
Value of cardiopulmonary exercise testing in the assessment of symptoms and quality of life in Asian patients with chronic obstructive pulmonary disease
Highlight box
Key findings
• Among the parameters of cardiopulmonary exercise testing (CPET), maximal oxygen uptake (VO2 max) and physiological dead space/tidal volume ratio at peak exercise (VD/VT peak) comprehensively reflect symptoms and quality of life in patients with COPD.
What is known and what is new?
• Previous studies assessed the correlation between CPET parameters and symptoms and quality of life by using complex instruments, and the correlation was weak. Also, no studies were conducted with Asian patients with COPD.
• We discovered that VO2 max and VD/VT peak significantly correlate with the mMRC grade and COPD Assessment Test score. In addition, we proposed the optimal cut-off values of VO2 max and VD/VT peak for predicting the onset of clinically significant dyspnea and poor quality of life.
What is the implication, and what should change now?
• VO2 max and VD/VT peak are reliable indicators of symptoms and health-related quality of life in patients with COPD and would suggest when to consider more aggressive treatment.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation (1) and many accompanying chronic respiratory symptoms (2). These symptoms worsen with disease progression, resulting in functional limitations and reduced quality of life (3). Therefore, it is important to evaluate the subjective complaints of patients with COPD. Several methods for assessing symptoms are available, among which the modified Medical Research Council (mMRC) dyspnea scale is mainly used to estimate the severity of dyspnea (1,4). The mMRC dyspnea scale is known to be strongly correlated with other methods of assessing the health status of patients (5) and is useful for predicting mortality (6). The COPD Assessment Test (CAT) is also a widely used method for evaluating symptoms (7). Rather than simply measuring breathlessness, CAT evaluates other respiratory symptoms and the ability to perform daily activities, thereby estimating the quality of life (8). The mMRC dyspnea scale and CAT are relatively simple measures that are included in the criteria for determining the treatment of COPD in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (1).
Cardiopulmonary exercise testing (CPET) is an important tool for diagnosis and functional evaluation in patients with cardiopulmonary diseases (9-12). CPET parameters are used to evaluate integrative physiological responses during maximal exercise and help identify factors that may be causing exercise limitations (13). In many cases, multiple factors are involved, including cardiopulmonary, musculoskeletal, neurological, metabolic, and psychological factors (13). In particular, as multiple factors often contribute to exercise intolerance in patients with COPD (9,14), CPET can provide useful information that can guide the diagnosis and subsequent treatment of COPD.
Thus far, only a few studies have examined the relationship of CPET parameters to symptoms and quality of life in patients with COPD. Previous studies assessed symptoms and quality of life by using instruments such as the St. George’s Respiratory Questionnaire (SGRQ) (15), 20-item Chronic Respiratory Disease Questionnaire (16), and Nijmegen Integral Assessment Framework (NIAF) (17), all of which are too complex to use in daily practice. In those studies, only maximal oxygen uptake (VO2 max), breathing reserve, and VO2/work rate ratio were found to be correlated with some of the instruments used, and the degree of relationship were weak. Furthermore, no study has investigated the utility of CPET parameters in evaluating subjective complaints in Asian patients with COPD.
In this study, we investigated which CPET parameters can explain and reflect the symptoms and quality of life of Asian patients with COPD, as assessed using the mMRC grade and CAT score. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-185/rc).
Methods
Patients
This retrospective single-center study enrolled 681 adult patients who underwent CPET at Asan Medical Center (Seoul, Republic of Korea) between January 2020 and June 2022. Patients in whom COPD had been diagnosed on the basis of compatible clinical symptoms and confirmed using spirometry with a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio of <0.7 were included. Patients were excluded for the following reasons: (I) lack of medical records, (II) presence of relevant comorbidities (stage IV lung cancer based on tumour-node-metastasis stage (18), heart failure, history of lung resection, interstitial lung disease, and neuromuscular disease), (III) presence of features of asthma, (IV) a submaximal test defined by a maximal respiratory exchange ratio of <1.15, and (V) any COPD exacerbation within the previous two months. Clinical data, including age, sex, body weight, height, body mass index, and symptoms, were reviewed from medical records.
Assessment of symptoms
The mMRC dyspnea scale was administered to assess the degree of dyspnea. The mMRC scale categorizes patients into one of five grades (0–4) based on the degree of dyspnea (1). CAT was used to evaluate the patients’ quality of life, including respiratory symptoms (e.g., cough and chest tightness), ability to perform daily activities, and sleep. Each item is scored from 0 to 5 points, with a higher score indicating a worse outcome. As the CAT questionnaire contains a total of eight items, the worst possible score is 40 points (8). Previous studies have demonstrated that CAT scores are strongly associated with SGRQ scores (7). Patients were stratified into two groups based on mMRC grade (either <2 or ≥2) and CAT score (either <10 or ≥10), differentiating those with less subjective and more subjective complaints.
CPET
CPET was performed using an electronically braked cycle ergometer (VIAsprint 150P; CareFusion Inc., San Diego, CA, USA) (19). A continuous incremental protocol based on the American Thoracic Society/American College of Chest Physicians guidelines on CPET was selected, and the test was performed under medical supervision (13,20). Inhaled and exhaled gas concentrations were measured through a face mask (V2TM Oro-Nasal Mask; Hans Rudolph Inc., Kansas City, MO, USA) and analyzed using the breath-by-breath method (13). All patients underwent spirometry before CPET on the same day. During CPET, electrocardiography, arterial saturation, heart rate, and blood pressure were monitored.
The test consisted of four phases: resting, warm-up, exercise, and recovery. During the resting phase, the patients rested for 2 min without pedalling and baseline data were collected. In the warm-up phase, the patients started pedalling without a load for 1 min and 30 s at a speed of 30–40 revolutions per minute (RPM). In the exercise phase, the patients pedalled at 65 RPM with increasing load at an individualized speed of 5–15 W/min, depending on fitness and spirometry results. The patients were encouraged to exercise up to their maximum capacity, for >10 min if possible. However, even if the maximum capacity was not reached, the test was immediately terminated on the patients’ request or in case of abnormal monitoring results. In the recovery phase, the patients pedalled at 30–40 RPM with no load until stabilization of vital signs.
Statistical analysis
Continuous variables are presented as means and standard deviations, and categorical variables are described as counts and percentages. The comparison of CPET parameters among groups stratified by symptom severity was conducted using Student’s t-test. The Kolmogorov-Smirnov test was used to test data normality. For variables with a normal distribution, we used Pearson’s correlation coefficients. For variables that did not follow a normal distribution, Spearman’s correlation coefficients were employed. Receiver operating characteristic (ROC) analysis and the Youden index were used to determine cut-off values. In all analyzes, statistical significance was determined at P<0.05 and 95% confidence interval. IBM Statistical Package for the Social Sciences (version 24.0; SPSS Inc., Chicago, IL, USA) was used for data analysis.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) of the Asan Medical Center (approval No. 2021-0915), and individual consent for this retrospective analysis was waived.
Results
Between January 2020 and June 2022, a total of 681 adult patients underwent CPET at Asan Medical Center. Of these patients, 268 had been diagnosed with COPD. The most common indications for CPET were pre-operative evaluation and investigation of dyspnea. Of the 268 patients with COPD, 73 patients were excluded according to the exclusion criteria. Finally, 195 patients were included in the analysis (Figure 1).

The patients’ baseline characteristics, spirometry results, mMRC grades, and CAT scores are shown in Table 1, while CPET parameter values are summarized in Table 2.
Table 1
| Baseline characteristics | Values |
|---|---|
| No. | 195 |
| Age, years | 68.3±9.0 |
| BMI, kg/m2 | 24.0±3.4 |
| Height, cm | |
| Male | 166.7±5.9 |
| Female | 153.9±5.3 |
| Weight, kg | |
| Male | 66.8±9.9 |
| Female | 55.4±13.8 |
| Sex | |
| Male | 185 (94.8) |
| Female | 10 (5.2) |
| FEV1, L | 1.92±0.48 |
| FEV1, % predicted | 64.1±13.2 |
| GOLD 1 | 14 (7.2) |
| GOLD 2 | 147 (75.4) |
| GOLD 3 | 31 (15.9) |
| GOLD 4 | 3 (1.5) |
| FEV1/FVC, % | 57.1±10.5 |
| DLCO, % predicted | 67.3±18.6 |
| mMRC dyspnea scale | |
| Grade 0 | 115 (58.9) |
| Grade 1 | 55 (28.2) |
| Grade 2 | 17 (8.7) |
| Grade 3 | 4 (2.1) |
| Grade 4 | 4 (2.1) |
| CAT score | 8.9±5.1 |
| 0–5 | 51 (26.2) |
| 6–10 | 84 (43.0) |
| 11–15 | 41 (21.0) |
| 16–20 | 13 (6.7) |
| 21–40 | 6 (3.1) |
Continuous variables are presented as mean ± standard deviation, and categorical variables are described as counts and percentages. BMI, body mass index; FEV1, forced expiratory volume in 1 s; GOLD, Global initiative for Chronic Obstructive Lung Disease; FVC, forced vital capacity; VC, vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; mMRC, modified Medical Research Council; CAT, chronic obstructive pulmonary disease Assessment Test.
Table 2
| CPET parameters | Values |
|---|---|
| VO2 max, L/min | 1.11±0.34 |
| VO2 max, mL/kg/min | 16.8±4.7 |
| AT, L/min | 0.67±0.24 |
| AT, % predicted VO2 max | 39.9±13.9 |
| WR max, W | 87.5±28.8 |
| HR max, beats/min | 134.0±20.4 |
| HRR, beats/min | 25.4±17.7 |
| O2 pulse max, mL/beat | 8.84±4.93 |
| O2 pulse max, % predicted | 80.4±24.3 |
| Systolic BP max, mmHg | 200.8±32.69 |
| VE max, L/min | 51.3±15.4 |
| MVV, L/min | 81.3±25.2 |
| Peak PETCO2, mmHg | 36.8±5.3 |
| Breathing reserve, % | 34.1±17.8 |
| VD/VT at rest | 0.39±0.07 |
| VD/VT peak | 0.24±0.05 |
Data are presented as mean ± standard deviation. VO2 max, maximal oxygen uptake; AT, anaerobic threshold; WR, work rate; HR, heart rate; HRR, heart rate reserve; BP, blood pressure; SpO2, saturation of percutaneous oxygen; VE, minute ventilation; MVV, maximal voluntary ventilation; PETCO2, end-tidal PCO2; VD, dead space volume; VT, tidal volume.
The comparison of CPET results between groups with less subjective and more subjective complaints are shown in Tables 3,4. Age did not differ between the two groups based on both mMRC grade and CAT score. In the comparison according to the mMRC scale, the group with higher mMRC grades showed significantly lower VO2 max (1.15±0.32 vs. 0.81±0.30 L/min, P<0.001), lower maximal minute ventilation (VE max) (53.1±14.7 vs. 39.4±14.7 L/min, P<0.001), and higher VD/VT peak (0.23±0.04 vs. 0.27±0.06, P=0.006). Further, no significant difference in breathing reserve and VD/VT at rest was found between the two groups divided according to mMRC grade. In the comparison according to the CAT result, the group with higher CAT scores showed significantly lower VO2 max (1.18±0.33 vs. 0.99±0.33 L/min, P<0.001), lower VE max (53.6±15.1 vs. 47.3±15.0, L/min, P=0.005), higher VD/VT at rest (0.38±0.07 vs. 0.40±0.05, P=0.002), and higher VD/VT peak (0.23±0.04 vs. 0.25±0.05, P=0.001). CPET parameters exhibiting differences between the two groups were subjected to logistic regression analysis, and the findings are summarized in Tables S1,S2. Maximal voluntary ventilation (MVV) and VD/VT peak emerged as independent predictors of severe dyspnea.
Table 3
| CPET parameters | mMRC grade <2 | mMRC grade ≥2 | P value |
|---|---|---|---|
| Age, years | 68.3±7.8 | 68.0±15.0 | 0.895 |
| VO2 max, L/min | 1.15±0.32 | 0.81±0.30 | <0.001 |
| VO2 max, mL/kg/min | 17.4±4.6 | 12.9±4.19 | <0.001 |
| AT, L/min | 0.69±0.23 | 0.57±0.29 | 0.028 |
| AT, % predicted VO2 max | 40.5±13.3 | 36.2±17.1 | 0.163 |
| WR max, W | 91.8±26.6 | 58.1±26.7 | <0.001 |
| HR max, beats/min | 135.5±20.8 | 124.0±13.3 | 0.001 |
| HRR, beats/min | 24.0±18.0 | 34.8±12.8 | 0.004 |
| O2 pulse max, mL/beat | 9.15±5.12 | 6.77±2.63 | 0.024 |
| O2 pulse max, % predicted | 82.1±24.0 | 69.0±26.7 | 0.011 |
| Systolic BP max, mmHg | 201.3±32.2 | 196.8±36.0 | 0.515 |
| VE max, L/min | 53.1±14.7 | 39.4±14.7 | <0.001 |
| MVV, L/min | 84.9±23.8 | 57.2±21.0 | <0.001 |
| Peak PETCO2, mmHg | 37.1±4.9 | 34.3±6.6 | 0.012 |
| Breathing reserve, % | 34.7±18.1 | 29.3±15.5 | 0.120 |
| VD/VT at rest | 0.38±0.07 | 0.39±0.05 | 0.483 |
| VD/VT peak | 0.23±0.04 | 0.27±0.06 | 0.006 |
Data are presented as mean ± standard deviation. CPET, cardiopulmonary exercise testing; mMRC, modified Medical Research Council; VO2 max, maximal oxygen uptake; AT, anaerobic threshold; WR, work rate; HR, heart rate; HRR, heart rate reserve; BP, blood pressure; SpO2, saturation of percutaneous oxygen; VE, minute ventilation; MVV, maximal voluntary ventilation; PETCO2, end-tidal PCO2; VD, dead space volume; VT, tidal volume.
Table 4
| CPET parameters | CAT score <10 | CAT score ≥10 | P value |
|---|---|---|---|
| Age, years | 68.3±7.7 | 68.3±11.0 | 0.994 |
| VO2 max, L/min | 1.18±0.33 | 0.99±0.33 | <0.001 |
| VO2 max, mL/kg/min | 17.5±4.7 | 15.5±4.6 | 0.004 |
| AT, L/min | 0.70±0.23 | 0.62±0.24 | 0.023 |
| AT, % predicted VO2 max | 40.8±13.3 | 38.4±14.8 | 0.265 |
| WR max, W | 94.6±24.9 | 75.1±31.1 | <0.001 |
| HR max, beats/min | 136.3±21.7 | 130.0±17.3 | 0.027 |
| HRR, beats/min | 22.8±18.2 | 29.7±16.1 | 0.009 |
| O2 pulse max, mL/beat | 9.35±5.83 | 7.96±2.54 | 0.058 |
| O2 pulse max, % predicted | 81.8±25.6 | 78.0±21.7 | 0.294 |
| Systolic BP max, mmHg | 202.4±32.6 | 198.0±32.8 | 0.365 |
| VE max, L/min | 53.6±15.1 | 47.3±15.0 | 0.005 |
| MVV, L/min | 86.5±24.3 | 72.3±24.4 | <0.001 |
| Peak PETCO2, mmHg | 37.2±5.0 | 36.0±5.6 | 0.115 |
| Breathing reserve, % | 34.9±17.9 | 32.5±17.8 | 0.364 |
| VD/VT at rest | 0.38±0.07 | 0.40±0.05 | 0.002 |
| VD/VT peak | 0.23±0.04 | 0.25±0.05 | 0.001 |
Data are presented as mean ± standard deviation. CPET, cardiopulmonary exercise testing; CAT, chronic obstructive pulmonary disease Assessment Test; VO2 max, maximal oxygen uptake; AT, anaerobic threshold; WR, work rate; HR, heart rate; HRR, heart rate reserve; BP, blood pressure; SpO2, saturation of percutaneous oxygen; VE, minute ventilation; MVV, maximal voluntary ventilation; PETCO2, end-tidal PCO2; VD, dead space volume; VT, tidal volume.
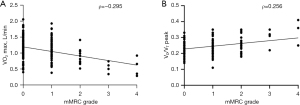
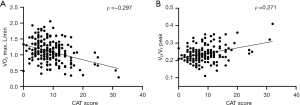
The correlations among mMRC grade, CAT score, CPET parameters, and FEV1 are described in Table 5. A correlation between mMRC grade and VO2 max (L/min) was observed (Spearman’s correlation coefficient ρ=−0.295, P<0.001), as well as between mMRC grade and VD/VT peak (ρ=0.256, P<0.001), as illustrated in Figure 2. Similarly, CAT score demonstrated a correlation with both VO2 max (L/min) (Spearman’s correlation coefficient ρ=−0.297, P<0.001) and VD/VT peak (ρ=0.271, P<0.001), depicted in Figure 3. The mMRC grade and CAT score had no significant correlation with breathing reserve (ρ=−0.108, P=0.13 and ρ=−0.122, P=0.089, respectively).
Table 5
| Variables | mMRC grade | CAT score | |||
|---|---|---|---|---|---|
| ρ† | P value | ρ† | P value | ||
| VO2 max, L/min | −0.295 | <0.001 | −0.297 | <0.001 | |
| VO2 max, mL/kg/min | −0.282 | <0.001 | −0.252 | <0.001 | |
| FEV1 | −0.387 | <0.001 | −0.299 | <0.001 | |
| O2 pulse | −0.195 | 0.006 | −0.142 | 0.047 | |
| Anaerobic threshold | −0.135 | 0.07 | −0.194 | 0.009 | |
| Breathing reserve | −0.108 | 0.13 | −0.122 | 0.089 | |
| ETCO2 | −0.131 | 0.068 | −0.145 | 0.043 | |
| VE max | −0.248 | <0.001 | −0.232 | 0.001 | |
| VD/VT peak | 0.256 | <0.001 | 0.271 | <0.001 | |
†, Spearman’s correlation coefficient. VO2 max, maximal oxygen uptake; mMRC, modified Medical Research Council; CAT, chronic obstructive pulmonary disease Assessment Test; CPET, cardiopulmonary exercise testing; FEV1, forced expiratory volume in 1 s; VE, minute ventilation; VD, dead space volume; VT, tidal volume.
Additionally, we analyzed whether FEV1 has a correlation with VO2 max and VD/VT peak. The results showed that FEV1 (L) was correlated with VO2 max (L/min) (Pearson’s correlation coefficient r=0.450, P<0.001) and VD/VT peak (r=−0.466, P<0.001).
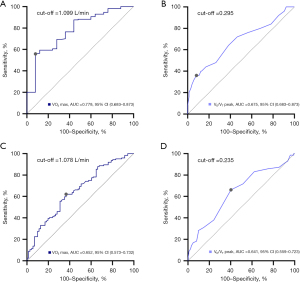
ROC curves for VO2 max and VD/VT peak were generated to determine the optimal cut-off values for predicting symptoms and quality of life in patients with COPD (Figure 4). VO2 max =1.099 L/min and VD/VT peak =0.295 were optimal predictors of dyspnea assessed using the mMRC scale. VO2 max =1.078 L/min and VD/VT peak =0.235 were also optimal thresholds for predicting quality of life estimated using the CAT score in patients with COPD.
Discussion
In this retrospective study, we evaluated the utility of CPET parameters in predicting symptoms and quality of life in Asian patients with COPD. To our knowledge, this is the first study to evaluate the correlation between CPET parameters and patients’ symptoms using the mMRC scale and CAT. Among CPET parameters, VO2 max (L/min) and VD/VT peak showed the most significant correlation with both mMRC grade and CAT score. Further, we determined the optimal cut-off values of VO2 max and VD/VT peak for predicting clinically significant symptoms and poor quality of life.
VO2 max can predict 5-year mortality and is generally considered the most important CPET parameter (21). It is determined by how much oxygen the body actually uses through the process of receiving oxygen from respiration and transporting it to the tissues (22). Therefore, various factors are involved in this process, including arterial blood oxygen partial pressure, blood hemoglobin concentration, cardiac output, and tissue perfusion (13). As dyspnea and decreased exercise capacity in patients with COPD are frequently due to extrapulmonary factors, VO2 max can be considered an important parameter in COPD (9).
A previous study reported that VO2 max improved the prediction of self-reported function and health-related quality of life after controlling FEV1 (16). Another study assessed the relationship between the NIAF health status and VO2 max in patients with COPD, and found a weak correlation (17). In accordance with previous studies, our study showed that VO2 max had a weak correlation with mMRC grade and CAT score. Because most patients in this study had mild symptoms, the relationship may have been underestimated. Therefore, we divided the study population into two groups based on mMRC grade (<2 or ≥2) and CAT score (<10 or ≥10). We observed significant differences in VO2 max between the two groups. This suggests that VO2 max is not only an indicator of prognosis in patients with COPD but is also a valuable parameter reflecting actual symptoms and quality of life in these patients. We also created ROC curves of VO2 max to determine the optimal cut-off value for predicting the onset of clinically significant symptoms and impaired quality of life. The extent to which CPET parameters vary as a disease progresses, and the clinical significance of such changes, remain inadequately understood compared to other objective measurements like FEV1 in spirometry. This lack of data contributes to a reluctance to utilize CPET, despite its proven usefulness. A decrease in VO2 max below the cut-off value during the patients’ follow-up should prompt physicians to consider more aggressive treatment, such as medication or rehabilitation, to control symptoms and ultimately improve quality of life. Additionally, improved interpretation of CPET results would facilitate its broader application in clinical practice.
In addition, we found that VO2 max was closely correlated with FEV1, which is a well-established prognostic factor and an important parameter for evaluating lung function in patients with COPD (23). The significant correlation of VO2 max with mMRC grade, CAT score, and FEV1 suggests that VO2 max can comprehensively reflect the lung function, symptoms, quality of life, and actual exercise capacity of patients with COPD.
In this study, VD/VT peak was more strongly correlated with mMRC grade and CAT score than other CPET parameters. COPD is characterized by airflow limitation, which becomes aggravated during exercise and leads to lung hyperinflation and increased dead space volume (24). When we compared VD/VT between the mMRC grade <2 and ≥2 groups, we observed a significant difference in VD/VT peak but not in VD/VT at rest between the two groups. This suggests that sudden hyperinflation of the lungs during exercise is one of the factors contributing to severe dyspnea during exercise, eventually causing exercise intolerance (25). The significant correlation of VD/VT peak with mMRC grade and CAT score implies that dyspnea and poor quality of life partially result from an increase in dead space during exercise.
VD/VT was also correlated with FEV1, with a stronger correlation between FEV1 and VD/VT peak than between FEV1 and VD/VT at rest. This suggests that air trapping during exercise and dynamic lung hyperinflation highly depend on the degree of airflow limitation.
In this study, we assessed dyspnea and quality of life in patients with COPD using the mMRC grade and the CAT score, both of which are readily available in clinical settings. The mMRC grade and CAT score demonstrated a significant correlation with VO2 max and VD/VT peak, substantiating the validity of these two straightforward questionnaires. Therefore, we advocate for the increased utilization of these tools by physicians when assessing patients with COPD.
This retrospective study conducted at a single center is limited by the existence of relevant bias. Moreover, the generalizability of our findings is limited, as the majority of patients were male and the research was conducted exclusively on an Asian population. Also, the number of patients with severe symptoms (mMRC grade ≥2 or CAT score ≥10) was relatively small. Among patients with severe symptoms, few can undergo CPET and those who can frequently perform submaximal exercise owing to several reasons, including dyspnea and deconditioning. As the correlation is expected to be stronger if more patients with severe symptoms are analyzed, a larger prospective study involving such patients is required.
Conclusions
In this study, among CPET parameters, VO2 max and VD/VT peak demonstrated significant correlations with mMRC grade and CAT score. This suggests that VO2 max and VD/VT peak are reliable indicators of symptoms and health-related quality of life in patients with COPD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-185/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-185/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-185/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-185/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Asan Medical Center (approval No. 2021-0915) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2022 report [Internet]. c2022 [cited 2022 28 November]. Available online: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf.
- Ahmed MS, Neyaz A, Aslami AN. Health-related quality of life of chronic obstructive pulmonary disease patients: Results from a community based cross-sectional study in Aligarh, Uttar Pradesh, India. Lung India 2016;33:148-53. [Crossref] [PubMed]
- Rennard S, Decramer M, Calverley PM, et al. Impact of COPD in North America and Europe in 2000: subjects' perspective of Confronting COPD International Survey. Eur Respir J 2002;20:799-805. [Crossref] [PubMed]
- Hajiro T, Nishimura K, Tsukino M, et al. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1185-9. [Crossref] [PubMed]
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [Crossref] [PubMed]
- Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002;121:1434-40. [Crossref] [PubMed]
- Karloh M, Fleig Mayer A, Maurici R, et al. The COPD Assessment Test: What Do We Know So Far?: A Systematic Review and Meta-Analysis About Clinical Outcomes Prediction and Classification of Patients Into GOLD Stages. Chest 2016;149:413-25. [Crossref] [PubMed]
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648-54. [Crossref] [PubMed]
- ERS Task Force. Recommendations on the use of exercise testing in clinical practice. Eur Respir J 2007;29:185-209. [PubMed]
- Ferrazza AM, Martolini D, Valli G, et al. Cardiopulmonary exercise testing in the functional and prognostic evaluation of patients with pulmonary diseases. Respiration 2009;77:3-17. [Crossref] [PubMed]
- DeCato TW, Haverkamp H, Hegewald MJ. Cardiopulmonary Exercise Testing (CPET). Am J Respir Crit Care Med 2020;201:1-2. [Crossref] [PubMed]
- Laveneziana P, Di Paolo M, Palange P. The clinical value of cardiopulmonary exercise testing in the modern era. Eur Respir Rev 2021;30:200187. [Crossref] [PubMed]
- American Thoracic Society. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211-77. [Crossref] [PubMed]
- Franssen FM, Rochester CL. Comorbidities in patients with COPD and pulmonary rehabilitation: do they matter? Eur Respir Rev 2014;23:131-41. [Crossref] [PubMed]
- Mirdamadi M, Rahimi B, Safavi E, et al. Correlation of cardiopulmonary exercise testing parameters with quality of life in stable COPD patients. J Thorac Dis 2016;8:2138-45. [Crossref] [PubMed]
- Berry MJ, Adair NE, Rejeski WJ. Use of peak oxygen consumption in predicting physical function and quality of life in COPD patients. Chest 2006;129:1516-22. [Crossref] [PubMed]
- Verhage TL, Vercoulen JH, van Helvoort HA, et al. Maximal exercise capacity in chronic obstructive pulmonary disease: a limited indicator of the health status. Respiration 2010;80:453-62. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Jeong D, Oh YM, Lee SW, et al. Comparison of Predicted Exercise Capacity Equations in Adult Korean Subjects. J Korean Med Sci 2022;37:e113. [Crossref] [PubMed]
- Myers J, Buchanan N, Walsh D, et al. Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol 1991;17:1334-42. [Crossref] [PubMed]
- Oga T, Nishimura K, Tsukino M, et al. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003;167:544-9. [Crossref] [PubMed]
- Albouaini K, Egred M, Alahmar A, et al. Cardiopulmonary exercise testing and its application. Heart 2007;93:1285-92. [PubMed]
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179-91. [Crossref] [PubMed]
- Díaz O, Villafranca C, Ghezzo H, et al. Breathing pattern and gas exchange at peak exercise in COPD patients with and without tidal flow limitation at rest. Eur Respir J 2001;17:1120-7. [Crossref] [PubMed]
- O'Donnell DE, D'Arsigny C, Fitzpatrick M, et al. Exercise hypercapnia in advanced chronic obstructive pulmonary disease: the role of lung hyperinflation. Am J Respir Crit Care Med 2002;166:663-8. [Crossref] [PubMed]


