
Systematic review and meta-analysis of segmentectomy vs. lobectomy for stage IA non-small cell lung cancer
Highlight box
Key findings
• Lobectomy showed superior OS, RFS, and DFS outcomes compared to segmentectomy in the stage IA of the 2–3 cm subgroup.
What is known and what is new?
• In the stage IA ≤2 cm subgroup, lobectomy and segmentectomy had similar OS, RFS or DFS outcomes.
• The final results, depending on sensitivity analysis, showed that lobectomy had a survival advantage in the entire IA stage NSCLC population, whether in the stage IA ≤2 cm subgroup or the stage IA of the 2–3 cm subgroup.
What is the implication, and what should change now?
• Segmentectomy is appropriate for carefully selected stage IA NSCLC patients with tumor sizes of 2 cm or less, and lobectomy is recommended for stage IA patients with tumor size of 2 to 3 cm.
Introduction
Lung cancer is one of most common cancers worldwide and the major cause of cancer-related deaths in both the United States and China in 2022 according to cancer statistics (1). Surgical treatment is the first choice for early-stage non-small cell lung cancer (NSCLC). Lobectomy became the standard treatment for early-stage lung cancer following the randomized controlled trial (RCT) by Ginsberg et al. (2) in 1995. The prevalence of computed tomography (CT) screening equipment has led to an increase in the detection of small peripheral pulmonary nodules, particularly ground glass nodules (GGNs), which has decreased tumor-related fatalities (3). Sublobectomy, particularly segmentectomy, has regained the interest of thoracic surgeons in recent years. In fact, anatomical sublobectomy (segmentectomy) allows for more precise removal of the cancerous tissue compared to nonanatomical sublobectomy (wedge resection), which can lead to better survival outcomes and increased chances of cure (4,5). Many studies (6-10) have shown that segmentectomy is not inferior to lobectomy for peripheral lung GGNs of 2 cm or less. In addition, segmentectomy preserves more lung function than lobectomy (11-13). The multicenter RCT by the Japan Clinical Oncology Group and West Japan Oncology Group [JCOG0802/WJOG4607L (14)] showed that the overall survival (OS) of the segmentectomy group was better than that of the lobectomy group. However, the lobectomy group reported more deaths due to other diseases, and the local recurrence rate of the segmentectomy group was significantly higher than that of the lobectomy group. Some studies (15-18) have shown lobectomy has a survival advantage over segmentectomy for stage IA NSCLC, especially tumors 2–3 cm in size (17).
Thus, it is still controversial whether segmentectomy is appropriate for stage IA NSCLC, especially for stage IA NSCLC with a tumor size of 2–3 cm. To draw further conclusions, we performed this systematic review and meta-analysis to compare segmentectomy and lobectomy for stage IA of 2–3 cm and IA ≤2 cm lung cancer in terms of perioperative outcomes (perioperative morbidity, 30- or 90-day mortality, intraoperative blood loss, operative time and mean number of lymph nodes harvested) and survival outcomes [OS, recurrence-free survival (RFS), or disease-free survival (DFS)]. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-410/rc).
Methods
Search strategy
Two investigators independently searched online databases, including PubMed, Embase, Web of Science, and Cochrane Library, for relevant articles published until December 2022. The medical subject headings and entry terms used were as follows: “Lung Cancer OR Lung Neoplasms OR Pulmonary Neoplasms OR Pulmonary Cancer”, “segmentectomy OR segment resection OR sublobectomy OR sublobar resection”, “lobectomy”, and “randomized controlled trials (RCTs) OR prospective OR retrospective”. The comprehensive search strategy of PubMed is explained in Table S1. The other databases also used similar search terms. In addition, the literature of the included articles were also searched to identify additional potential articles.
Selection criteria
The selection criteria for the included studies were as follows: (I) the patients with clinical stage IA NSCLC were included; (II) written in English; (III) the study compared segmentectomy and lobectomy with the reported results of perioperative and/or 5-year survival outcomes; (IV) the survival outcomes should include at least one of 5-year DFS, 5-year RFS, and 5-year OS; (V) to reduce potential selection and other confounding biases, the study design of a RCT or case-control (prospective or retrospective) trial with propensity score matching was included; (VI) the full text of the studies can be downloaded.
To prevent duplication of the same data, only the most recent study from the Surveillance, Epidemiology, and End Results (SEER) database was included. Articles with 3-year survival outcomes were excluded. Letters to the editor, reviews, case reports, meta-analyses, trial protocols, meeting abstracts, and animal studies were excluded.
Date extraction
The first step was to use Endnote (version X9.3.2, Clarivate Analytics, USA) to delete duplicates. Irrelevant literature was excluded by reading titles and abstracts according to the selection criteria above. Afterwards, the full content was read to further exclude literature that did not meet the selection criteria. These processes were completed independently by two investigators. When there were inconsistencies in the screening results, the third investigator decided whether to include the study in light of the selection criteria. The characteristics of interest extracted from final articles included name of first author, year, study design, sex, age, lung cancer stage, tumor size, number of patients who underwent segmentectomy and lobectomy, perioperative morbidity, 30- or 90-day mortality, intraoperative blood loss, operative time, mean number of lymph nodes harvested, 5-year DFS, 5-year RFS, and 5-year OS. The baseline characteristics of all included studies were well balanced in all retrospective postmatched and prospective studies.
Quality and risk of bias assessment
Two investigators independently assessed the quality of the included studies via the Methodological Index for Nonrandomized Studies (MINORS) items. Each of the 12 entries in MINORS is graded from 0 to 2 points, for a total of 24 points. Literature with a score of 0–8 was of low quality, 9–16 was of moderate quality, and 17–24 was of high quality. Studies with a score of less than 12 were not included in the meta-analysis. The risk of bias assessment included the following six aspects: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias. Bias assessment was performed by Review Manager (version 5.3 for Windows, Cochrane Collaboration, Oxford, UK).
Statistical analysis
Review Manager and Stata (version 14.0, Stata Corp, College Station, USA) were used to pool effect sizes, calculate the bias of publication and perform sensitivity analysis. Egger’s test was used to assess publication bias, with P<0.1 showing significant publication bias. Mean difference (MD), risk ratio (RR), and hazard ratio (HR) with 95% confidence intervals (CIs) were used to calculate continuous variables, dichotomous variables, and survival outcomes, respectively. The inverse variance-weighted and Mantel-Haenszel approaches were used to pool HR, MD, and RR for survival outcomes, continuous variables, and dichotomous variables, respectively. P<0.05 was considered to be significant. If direct means and standard deviations were not available in the full text for extraction, calculations were performed by sample size, median, interquartile range, or range as provided by Luo and Shi et al. (19,20). If the HR data could not be extracted directly from the articles, the HR was calculated by extracting the data from the survival curves using Engauge Digitizer (version 11.0) and Microsoft Excel (version 16.49) according to the method of Tierney et al. (21). The Higgins I2 statistics, which are calculated by the χ2 test, were used to assess the heterogeneity among the included studies. If I2<50% and P>0.1, there was homogeneity among studies, and the fixed effect model was used; if I2<50% and P<0.1, there was acceptable heterogeneity among the included studies, and the fixed effect model was used as well. When I2>50% and P<0.1, the included studies exhibited a large degree of heterogeneity, and the random effect model was used to pool the data.
Subgroup analysis
To draw more representative conclusions, we divided the population of included studies into two subgroups (one was stage IA of 2–3 cm, and the other was stage IA ≤2 cm) to pool survival effect size according to tumor size.
Results
Included studies
After searching databases including PubMed, Embase, Web of Science, and Cochrane Library, a total of 10 (14-17,22-27) articles were included for the final analysis from the initial 1,209 articles. The screening process is detailed in Figure 1. There were 8 retrospective studies with propensity score matching (15-17,22-26) and 2 RCTs (14,27). Six articles focused on stage IA ≤2 cm lung cancer, and only one article focused on stage IA of 2–3 cm lung cancer; the other three articles performed subgroup analysis for stage IA lung cancer, which was consistent with our meta-analysis. Table 1 presents the characteristics of the 10 articles in detail. The baseline parameters of all included studies were statistically balanced between the segmentectomy group and the lobectomy group. The detailed results of bias analyses are shown in Figures 2,3. The MINORS tool was used for quality assessment of the included articles. Four articles were graded as moderate quality, and six were graded as high quality (Table 2). The 10 articles involved a total of 4,935 patients, with 2,344 patients who undergoing segmentectomy and 2,591 patients who undergoing lobectomy.
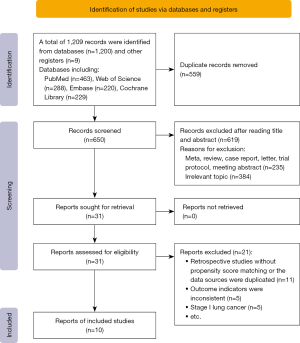
Table 1
| Author | Year | Study design | Stage | Seg, n | Lob, n | Tumor size* (cm), seg vs. lob | Sex* (male/female), seg vs. lob | Age* (years), seg vs. lob | Blood loss* (mL), seg vs. lob | Operative time* (min), seg vs. lob | No. of lymph nodes harvested*, seg vs. lob | Postoperative morbidity*, seg vs. lob | 30- or 90-day mortality*, seg vs. lob | 5-year RFS, HR (seg vs. lob), 95% CI) | 5-year DFS, HR (seg vs. lob), 95% CI) | 5-year OS, HR (seg vs. lob), 95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yamashita et al. (22) | 2012 | PM, RS | IA ≤2 cm | 76 | 72 | 1.5 [0.7–3.0] vs. 2.0 [0.9–3.0] | 41/49 vs. 73/51 | 69 [31–87] vs. 68 [50–90] | 132±181 vs. 202±437 | 257±91 vs. 276±82 | 12.1±9.4 vs. 21±9.1 | 17 (18.9) vs. 28 (22.6) | Not reported | – | 1.39 (0.28–7.02) | 1.49 (0.12–2.548) |
| IA >2 cm | 14 | 52 | 1.31 (0.43–4.01) | 1.28 (0.24–6.79) | ||||||||||||
| Deng et al. (16) | 2014 | PM, RS | IA ≤2 cm | 74 | 222 | – | 37/37 vs. 110/112 | 69.8±11.9 vs. 69.8±10.1 | Not reported | Not reported | Not reported | 68 (32.1) vs. 250 (39.3) | 1 (0.4) vs. 2 (0.3) | – | 1.72 (0.95–3.09) | 1.80 (0.96–3.38) |
| IA >2 cm | 31 | 93 | – | 17/14 vs. 48/45 | 71.5±8.1 vs. 71.4±7.7 | Not reported | Not reported | Not reported | 1.93 (0.99–3.77) | 1.92 (0.84–4.36) | ||||||
| Khullar et al. (15) | 2015 | PM, RS | IA ≤2 cm | 987 | 987 | 1.46±0.40 vs. 1.52±0.39 | – | – | Not reported | Not reported | Not reported | Not reported | 15 (1.55) vs. 16 (1.6) | Not reported | Not reported | 1.45 (1.10–1.91) |
| Kodama et al. (23) | 2016 | PM, RS | IA ≤2 cm | 69 | 69 | 1.5±0.4 vs. 1.67±0.3 | 33/36 vs. 32/37 | 62.6±7.81 vs. 62.1±9.52 | Not reported | Not reported | Not reported | Not reported | Not reported | 1.42 (0.24–8.28) | – | 0.69 (0.24–1.95) |
| Nishio et al. (25) | 2016 | PM, RS | IA ≤2 cm | 59 | 59 | 1.7 [1.3–1.8] vs. 1.7 [1.4–1.8] | 38/21 vs. 38/21 | 64 [58–71] vs. 61 [57–70.5] | 161±128 vs. 132±96 | 191±47 vs. 148±29 | Not reported | Not reported | Not reported | – | 1.65 (0.89–3.08) | 1.43 (0.64–3.20) |
| Koike et al. (24) | 2016 | PM, RS | IA ≤2 cm | 87 | 87 | 1.6 [0.6–2.0] vs. 1.6 [0.8–2.0] | 51/36 vs. 48/39 | 68 [42–83] vs. 68 [37-81] | Not reported | Not reported | 10±6 vs. 16±8 | Not reported | Not reported | – | 1.08 (0.63–1.88) | 0.99 (0.54–1.82) |
| Chan et al. (26) | 2020 | PM, RS | IA >2 cm | 90 | 90 | – | 44/46 vs. 132/147 | 71.5±8.6 vs. 68.8±9.1 | Not reported | Not reported | 10±1 vs. 18 ±1 | 23 (25.8) vs. 104 (37.3) | 1 (1.1), 2 (2.2) vs. 5 (1.8%), 6 (2.1) | 1.23 (0.82–1.85) | – | 1.23 (0.91–1.82) |
| Saji et al. (14) | 2022 | MRCT | IA ≤2 cm | 552 | 554 | 1.6 [0.6–2.0] vs. 1.6 [0.6–2.0] | 290/262 vs. 293/261 | 67 [32–83] vs. 67 [35–85] | 60±131 vs. 59±147 | 204±61 vs. 179±82 | 18±11 vs. 18±7 | 148 (26.8) vs. 142 (25.6) | Not reported | 0.998 (0.753–1.323) | – | 0.663 (0.474–0.927) |
| Shao et al. (17) | 2022 | PM, RS | IA >2 cm | 78 | 78 | – | 107/147 vs. 1,642/1,335 | – | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 1.578 (0.9865–2.525) |
| IA 1–2 cm | 138 | 138 | 1.895 (1.332–2.697) | |||||||||||||
| IA ≤1 cm | 36 | 36 | 1.287 (0.6264–2.644) | |||||||||||||
| Stamatis et al. (27) | 2022 | MRCT | IA ≤2 cm | 53 | 54 | 1.5 [0.5–2.5] vs. 1.5 [0.6–2.0] | 32/21 vs. 30/24 | 69 [42–80] vs. 66 [52–79] | Not reported | Not reported | Not reported | 7 (13.2) vs. 7 (13.0) | Not reported | – | 1.50 (0.60–3.76) | 0.61 (0.23–1.66) |
*, data are presented as median [range], mean ± SD, n, or n (%). Seg, segmentectomy; lob, lobectomy; RFS, recurrence-free survival; HR, hazard ratio; CI, confidence interval; DFS, disease-free survival; OS, overall survival; PM, propensity match; RS, retrospective study; MRCT, multicenter randomized controlled trial; SD, standard deviation.


Table 2
| Study | Purpose of study | Coherence of patients included | Collection of expected date | Endpoint indicators appropriately reflect the purpose of study | Objective of endpoint indicators | Adequacy of follow-up time | Loss to follow-up rate less than 5% | Sample size evaluation | Inclusion criteria of control group | Parallel experiments at the same time | Baseline comparability between groups | Whether the statistical analysis is appropriate | MINORS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yamashita et al. | 1 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 0 | 2 | 1 | 2 | 13 |
| Deng et al. | 1 | 1 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | 1 | 2 | 2 | 16 |
| Khullar et al. | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 1 | 1 | 2 | 16 |
| Kodama et al. | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 0 | 2 | 1 | 2 | 2 | 17 |
| Nishio et al. | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 20 |
| Koike et al. | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 17 |
| Chan et al. | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 19 |
| Shao et al. | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 16 |
| Saji et al. | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 23 |
| Stamatis et al. | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 21 |
*, literature with a score of 0–8 was of low quality, 9–16 was of moderate quality, and 17–24 was of high quality. Literature with a MINORS score of less than 12 should not be included in the meta-analysis. MINORS, Methodological Index for Nonrandomized Studies.
Perioperative outcomes
A total of 9 studies reported data on perioperative morbidity and 30- or 90-day mortality. Heterogeneity was not detected among these studies (I2=21%, P=0.28; I2=0%, P=0.96, respectively). The results of the meta-analysis showed that the combined RR for perioperative morbidity was 0.90 (95% CI: 0.79–1.02, P=0.10). The 30- or 90-day mortality was 0.94 (95% CI: 0.52–1.70, P=0.84) (Figure 4). Egger’s test showed that publication bias could not be found, with P=0.616 and P=0.880 for perioperative morbidity and 30- or 90-day mortality, respectively.
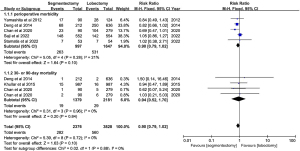
Three articles reported data on intraoperative blood loss and operative time. Apparent heterogeneity across included studies was detected (I2=63%, P=0.07; I2=91%, P<0.0001), so a random effect model was used to pool the data. The results of the meta-analysis showed that the intraoperative blood loss and operative time of the segmentectomy and lobectomy groups were comparable, with a MD of 3.07 (95% CI: −29.99 to 36.13, P=0.86) for intraoperative blood loss and a MD of 18.99 (95% CI: −5.71 to 43.68, P=0.13) for operative time (Figure 5). Egger’s test showed that publication bias could not be found, with P=0.735 and P=0.780 for intraoperative blood loss and operative time, respectively.
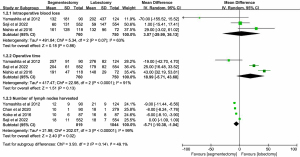
Four articles reported data on the mean number of lymph nodes harvested. A random effect model was used to pool data due to heterogeneity across the included studies (I2=99%, P<0.00001). The number of lymph nodes harvested was lower in segmentectomy patients, with a statistically significant MD of −5.71 (95% CI: −10.38 to −1.04, P=0.02, Figure 5). Egger’s test showed that publication bias could not be found with P=0.228.
Five-year OS
A total of 10 studies reported data on the 5-year OS for stage IA of 2–3 cm and IA ≤2 cm NSCLC. Heterogeneity among these studies was detected (I2=53%>50%, P=0.01<0.1), and a random effect model was applied. There was a significant difference in overall 5-year OS with a pooled HR of 1.26 (95% CI: 1.01–1.57, P=0.04), indicating the inferiority of segmentectomy. The subgroup analysis showed that a significant difference was not found for IA ≤2 cm NSCLC, with a combined HR of 1.18 (95% CI: 0.87–1.60, P=0.29). The OS difference for stage IA of 2–3 cm was statistically significant (HR =1.39, 95% CI: 1.07–1.81, P=0.01), with lobectomy showing a survival benefit (Figure 6). Egger’s test showed that there was no publication bias, with P values of 0.533 and 0.712 for IA (2–3 cm) and IA ≤2 cm NSCLC, respectively.

Five-year DFS or RFS
A total of 8 studies reported results of the 5-year DFS or RFS for stage IA of 2–3 cm and IA ≤2 cm NSCLC. Heterogeneity was not detected among these studies (I2=0%, P=0.70), and a fixed effect model was applied. There was a significant difference in overall 5-year DFS or RFS with a pooled HR of 1.23 (95% CI: 1.03–1.47, P=0.02), indicating the inferiority of segmentectomy. A significant difference was not found for stage IA ≤2 cm NSCLC with a combined HR of 1.18 (95% CI: 0.96–1.45, P=0.12). The difference was almost statistically significant (P=0.06) between segmentectomy and lobectomy for stage IA of 2–3 cm, with a pooled HR of 1.38 (95% CI: 0.99–1.93), indicating the survival benefit of lobectomy (Figure 7). Egger’s test revealed no publication bias with P values of 0.707 and 0.137 for stage IA (2–3 cm) and IA ≤2 cm NSCLC, respectively.

Publication bias and sensitivity analysis
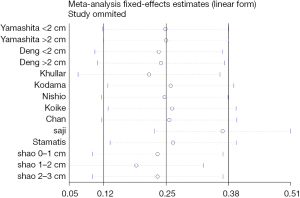
According to the results of Egger’s test, there was no publication bias in the included studies. Sensitivity analysis revealed that the study by Saji et al. (14) exhibited significant heterogeneity with other studies (Figure 8). After this study was eliminated, heterogeneity was not detected among the remaining studies (I2=0%, P=0.56), and the final result of the 5-year OS group was slightly affected, but the OS result for stage IA ≤2 cm was significantly impacted, with lobectomy showing a survival benefit (HR =1.44, 95% CI: 1.21–1.72, P<0.0001, Figure 9). The heterogeneity of intraoperative blood loss and operative time among the included studies may be mainly due to the learning curve of segmentectomy. Some of the included studies (22,25) were carried out when the segmentectomy technique was not mature and the surgeons were in the rising stage of the learning curve. The heterogeneity of the mean number of lymph nodes harvested can be explained by the different lymph node dissection methods of different studies.

Discussion
With the spread of low-dose CT, an increasing number of lung nodules are detected in the early stage, reducing mortality related to lung cancer (3). Surgical treatment has long been the first choice for stage IA lung cancer. In recent years, segmentectomy has been increasingly used to remove small nodules located in the peripheral lung. The role of segmentectomy in early-stage lung cancer has been debated for decades. The conclusions of many meta-analyses regarding segmentectomy and lobectomy are also inconsistent, with meta-analyses of Bao (28) and Cao et al. (29) showing that lobectomy and segmentectomy had comparable oncological results for lung cancer patients with stage IA ≤2 cm, while one study (30) showed that for stage IA ≤2 cm, segmentectomy had poorer OS and lung cancer-specific survival. A meta-analysis by Zheng et al. (31) also noted that segmentectomy was associated with shorter OS in stage I lung cancer. The differences between segmentectomy and lobectomy in terms of survival outcomes, postoperative complications, and perioperative outcomes have not been adequately studied. Recently, the multicenter, RCT JCOG0802/WJOG4607L (14) on the perioperative outcomes and survival outcomes of segmentectomy and lobectomy revealed the OS advantage of segmentectomy in stage IA ≤2 cm NSCLC. However, controversy remains regarding the role of segmentectomy for stage IA NSCLC 2–3 cm in size.
Our meta-analysis included six high-quality studies, including JCOG0802/WJOG4607L (14), and four moderate-quality studies, with 2,344 patients in the segmentectomy group and 2,591 patients in the lobectomy group. To better explore the perioperative outcomes and survival outcomes of segmentectomy for stage IA lung cancer with a size of 2–3 cm, we divided the study population into two subgroups: one was stage IA of 2–3 cm, and the other was stage IA ≤2 cm. Furthermore, to minimize reporting bias, we excluded studies reporting only 3-year survival outcomes and included studies reporting survival outcomes of 5 years or longer.
Our findings suggested that lobectomy showed superior OS outcomes compared to segmentectomy in the stage IA of the 2–3 cm subgroup. In the stage IA ≤2 cm subgroup, lobectomy and segmentectomy had similar OS outcomes. When we excluded the study with heterogeneity according to the plot of the sensitivity analysis, the final results showed that lobectomy had a survival advantage in the entire IA stage NSCLC population, whether in the stage IA ≤2 cm subgroup or the stage IA of the 2–3 cm subgroup, which was consistent with the meta-analysis results of Bao (28) and Cao et al. (29), Qu (32), Nakamura (33), and Fan et al. (34) had a different opinion that segmentectomy provides a comparable oncological prognosis with lobectomy for entire stage IA lung cancer. However, they did not use stratified data based on different tumor sizes to compare segmentectomy and lobectomy. Tumors with a size of 2–3 cm may be more aggressive in terms of oncology. Their findings are limited because more data have been reported in recent years. Our study is mainly based on the updated data in the last 10 years to compare segmentectomy and lobectomy by stratified meta-analysis.
In terms of RFS or DFS, our results concluded that lobectomy was associated with longer RFS or DFS in stage IA of the 2–3 cm subgroup. In the IA ≤2 cm subgroup, lobectomy and segmentectomy had comparable RFS or DFS. This may be because in the larger 2–3 cm subgroup, some patients who were originally intended for lobectomy underwent compromised segmentectomy due to smoking status, comorbidities and poor cardiopulmonary function in the included studies by Koike (24), Deng (16), and Yamashita et al. (22) The DFS or RFS of these patients would be affected, resulting in the advantage balance leaning toward lobectomy. In addition, many included studies did not disclose the percentage of solid and ground-glass components. Previous study (35) have shown that solid nodules increase the probability of spread through air spaces, and the recurrence rate of larger nodules may increase significantly after segmentectomy. In JCOG0802/WJOG4607L, the 5-year RFS of the two groups were almost identical (88.0% vs. 87.9%, P=0.988), and approximately 12% of the patients in both the segmentectomy and lobectomy groups had recurrence within 5 years. Unfortunately, the subgroup analysis did not provide data for comparing RFS outcomes in the two groups for patients with pure solid nodules. Recently, the results of the North American trial (36) (CALGB 140503) demonstrated that the DFS of the sublobar resection group was not inferior to lobar resection, and the difference in locoregional relapses between the two groups was not significant. Thus, it can be concluded that segmentectomy is comparable to lobectomy in terms of RFS or DFS in stage IA ≤2 cm lung cancer. However, more studies are needed to explain the benefit of segmentectomy for stage IA lung cancer in the 2–3 cm subgroup, especially pure solid nodules.
Considering the perioperative outcomes of the two surgical treatments, our meta-analysis showed that there were no statistically significant differences in perioperative morbidity, 30- or 90-day mortality, intraoperative blood loss, or operative time. The number of lymph nodes harvested was statistically lower in segmentectomy than in lobectomy. This may be related to the difference in the number of intrasegmental and intersegmental lymph node dissections and to the fact that lymph node sampling was more adopted in segmentectomy than in lobectomy (24,26).
There are some limitations to our study. First, 8 of the 10 included studies were retrospective, with the potential for selection and reporting bias. We limited our screening to retrospective studies with propensity matching analysis and prospective randomized trials to reduce bias. Second, the clinical staging procedures of lung cancer patients in certain studies were not explained in the text, which may compromise the credibility of the final conclusions. We did not report the pooled effect sizes of postoperative changes in pulmonary function because there were insufficient data to analyze. Finally, there was heterogeneity in the subgroup studies of OS for stage IA ≤2 cm and in the perioperative outcomes of intraoperative blood loss, operative time, and number of lymph nodes harvested. Thus, the findings must be interpreted with caution, and future studies are still needed to further interpret the role of segmentectomy in stage IA lung cancer of 2–3 cm in size. Additionally, we did not further divide the stage IA ≤2 cm group into stage IA ≤1 cm and stage IA of 1–2 cm because there were insufficient data to analyze, but the studies (4,17,30,37) have demonstrated that sublobectomy can provide similar disease control and oncological outcomes as lobectomy for patients with stage IA ≤1 cm NSCLC.
Conclusions
Our meta-analysis demonstrated that segmentectomy showed inferior results for stage IA NSCLC with a tumor size of 2–3 cm compared with lobectomy. For stage IA patients with tumor sizes less than 2 cm, the oncological outcomes of segmentectomy and lobectomy were comparable. There were no differences in perioperative outcomes between segmentectomy and lobectomy for stage IA NSCLC. We suggest that segmentectomy is appropriate for carefully selected stage IA NSCLC patients with tumor sizes of 2 cm or less, and lobectomy is recommended for stage IA patients with tumor size of 2 to 3 cm.
Acknowledgments
We would like to show our highest appreciation to the reviewer who made changes to this article.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-410/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-410/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-410/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135:584-90. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Cao J, Yuan P, Wang Y, et al. Survival Rates After Lobectomy, Segmentectomy, and Wedge Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:1483-91. [Crossref] [PubMed]
- Smith CB, Swanson SJ, Mhango G, et al. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:73-8. [Crossref] [PubMed]
- Winckelmans T, Decaluwé H, De Leyn P, et al. Segmentectomy or lobectomy for early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2020;57:1051-60. [Crossref] [PubMed]
- Sienel W, Dango S, Kirschbaum A, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg 2008;33:728-34. [Crossref] [PubMed]
- Moon MH, Moon YK, Moon SW. Segmentectomy versus lobectomy in early non-small cell lung cancer of 2 cm or less in size: A population-based study. Respirology 2018;23:695-703. [Crossref] [PubMed]
- Nomori H, Mori T, Shiraishi A, et al. Long-Term Prognosis After Segmentectomy for cT1 N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1500-6. [Crossref] [PubMed]
- Hennon M, Landreneau RJ. Role of Segmentectomy in Treatment of Early-Stage Non-Small Cell Lung Cancer. Ann Surg Oncol 2018;25:59-63. [Crossref] [PubMed]
- Nomori H, Shiraishi A, Cong Y, et al. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018;53:640-7. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Deng B, Cassivi SD, de Andrade M, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg 2014;148:1186-1192.e3. [Crossref] [PubMed]
- Shao S, Song G, Wang Y, et al. Selection of the surgical approach for patients with cStage IA lung squamous cell carcinoma: A population-based propensity score matching analysis. Front Oncol 2022;12:946800. [Crossref] [PubMed]
- Bertolaccini L, Solli P. COUNTERPOINT: Should Segmentectomy Rather Than Lobectomy Be the Operation of Choice for Early-Stage Non-small Cell Lung Cancer? No. Chest 2018;153:592-5. [Crossref] [PubMed]
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785-805. [Crossref] [PubMed]
- Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods 2020;11:641-54. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg 2012;42:83-8. [Crossref] [PubMed]
- Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:504-11. [Crossref] [PubMed]
- Koike T, Kitahara A, Sato S, et al. Lobectomy Versus Segmentectomy in Radiologically Pure Solid Small-Sized Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1354-60. [Crossref] [PubMed]
- Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1702-10. [Crossref] [PubMed]
- Chan EG, Chan PG, Mazur SN, et al. Outcomes with segmentectomy versus lobectomy in patients with clinical T1cN0M0 non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:1639-1648.e2. [Crossref] [PubMed]
- Stamatis G, Leschber G, Schwarz B, et al. Survival outcomes in a prospective randomized multicenter Phase III trial comparing patients undergoing anatomical segmentectomy versus standard lobectomy for non-small cell lung cancer up to 2 cm. Lung Cancer 2022;172:108-16. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤1 cm or >1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Zheng YZ, Zhai WY, Zhao J, et al. Oncologic outcomes of lobectomy vs. segmentectomy in non-small cell lung cancer with clinical T1N0M0 stage: a literature review and meta-analysis. J Thorac Dis 2020;12:3178-87. [Crossref] [PubMed]
- Qu X, Wang K, Zhang T, et al. Long-term outcomes of stage I NSCLC (≤3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: a PSM based analysis. J Thorac Dis 2017;9:4561-73. [Crossref] [PubMed]
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer 2005;92:1033-7. [Crossref] [PubMed]
- Fan J, Wang L, Jiang GN, et al. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol 2012;19:661-8. [Crossref] [PubMed]
- Song T, Jiang L, Zhuo Z, et al. Impacts of thoracoscopic surgery and high grade histologic subtypes on spread through air spaces in small stage I lung adenocarcinomas. J Cancer Res Clin Oncol 2019;145:2375-82. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Moon Y, Choi SY, Moon MH. Prognosis of wide wedge resection in patients with stage IA1 and IA2 lung adenocarcinoma with total tumor size including the lepidic component greater than 2 cm: a single center retrospective study. J Thorac Dis 2020;12:4731-41. [Crossref] [PubMed]


