahashi M, Suzuki E, et al. Pathologically noninvasive cancer predictors and surgical procedure for peripheral lung cancer. Thorac Cancer 2023;14:289-97. [Crossref] [PubMed]
- Shimomura M, Iwasaki M, Ishihara S, et al. Volume-Based Consolidation-to-Tumor Ratio Is a Useful Predictor for Postoperative Upstaging in Stage I and II Lung Adenocarcinomas. Thorac Cardiovasc Surg 2022;70:265-72. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Nakamura S, Fukui T, Taniguchi T, et al. Prognostic impact of tumor size eliminating the ground glass opacity component: modified clinical T descriptors of the tumor, node, metastasis classification of lung cancer. J Thorac Oncol 2013;8:1551-7. [Crossref] [PubMed]
- Yoshida Y, Sakamoto M, Maeda E, et al. Can image analysis on high-resolution computed tomography predict non-invasive growth in adenocarcinoma of the lung? Ann Thorac Cardiovasc Surg 2015;21:8-13. [Crossref] [PubMed]
- Liu Y, Sun H, Zhou F, et al. Imaging features of TSCT predict the classification of pulmonary preinvasive lesion, minimally and invasive adenocarcinoma presented as ground glass nodules. Lung Cancer 2017;108:192-7. [Crossref] [PubMed]
- Shikuma K, Menju T, Chen F, et al. Is volumetric 3-dimensional computed tomography useful to predict histological tumour invasiveness? Analysis of 211 lesions of cT1N0M0 lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;22:831-8. [Crossref] [PubMed]
- Oda S, Awai K, Murao K, et al. Volume-doubling time of pulmonary nodules with ground glass opacity at multidetector CT: Assessment with computer-aided three-dimensional volumetry. Acad Radiol 2011;18:63-9. [Crossref] [PubMed]
- Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg 2011;92:1819-23; discussion 1824-5. [Crossref] [PubMed]
- Lee SM, Park CM, Paeng JC, et al. Accuracy and predictive features of FDG-PET/CT and CT for diagnosis of lymph node metastasis of T1 non-small-cell lung cancer manifesting as a subsolid nodule. Eur Radiol 2012;22:1556-63. [Crossref] [PubMed]
- Kagimoto A, Tsutani Y, Handa Y, et al. Patient Selection of Sublobar Resection Using Visual Evaluation of Positron-Emission Tomography (PET) for Early-Stage Lung Adenocarcinoma. Ann Surg Oncol 2021;28:2068-75. [Crossref] [PubMed]
- Nair VS, Barnett PG, Ananth L, et al. PET scan 18F-fluorodeoxyglucose uptake and prognosis in patients with resected clinical stage IA non-small cell lung cancer. Chest 2010;137:1150-6. [Crossref] [PubMed]


