
High level of C-reactive protein as a predictive factor for immune-related adverse events of immune checkpoint inhibitors in non-small cell lung cancer: a retrospective study
Highlight box
Key findings
• We showed that high CRP levels were associated with a high incidence of irAEs and poor prognosis by assessing CRP levels prior to ICI monotherapy.
What is known and what is new?
• Several risk factors for the irAEs before treatment with ICIs have been reported, of which high levels of pro-inflammatory markers and pro-inflammatory cytokines are candidates.
• Although previous reports have investigated changes in CRP levels from the baseline of ICIs treatment to the occurrence of irAEs, it has been still unknown that the frequency and severity of irAEs in patients with high CRP levels at baseline.
What is the implication, and what should change now?
• The data contribute to the prediction of the development of irAEs and the survival of patients treated with ICIs, and CRP may be one of the candidate biomarkers for predicting the development of irAEs and treatment response to ICIs treatment.
Introduction
Immune checkpoint inhibitors (ICIs) are effective for a wide variety of cancers including lung cancer (1-3). However, a major proportion of patients with non-small cell lung cancer (NSCLC) treated by ICIs are non-responders, and more than two thirds of patients develop acquired resistance during ICIs treatments (4). Therefore, reproducible predictive biomarkers need to be developed in order to improve patient selection, to maximize treatment benefit and to decrease serious toxicities of ICIs treatment. To date, validated predictive markers of ICIs responsiveness include Eastern Cooperative Oncology Group performance status (ECOG PS) and programmed cell death ligand 1 (PD-L1) tumor proportion score (TPS) (5).
ICIs treatments might cause immune-related adverse events (irAEs), when an immune response is extended to normal tissue (6). These irAEs can occur in any organ system and induce variety of symptoms. Some patients might experience higher grades of irAEs that require hospitalization or termination of treatments and irAEs often are life-threatening (7). Therefore, development of biomarkers capable of early detection and monitoring of irAEs is also needed.
Several risk factors for the irAEs before treatment with ICIs have been reported, of which high levels of pro-inflammatory markers and pro-inflammatory cytokines are candidates (8). C-reactive protein (CRP), a popular biomarker of the inflammatory response, as an acute phase protein of hepatic origin, has been strongly associated with poor prognosis for NSCLC patients (9,10). Therefore, we considered that CRP could be promising predictors of irAEs. Although previous reports have investigated changes in CRP levels from the baseline of ICIs treatment to the occurrence of irAEs (11,12), it has been still unknown that the frequency and severity of irAEs in patients with high CRP levels at baseline.
We aim to evaluate CRP levels before ICIs treatments as potential predictive biomarkers of irAEs incidence rate and overall survival (OS) in patients with advanced NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-85/rc).
Methods
Patient population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Iwate Medical University Graduate School and Faculty of Medicine (https://www.iwate-med.ac.jp) (approval No. MH2021-144), and informed consent from each patient was waived due to the retrospective nature of this study. Potentially eligible patients were identified from the database of the Iwate Medical University Hospital. The following main inclusion criteria were applied: (Ⅰ) a diagnosis of NSCLC by histology or cytology; (Ⅱ) monotherapy with an anti-PD-1 antibody (nivolumab, pembrolizumab), anti-PD-L1 antibody (atezolizumab, durvalumab); (Ⅲ) immunoserological tests including CRP have been measured before ICI treatments. The exclusion criteria included: (Ⅰ) patients experiencing active autoimmune disease or history of autoimmune disease; (Ⅱ) chemotherapy such as ICIs and platinum-based preparations is used in combination; (Ⅲ) have an infection that requires systemic administration of antibacterial, antifungal or antiviral drugs; (Ⅳ) history of severe interstitial pneumonia, including radiation pneumonia; (Ⅴ) patients treated with dual immunotherapy.
Study design
Between December 1, 2015 to December 31, 2019, we retrospectively included adult patients (≥20 years old) with NSCLC patients met all those meeting inclusion criteria during the defined study period were included. The patients were categorized into low and high groups with a cut-off value of 10 mg/L as the baseline level of CRP before the ICI treatment (13). The primary endpoint was relationship between CRP levels at baseline and incidence of irAEs. The secondary endpoints were the relationship of progression-free survival (PFS) and OS. Patients characteristics including age, gender, body mass index (BMI), histological type, epidermal growth factor receptor (EGFR) mutation, PD-L1 expression, smoking status, clinical stage, ECOG performance status, incidence of irAEs were collected from medical records. Data of according to baseline CRP levels PFS and OS were evaluated with computed tomography (CT) scans and MRI and medical records. The date of the follow-up cutoff was December 31, 2021.
Clinical assessment
The CRP levels of within 14 days before the start of ICIs treatment was considered as the baseline CRP levels. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 published by the National Institute of Health in 2010. PFS was evaluated for the period from the date of start of ICI treatment to the date when progression of disease or death occurred. OS was evaluated for the period from the date of start of ICI treatment to the date of death.
Statistical analysis
The irAEs incidence rate among CRP levels was compared between the two groups with chi-square test. We performed a stepwise backwards (Wald) method univariate and multivariate logistic regression analyses to identify variables associated with irAEs incidence. Kaplan-Meier survival curves were drawn for PFS and for OS, difference between high CRP group and the low CRP group were analyzed by means of a log-rank test. Hazard ratios (and 95% confidence intervals) were calculated with the use of a Cox proportional-hazards analysis. Each analysis was performed with the use of a two-sided, 5% significance level and a 95% confidence interval by means of SPSS 28.0 (SPSS Inc., Chicago, IL, USA).
Results
Basic information on patients with different CRP levels
Between December 1, 2015 to December 31, 2019, 274 patients with NSCLC treated with chemotherapy or immunotherapy were collected for the trial. ICI monotherapies were administered to 178 patients and 15 patients were excluded according to criteria as shown in Figure 1. Eighty-four patients were assigned to the low CRP group and 79 patients to the high CRP group (Figure 1). There were no missing values for all the relevant variables. The baseline characteristics of these patients are listed in Table 1. In eligible patients, a mean age was 67.6 years (range, 38–89), male was 79.1%, and 59.5% patients had adenocarcinomas. PD-L1 expression was categorized into four groups (<1%, 1–49%, ≥50% and unknown). Distribution was not difference among PD-L1 expression. Of the analysed sample, 132 (81.0%) were current and former smokers and 31 (19.0%) were never smokers. In comparison between high and low CRP groups, squamous cell carcinoma, smoker, and poor PS were more in the high CRP group, and more EGFR mutated tumor in the low CRP group. These factors were managed by multivariate analyses.

Table 1
| Variables | Total (n=163) | Low CRP group (n=84) | High CRP group (n=79) |
|---|---|---|---|
| Age (years), mean ± SD | 67.6±9.0 | 68.1±9.1 | 67.1±10.0 |
| Male, n (%) | 129 (79.1) | 60 (71.4) | 69 (87.3) |
| BMI (kg/m2), mean ± SD | 21.4±3.6 | 21.8±3.3 | 20.9±3.9 |
| Histological type, n (%) | |||
| Squamous cell carcinoma | 50 (30.7) | 17 (20.2) | 33 (41.8) |
| Adenocarcinoma | 97 (59.5) | 63 (75.0) | 34 (43.0) |
| Other | 16 (9.8) | 4 (4.8) | 12 (15.2) |
| EGFR mutation | 12 (7.4) | 11 (13.1) | 1 (1.3) |
| PD-L1 expression, n (%) | |||
| Negative | 24 (14.7) | 12 (14.3) | 12 (15.2) |
| 1–49% | 26 (16.0) | 10 (11.9) | 16 (20.2) |
| ≥50% | 30 (18.4) | 17 (20.2) | 13 (16.5) |
| Unknown | 83 (50.9) | 45 (53.6) | 38 (48.1) |
| Smoking status, n (%) | |||
| Current/former | 132 (81.0) | 58 (69.0) | 74 (93.7) |
| Never | 31 (19.0) | 26 (31.0) | 5 (6.3) |
| Clinical stage, n (%) | |||
| III | 15 (9.2) | 10 (11.9) | 5 (6.3) |
| IV | 148 (90.8) | 74 (88.1) | 74 (93.7) |
| Treatment lines, n (%) | |||
| 1 | 23 (14.1) | 7 (0.83) | 16 (20.3) |
| 2 | 95 (58.3) | 50 (59.5) | 45 (56.9) |
| ≥3 | 45 (27.6) | 27 (32.2) | 18 (22.8) |
| ECOG PS, n (%) | |||
| 0/1 | 150 (92.0) | 82 (97.6) | 68 (86.1) |
| ≥2 | 13 (8.0) | 2 (2.4) | 11 (13.9) |
| Immunotherapeutic agent, n (%) | |||
| Nivolumab | 95 (58.3) | 45 (53.6) | 50 (63.3) |
| Pembrolizumab | 33 (20.2) | 12 (14.3) | 21 (26.6) |
| Atezolizumab | 20 (12.3) | 15 (17.8) | 5 (6.3) |
| Durvalumab | 15 (9.2) | 12 (14.3) | 3 (3.8) |
CRP, C-reactive protein; SD, standard deviation; BMI, body mass index; EGFR, epidermal growth factor receptor; PD-L1, programmed cell death ligand 1; ECOG PS, Eastern Cooperative Oncology Group performance status.
The correlation between CRP level and irAEs
We first analyzed the incidence rates for each irAEs. The median duration of ICI administration for patients included in the study was 2.6 months (2.0 months for the high CRP and 3.0 months low CRP groups; P=0.017). All incidence of irAEs was significantly higher in the high CRP group compared to the low CRP group (54.4% vs. 34.5%, respectively, P=0.003) (Figure 2). A total of 101 irAEs, and 25 severe irAEs were observed (Figure 3). The most frequent irAEs were skin rush (28.8%), followed by pneumonitis (19.2%), hypothyroidism (15.4%), and hepatotoxicity (9.6%). The most common grade 3 or 4 irAEs was pneumonitis (7.9%), which tended to be more frequent in the high CRP group. Multivariate regression analysis was performed to assess the risk factors for irAEs (Table 2). The variables uterized in the multivariate regression model were selected according to the univariate analysis results. Of the 163 patients, 72 developed irAEs of any grade during ICI treatment. In univariate analysis, high CRP and non-adenocarcinoma were associated with a high risk of incidence irAEs, with an adjusted odds ratio of 2.35 in the high CRP group (95% CI: 1.24–4.43, P=0.004) and 1.89 in non-adenocarcinoma (95% CI: 1.00–3.58, P=0.056). In multivariate analysis, patients with high CRP levels had an adjusted OR of 2.41 and were associated with an increased risk of developing irAEs (95% CI: 1.16–4.43, P=0.020).

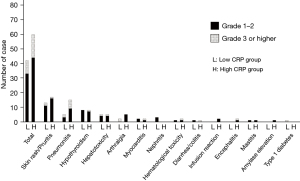
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (years) | 0.075 | ||||
| <65 | 1.00 (reference) | ||||
| ≥65 | 1.89 (0.95–3.78) | ||||
| Gender | 0.976 | ||||
| Female | 1.00 (reference) | ||||
| Male | 1.02 (0.47–2.18) | ||||
| BMI (kg/m2) | 0.234 | ||||
| <22.0 | 1.00 (reference) | ||||
| ≥22.0 | 0.67 (0.35–1.29) | ||||
| Baseline CRP (mg/L) | 0.004 | 0.020 | |||
| <10 | 1.00 (reference) | 1.00 (reference) | |||
| ≥10 | 2.35 (1.24–4.43) | 2.41 (1.16–4.43) | |||
| Histology | 0.056 | 0.423 | |||
| Adenocarcinoma | 1.00 (reference) | 1.00 (reference) | |||
| Non-adenocarcinoma | 1.89 (1.00–3.58) | 1.36 (0.65–2.85) | |||
| PD-L1 expression | 0.152 | ||||
| <50% | 1.00 (reference) | ||||
| ≥50% | 0.54 (0.23–1.26) | ||||
| Smoking status | 0.135 | ||||
| Never | 1.00 (reference) | ||||
| Current/former | 1.89 (0.82–4.32) | ||||
| Immunotherapeutic agent | |||||
| Anti-PD-1 therapy | 1.00 (reference) | 0.092 | |||
| Anti-PD-L1 therapy | 0.50 (0.23–1.11) | ||||
| ECOG PS | 0.129 | ||||
| 0–1 | 1.00 (reference) | ||||
| >2 | 0.35 (0.09–1.31) | ||||
| Clinical stage | 0.243 | ||||
| III | 1.00 (reference) | ||||
| IV | 1.59 (0.73–3.49) | ||||
| EGFR | 0.179 | ||||
| Wild type | 1.00 (reference) | ||||
| Mutant | 0.39 (0.10–1.50) | ||||
irAEs, immune-related adverse events; OR, odds ratio; CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; PD-L1, programmed cell death ligand 1; PD-1, programmed cell death protein-1; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
The correlation between CRP level and survival time
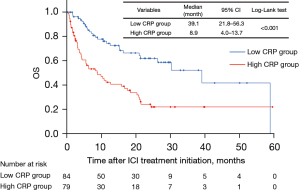
The results of Kaplan-Meier survival analysis of PFS and OS were shown in Figures 4,5, respectively. As for reasons for discontinuation of ICIs, 45 patients (53.6%) in the low CRP group and 43 patients (54.4%) in the high CRP group discontinued due to disease progression. Discontinuations due to irAEs were 15 patients (17.9%) in the low CRP group and 18 patients (22.8%) in the high CRP group. The median follow-up duration was 8.9 months. The high CRP group was related with shorter PFS compared to the low CRP group (2.2 vs. 3.3 months, respectively, P=0.006). The high CRP group were also related with shorter OS compared to the low CRP group (8.9 vs. 39.1 months, respectively, P<0.001).

In different subgroups, we used Cox’s proportional hazard model to analyze the relationship between various factors and OS (Table 3). The higher level of CRP was associated with the worse prognosis and the hazard ratio was 2.45 (95% CI: 1.53–6.39, P<0.001). Compared between ECOG PS =0–1, ECOG PS ≥2, PS >2 was more likely to reflect the patient’s survival (hazard ratio =3.12; 95% CI: 1.52–6.39, P=0.002).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.251 | ||||
| <65 | 1.00 (reference) | ||||
| ≥65 | 0.76 (0.48–1.20) | ||||
| Gender | 0.922 | ||||
| Male | 1.00 (reference) | ||||
| Female | 1.02 (0.60–1.75) | ||||
| BMI (kg/m2) | 0.858 | ||||
| <22.0 | 1.00 (reference) | ||||
| ≥22.0 | 0.95 (0.61–1.51) | ||||
| Baseline CRP (mg/L) | <0.001 | <0.001 | |||
| <10 | 1.00 (reference) | 1.00 (reference) | |||
| ≥10 | 2.71 (1.71–4.30) | 2.45 (1.53–6.39) | |||
| Histology | 0.137 | ||||
| Adenocarcinoma | 1.00 (reference) | ||||
| Non-adenocarcinoma | 1.40 (0.90–2.18) | ||||
| PD-L1 expression | 0.157 | ||||
| <50% | 1.00 (reference) | ||||
| ≥50% | 0.54 (0.23–1.26) | ||||
| ECOG PS | 0.004 | 0.002 | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | |||
| ≥2 | 4.34 (2.17–8.70) | 3.12 (1.52–6.39) | |||
| irAEs incidence | 0.236 | ||||
| No | 1.00 (reference) | ||||
| Yes | 0.94 (0.60–1.48) | ||||
| Clinical stage | 0.181 | ||||
| III | 1.00 (reference) | ||||
| IV | 1.96 (1.01–3.80) | ||||
| EGFR | 0.823 | ||||
| Wild type | 1.00 (reference) | ||||
| Mutant | 0.52 (0.19–1.46) | ||||
HR, hazard ratio; CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; PD-L1, programmed cell death ligand 1; ECOG PS, Eastern Cooperative Oncology Group performance status; irAEs, immune-related adverse events; EGFR, epidermal growth factor receptor.
Discussion
In this study, we showed that high CRP levels were associated with a high incidence of irAEs and poor prognosis by assessing CRP levels prior to ICI monotherapy. These data contribute to the prediction of the development of irAEs and the survival of patients treated with ICIs, and CRP may be one of the candidate biomarkers for predicting the development of irAEs and treatment response to ICIs treatment.
First, the cutoff point for CRP elevation was determined to be 10 mg/L, based on a systematic review of the relationship between CRP levels and prognosis in solid tumors (13). Although there have been several reports of the association between CRP levels and irAEs, those reports analyzed CRP levels after the development of irAEs during ICI treatment (11,12). One report focused on distinguishing methods to distinguish between irAEs and infection and irAEs (12). Previous studies have investigated CRP before and after the onset of irAEs. The novelty of this study is that we focused on baseline CRP before ICI administration and examined its correlation with the onset of irAEs, duration of ICI treatment, and OS. The pathogenesis of irAEs is not fully understood. It is hypothesized that irAEs development is induced by promoting the production of inflammatory cytokines IL-1, IL-6, IL-12, and TNF-α and inhibiting regulatory T cells that act negatively against inflammation (14). The correlation between CRP and IL-6 has already been demonstrated (15). Tocilizumab, an IL-6 receptor antagonist, has been shown to be involved in the control of irAEs by activating regulatory T cells (12). Patients with melanoma had elevated CRP when irAEs occurred, with patients with CRP >2 times the ULN being more likely to have irAEs than those with CRP below the ULN (11). Therefore, we speculate that ICIs treatment in patients with an already high inflammatory state may increase the incidence of irAEs by stimulating the production of proinflammatory cytokines.
In addition, reports on the survival of patients treated with CRP and ICIs have shown that CRP is a poor prognostic factor in various carcinomas (16-18). Suzuki et al. showed a strong association between elevated pre-treatment CRP levels and worse OS in patients with metastatic renal cell carcinoma treated with nivolumab. They further showed that a reduction in CRP ≥25% during ICI treatment predicted improved treatment response (19). In the present study, shorter PFS and OS were observed in patients with higher CRP levels prior to ICIs treatment, similar to previous reports (20). PFS was very limited, with 3.3 months in the low CRP group and 2.2 months in the high CRP group. Previous studies have shown a PFS of 3.5 months (95% CI: 2.1–4.9 months) in the nivolumab-treated group in the Checkmate-017 study in non-squamous cell carcinoma, which was similar to the results of this study (21). A meta-analysis reported that the development of irAEs during ICIs correlated with a better prognosis (22). Although the precise mechanisms by which irAEs occur have not been fully uncovered, they are thought to represent effects from activated T-cells and are consistent with the mechanism of action of ICIs (23,24). One set of studies suggests that perhaps irAEs are triggered by antigens that are common to both tumor and inflamed organ (25). No significant difference was found in the correlation between the development of irAEs and survival in this study (Figure S1).
When OS was compared between high and low CRP levels in patients with irAEs only, a significantly shorter survival was suggested in the group with higher CRP levels (Figure S2). A reason for marked difference in OS than PFS in this study may be the influence of CRP on OS as a prognostic factor (26,27). These results suggest that patients in an inflammatory state may be less likely to benefit positively from the occurrence of irAEs in ICIs.
This study has several limitations. First, this study was based on retrospective data collection from a single institution. Data bias was inevitable and it was difficult to accumulate a sufficient number of cases. Second, CRP may be elevated not only by inflammation but also by infection. In this study, patients who received antimicrobials were excluded to avoid enrolling patients with infections, but not all patients with infections could be completely excluded.
The CRP has potential predictive biomarkers for irAEs, measurement of CRP prior to ICIs treatment can screen out and exclude those who are not suitable for immunotherapy. In future, the relevance of inflammatory markers such as CRP and IL-6, as well as routine blood parameters and other unidentified biomarkers, should be clarified in large prospective clinical trials. The best treatment option can then be offered within the ICIs treatment strategy.
Conclusions
This study has shown that CRP should be measured prior to ICI treatment and that patients with high CRP can’t benefit enough from ICI treatment. CRP is a key factor in the choice of treatment for NSCLC patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-85/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-85/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-85/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-85/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Iwate Medical University Graduate School and Faculty of Medicine (https://www.iwate-med.ac.jp) (approval No. MH2021-144), and informed consent from each patient was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020;37:443-55. [Crossref] [PubMed]
- Yoneda T, Sone T, Koba H, et al. Long-term survival of patients with non-small cell lung cancer treated with immune checkpoint inhibitor monotherapy in real-world settings. Clin Lung Cancer 2022;23:467-76. [Crossref] [PubMed]
- Khan S, Gerber DE. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: a review. Semin Cancer Biol 2020;64:93-101. [Crossref] [PubMed]
- Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721-8. [Crossref] [PubMed]
- Hommes JW, Verheijden RJ, Suijkerbuijk KPM, et al. Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events-A Comprehensive Review. Front Oncol 2021;10:585311. [Crossref] [PubMed]
- Nagano T, Kinoshita F, Hashinokuchi A, et al. Prognostic Impact of C-Reactive Protein-to-Lymphocyte Ratio in Non-small Cell Lung Cancer: A Propensity Score-Matching Analysis. Ann Surg Oncol 2023;30:3781-8. [Crossref] [PubMed]
- Azzoli C, Huynh L, Yi D, et al. Retrospective Study to Examine Prognostic Value of C-Reactive Protein in Patients With Surgically Resectable Non-Small-Cell Lung Cancer. Clin Lung Cancer 2023;24:329-38. [Crossref] [PubMed]
- Abolhassani AR, Schuler G, Kirchberger MC, et al. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol 2019;145:2625-31. [Crossref] [PubMed]
- Samson M, Greigert H, Ciudad M, et al. Improvement of Treg immune response after treatment with tocilizumab in giant cell arteritis. Clin Transl Immunology 2021;10:e1332. [Crossref] [PubMed]
- Shrotriya S, Walsh D, Bennani-Baiti N, et al. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS One 2015;10:e0143080. [Crossref] [PubMed]
- Stucci S, Palmirotta R, Passarelli A, et al. Immune-related adverse events during anticancer immunotherapy: Pathogenesis and management. Oncol Lett 2017;14:5671-80. [Crossref] [PubMed]
- Laino AS, Woods D, Vassallo M, et al. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer 2020;8:e000842. [Crossref] [PubMed]
- Thompson JC, Hwang WT, Davis C, et al. Gene signatures of tumor inflammation and epithelial-to-mesenchymal transition (EMT) predict responses to immune checkpoint blockade in lung cancer with high accuracy. Lung Cancer 2020;139:1-8. [Crossref] [PubMed]
- Liu C, Zheng S, Jin R, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett 2020;470:95-105. [Crossref] [PubMed]
- Bar N, Costa F, Das R, et al. Differential effects of PD-L1 versus PD-1 blockade on myeloid inflammation in human cancer. JCI Insight 2020;5:e129353. [Crossref] [PubMed]
- Suzuki K, Terakawa T, Furukawa J, et al. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int J Clin Oncol 2020;25:135-44. [Crossref] [PubMed]
- Riedl JM, Barth DA, Brueckl WM, et al. C-Reactive Protein (CRP) Levels in Immune Checkpoint Inhibitor Response and Progression in Advanced Non-Small Cell Lung Cancer: A Bi-Center Study. Cancers (Basel) 2020;12:2319. [Crossref] [PubMed]
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. [Crossref] [PubMed]
- Haratani K, Hayashi H, Nakagawa K. Association of immune-related adverse events with immune checkpoint inhibitor efficacy: real or imaginary? BMC Med 2020;18:111. [Crossref] [PubMed]
- Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther 2017;6:73-82. [Crossref] [PubMed]
- Passat T, Touchefeu Y, Gervois N, et al. Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment. Bull Cancer 2018;105:1033-41. [Crossref] [PubMed]
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. [Crossref] [PubMed]
- Gagnon B, Abrahamowicz M, Xiao Y, et al. Flexible modeling improves assessment of prognostic value of C-reactive protein in advanced non-small cell lung cancer. Br J Cancer 2010;102:1113-22. [Crossref] [PubMed]
- Koch A, Fohlin H, Sörenson S. Prognostic significance of C-reactive protein and smoking in patients with advanced non-small cell lung cancer treated with first-line palliative chemotherapy. J Thorac Oncol 2009;4:326-32. [Crossref] [PubMed]


