
Aumolertinib in NSCLC with leptomeningeal involvement, harbouring concurrent EGFR exon 19 deletion and TP53 comutation: a case report
Highlight box
Key findings
• Aumolertinib is effective in treating leptomeningeal metastatic (LM) non-small cell lung cancer (NSCLC) patients with EGFR 19Del, TP53 and EGFR amplification multi-mutations.
What is known and what is new?
• First-line aumolertinib treatment is effective in EGFR concurrent mutated NSCLC.
• We provided efficacy and survival outcomes of aumolertinib in EGFR co-mutated NSCLC with leptomeningeal metastasis.
What is the implication, and what should change now?
• The findings suggested that almonertinib resulted in long-period clinical improvement and tolerable safety in concurrent mutated LM NSCLC.
Introduction
Lung cancer is a malignant tumor, and according to data from the National Cancer Registry in 2015, it has the highest incidence and mortality rates in China (1). It is the most common cancer in males, and second only to breast cancer in females (1). Non-small cell lung cancer (NSCLC) accounts for about 80–85% of all lung cancers (2). Following the successive identification of a series of oncogenic driver genes for lung cancer, multi-term registered research has shown that targeted therapy drugs greatly improve and prolong the prognosis and survival of patients with NSCLC carrying the corresponding driver genes (3,4).
In Asian patients, 30–50% of lung adenocarcinomas are epidermal growth factor receptor (EGFR) mutation positive (5,6). The core genes that co-mutate with the EGFR mutation in lung adenocarcinoma include tumor protein p53 (TP53) (54.6–64.6%), Retinoblastomal (RB1) (9.5–10.3%), and PIK3CA (9–12.4%) (7). Studies have shown that co-mutations affect the tumor microenvironment and drug sensitivity (8,9). The use of EGFR tyrosine kinase inhibitors (TKIs) in the initial treatment of patients with the co-mutations of TP53 and EGFR has been shown to be associated with shorter progression-free survival (PFS) (8,9). In solid tumors, TP53 is the most common concomitant mutation of the EGFR amplification (10). The frequency of synergistic gene mutations in patients with EGFR amplification is significantly higher than that in patients without EGFR amplification, and patients with EGFR amplification are more likely to have multi-pathway activation (10).
In non-small cell carcinoma patients with the EGFR Ex19del or L858R mutations, gefitinib, which is a classic EGFR-TKI, continues to be the sensitive mutation treatment recommended by guidelines (11). However, resistance to EGFR-TKIs invariably occurs, and approximately 10% of advanced NSCLC patients are diagnosed with leptomeningeal metastases (LM) (12). The median overall survival (OS) of LM is 3–10 months from diagnosis with limited systemic treatments (13). Pre-clinical research has shown that the distribution of 3rd-generation EGFR-TKIs in brain tissue is higher than that of 1st- and 2nd-generation TKIs (14). The BLOOM study reported that advanced NSCLC patients who showed disease progression after being treated with 1st- or 2nd-generation EGFR-TKIs and leptomeningeal metastases following osimertinib treatment had an intracranial objective response rate (ORR) of 41% (as assessed by an investigator), and a median PFS of 8.6 months (15).
Aumolertinib, which is a novel 3rd-generation EGFR-TKI, highly inhibit sensitive EGFR mutations and resistance mutations, has shown excellent efficacy and safety in treating central nervous system (CNS) metastases (16,17). The intracranial ORR of aumolertinib in the treatment of EGFR T790M mutation-positive NSCLC patients with brain metastasis has been reported to be 60.9%, the intracranial Disease Control Rate (DCR) has been reported to be 91.3%, and intracranial median PFS has been reported to be 11.8 months (16). Elevation of creatine kinase has been previously reported in treatment with aumolertinib (17). A previous pharmacokinetic study has reported that aumolertinib had efficacy in an EGFR-mutant brain metastases model (18). In previous report, high-dose aumolertinib was treated for patients with catastrophic symptoms of LMs and poor performance status (19). Another study suggested that aumolertinib monotherapy or combination therapy demonstrated superior activity for LMs of advanced EGFR mutated NSCLC, ORR was 54.5%, DCR was 81.8% and median PFS was 8.1 months (20).
Against this background, we report the case of a NSCLC patient with leptomeninges metastases and the EGFR 19Del, TP53, and EGFR amplification multi-mutations, who after showing disease progression following the 1st-line treatment of gefitinib was then treated with aumolertinib. The patient underwent a cerebrospinal fluid (CSF) gene test using high-throughput sequencing to detect mutations related to the CNS lesions. We present this article in accordance with the CARE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-841/rc).
Case presentation
A 51-year-old man of Asian ethnicity underwent a radical resection to treat upper left lung cancer at the Shanghai Huadong Hospital in December 2017 (Figure 1). The patient’s postoperative pathology diagnosis was left upper lobe invasive adenocarcinoma, predominant papolla and micropapilla, with ipsilateral mediastinal and para-bronchial lymph node metastases, pathological staging pT2N2M0, IIIA (according to the American Joint Committee on Cancer 8th edition staging system). The patient underwent a lung biopsy using the illimina high-throughput sequencing method for whole-cancer 520 gene detection, and the results revealed that the patient had EGFR 19del (p.Leu747_Thr751del), TP53 (p.lys120fs) and EGFR amplification multiple mutations with a low tumor mutational burden (TMB).

After eliminating the contraindications, the patient was treated with pemetrexed combined with carboplatin postoperative adjuvant chemotherapy for 4 cycles. In December 2018, due to left supraclavicular lymph node enlargement, the patient underwent a puncture biopsy of the supraclavicular lymph node. Based on the histopathology results the patient was diagnosed with metastatic poorly differentiated carcinoma. The patient received subsequent TKI treatments in Zhejiang Provincial People’s Hospital. The 1st-generation TKI gefitinib (250 mg, qd) was administered for 10 months. After that, secondary malignancy of the lymph nodes was diagnosed.
In June 2020, the patient presented with enlarged supraclavicular and cervical lymph nodes, indicating disease progression. The patient was then treated with carboplatin combined with pemetrexed for 2 cycles with continued TKI. The duration of 1st-generation TKI was 16 months. After chemotherapy, on July 31, 2020, the patient underwent a routine review and metastases were found in the left frontal lobe (Figure 2A). At that time, the patient reported no headache, dizziness, nausea, vomiting, or other symptoms of intracranial hypertension. After excluding contraindications, gamma knife stereotactic radiotherapy was performed on August 3, 2020, and the patient reported a headache after the operation.

Whole body positron emission tomography–computed tomography (PET-CT) showed bilateral hilar and mediastinum metastasis (in the 2R area, 4R area, 5 area, and 7 area), bilateral neck metastasis (in the bilateral I–IV area and right neck V area), and bilateral clavicle area lymph nodes metastasis (Figure 2B). Head magnetic resonance imaging (MRI) revealed no abnormalities, and that the original frontal lobe metastases had disappeared (Figure 2C); however, the left frontal lobe and left cerebellar meninges were locally thickened and distinctly enhanced (Figure 2D,2E).
A lumbar puncture was performed, and the results were as follows: CSF pressure 350 mmH2O; CSF protein 21.3 mg/dL (normal value 0.0–45.0 mg/dL); sugar 3.96 mmol/L; and mammary gland 0.9 mmol/L (0.5–1.7 mmol/L). No tumor cells were found in the CSF smear. Based on the patient’s medical history, clinical manifestations, and CSF pressure and imaging findings, leptomeningeal metastases were confirmed by clinical diagnosis.
The patient subsequently received the 3rd-generation aumolertinib (110 qd) orally from August 31, 2020. After the administration of aumolertinib, the patient’s symptoms of dizziness was relieved, cervical and supraclavicular lymph nodes were reduced, and the efficacy evaluation was partial response (PR). The results of the laboratory tests showed that the tumor biomarkers, such as carcinoembryonic antigen (CEA), had also steadily decreased.
After 10 months of treatment with the medication, the patient’s condition remained persistently stable. A further cranial MRI showed the local thickening and enhancement of the left frontal lobe and left cerebellar meninges similar to that observed before (Figure 2F,2G). Another lumbar puncture was performed, and the results were as follow: CSF pressure 220 mmH2O. However, adenocarcinoma cells were found in the CSF smear (Figure 3A). Thus, the aumolertinib therapy was continued.

After 11 months administration of aumolertinib, the symptoms of dizziness, accompanied by nausea and vomiting re-appeared. On August 5, 2021, another lumbar puncture was performed, and the patient’s CSF pressure was 450 mmH2O, and adenocarcinoma cells were found in the CSF smear (Figure 3B). A CSF gene test was performed using probe hybridization and high-throughput sequencing. The results revealed EGFR p. L747_T751 del, TP53 p. K120fs, and EGFR amplification mutations with microsatellite stability; however, the plasma detection was negative, which indicated that the EGFR mutations in the plasma might have been cleared by the aumolertinib.
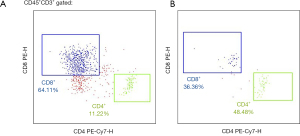
Further flow detections were implemented to investigate the mitochondria signatures of the T lymphocyte subpopulation from peripheral blood for 3 times and from CSF infiltrated immune cells for 2 times (flow cytometry instrument, NovoCyte 2060R, Agilent; Flow cytometry regent: From UB Biotechnology, Zhejiang Co., Ltd.). The absolute number of the cluster of differentiation (CD)3+ T lymphocytes and the subsets (i.e., the CD3+CD4+ subset and the CD3+CD8+ subset) in the peripheral blood increased over time, and the parameters of the single-cell mitochondrial mass (SCMM) showed a tendency of high to low, which suggested that the cell state had improved, and that the patient had increased immune cells and normal mitochondrial metabolism. These results showed that the clinical treatment had significantly improved the peripheral immune microenvironment of the patient (Figure 4). Additionally, the number of immune cells in the CSF had decreased from 43.6 to 1.47 cells/µL from July 2, 2021 to August 6, 2021 (Figure 5). There was a significant decrease in the immune cells in the brain, indicating a reduction in the inflammatory response, and local improvement in the intracranial environment of the patient.

With aumolertinib treatment, the patient achieved a PFS of 12 months after the diagnosis of leptomeningeal metastasis. However, the patient’s metastasis then progressed, and the patient was treated with mannitol and cortisol hormone dehydration to reduce the intracranial pressure, but his symptoms were not relieved. Thus, ventricular drainage and Ommaya capsule implantation were performed, and the symptom of cranial hypertension was relieved. After surgery, the dose of oral aumolertinib was increased to 165 mg qd and combined with an intravenous infusion of bevacizumab (7.5 mg/kg Q3W). The patient had a grade 2 rash, but no other obvious adverse events were observed. Following the diagnosis of leptomeningeal metastasis, the patient has survived for >16 months. The patient is still alive and has a good mental state, and the disease is under stable control.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
International Multidisciplinary Team (iMDT) discussion
At present, a variety of oncogenic driver genes have been found in NSCLC. In the past, the typing of lung cancer was classified by simple histopathology, and further subdivided into molecular subtypes based on driver genes. Multiple studies have shown the presence of concurrent alterations in NSCLC harbouring actionable mutations (21) and that the TP53 co-mutation is an adverse prognostic factor for EGFR-TKI therapy, resulting in shorter survival than patients with wild-type TP53 NSCLC (8,9). A meta-analysis of 1,342 patients in 15 studies showed that the TP53 co-mutation was a prognostic risk factor for the efficacy of EGFR-TKI therapy, and that TP53 was associated with shorter survival regardless of whether the patients underwent 1st-line or multi-line therapy and the histological type (22). The BENEFIT study, a prospective phase-II clinical trial, assessed the effect of TP53 mutations on gefitinib efficacy, and showed that compared to the TP53 wild-type patients, the TP53 mutation patients had a significantly shorter PFS time (8.4 vs. 12.81 months), while the patients with the EGFR exon 19 mutation combined with the TP53 mutation had a longer survival time than those with the EGFR L585R mutation combined with the TP53 mutation (23). Patients with T790M and TP53 co-mutations treated with a 3rd-generation EGFR-TKI had significantly shorter median PFS (8.9 vs. 12.8 months) and OS (17.8 vs. 26.6 months) than TP53 wild-type patients (9).
Improve our understanding on biodiversity of EGFR mutant NSCLC, is a priority in lung cancer setting considering its biological heterogeneity, due to coexpression of driver, passenger and co-occurring mutations (24).
Researchers examined the genomic landscape of the EGFR amplification and found that the TP53 mutation was the most common concomitant mutation of the EGFR amplification in solid tumors (10). EGFR amplification status was assessed among Guardant Health 28, 584 patients and 1,434 patients at the University of California San Diego (UCSD) with malignancies evaluated by next generation sequencing (NGS) of blood-derived deoxyribonucleic acid. In the UCSD cohort, patients with the EGFR amplification had a significantly higher frequency of synergistic gene mutations than those without the EGFR amplification, and the TP53 mutation was the most common concomitant mutation (65.3%) (10). The circulating tumor cell liquid biopsy can be used to rapidly and accurately assess and manage tumor advancement (25).
Ruiz-Patiño et al. found that 30.6% of lung adenocarcinoma patients have concomitant EGFR mutations and the EGFR amplification (26). Patients with EGFR mutations and EGFR amplifications have been shown to have better OS, PFS, CR, and PR responses to 1st-generation TKIs (26) than without EGFR amplifications. Zhou et al. suggested that patients whose tumors had a high abundance of EGFR mutations benefited more from EGFR-TKIs than those with a low abundance of EGFR mutations (27).
EGFR-mutated NSCLC is sensitive to 1st- and 2nd-generation TKI therapy. However, inevitably, lung tumors develop resistance and evolve into distant brain metastasis. The high incidence of brain metastasis in patients with positive driver genes may be related to different biological characteristics, the low blood-brain barrier penetration rate of 1st- and 2nd-generation TKIs, and the prolonged survival of such patients. After the first NGS revealed the EGFR 19del (p.Leu747_Thr751del), TP53 (p.lys120fs) and EGFR amplification multiple mutations, the patient in this case was treated with gefitinib and achieved a PFS of 10 months before disease progression occurred.
As a 3rd-generation EGFR-TKI, aumolertinib introduces a cyclopropyl in the structure that enhances the lipophilicity and increases the permeability of the blood-brain barrier. In the phase-III AENEAS study of aumolertinib in the treatment of 1st-line EGFR sensitive mutations, brain metastasis was analyzed as pre-determined stratification, compared to those treated with gefitinib, the brain metastasis patients significantly benefited from treatment with aumolertinib in terms of PFS, and the risk of disease progression was reduced by 62% (hazard ratio =0.38). Thus, aumolertinib achieves better disease control in patients with EGFR mutation-positive brain metastatic NSCLC (17). In this case after leptomeningeal metastasis occurred, aumolertinib was used as the 2nd-line treatment as the patient had developed resistance to the 1st-line treatment of gefitinib.
Zheng et al. suggested that CSF is effective at profiling intracranial mutational conditions and selecting patients who will benefit from a more sustained response from 3rd-generation TKIs (28). A 3rd-generation TKI is typically considered for EGFR-mutated NSCLC patients with brain metastasis but there are very little data about the efficacy and safety of 3rd-generation TKIs in the treatment of multiple co-mutation leptomeningeal metastasis. During aumolertinib treatment, the patient’s clinical symptoms were relieved. The CT showed that the patient’s cervical and supraclavicular lymph nodes were reduced. The flow detection results demonstrated a reduction in the inflammatory response, and a local improvement in the intracranial environment of the patient. The tumor biomarkers (e.g., carcinoembryonic antigen, carbohydrate antigen 15-3, and neuron-specific enolase) decreased steadily. After the patient had been treated for 12 months with aumolertinib, the flow detection results implied a reduction in the inflammatory response, but the patient’s disease progressed according to the clinical diagnosis.
In this case, aumolertinib has been shown to have efficacy in treating leptomeningeal metastatic NSCLC patients with EGFR 19Del, TP53 and EGFR amplification multi-mutations. The patient achieved PFS for 12 months and is still alive. The patient’s overall survival is significantly longer than that reported in previous research.
Discussion among physicians from Zhejiang Provincial People’s Hospital
Department of Medical Oncology
Patients with EGFR-sensitive mutated LMs should first be treated with 3rd-generation TKI drugs, because 3rd-generation TKI can better penetrate the blood-brain barrier and increase intracranial concentration. However, after the progression of 3rd-generation TKI, there is currently no effective therapy. The side effects of whole spinal cord irradiation and methotrexate intravaginal injection may be serious and the efficacy is uncertain, so the prognosis of patients is poor. A small sample clinical study suggested that intrathecal injection of pemetrexed might produce certain efficacy.
Department of Neurosurgery
Since cerebrospinal fluid obstruction leads to hydrocephalus in patients with LM, the patient may have repeated symptoms of cranial hypertension such as nausea, vomiting and headache, which significantly reduces the physical condition of the patient. Ommaya capsule implantation can quickly improve the symptoms of cranial hypertension, and it is convenient to extract cerebrospinal fluid and intracapsular administration, which can help patients with subsequent treatments.
Department of Pathology
The positive rate of cerebrospinal fluid in patients with LM is not high, which is about 50% that reported in the previous literature. In order to detect cerebrospinal fluid, more than 10 mL of cerebrospinal fluid needs to be taken, and even repeated sampling may also be required. The genetic status of cerebrospinal fluid is special. In this patient, EGFR mutation was not found in peripheral blood test after 3rd-generation TKI treatment, but EGFR combined with TP53 mutations were still found in cerebrospinal fluid, which again verified the central nervous system progression of 3rd-generation TKI treatment.
Several issues on the diagnosis and treatment of this patient were further discussed as follows
Question 1: Which generation of TKI will you choose for first-line EGFR-positive lung adenocarcinoma?
Expert opinion 1: Lorenzo Calvetti
Updated clinical data suggest the use of the 3rd-generation EGFR-TKI as the treatment of choice for first line EGFR-mut NSCLC for common alterations (ex19 e L858R. However new data suggest the use of the 3rd-generation EGFR-TKI in metastatic NSCLC harbouring uncommon EGFR mutations, with the exception of EGFR exon 20 insertion (ex20ins) mutations.
Expert opinion 2: Antonio Araujo
The 3rd-generation EGFR-TKI.
Expert opinion 3: Antonio Passaro
According to the international guideline, ASCO (29) and ESMO (30), and on the ESMO consensus statements on the management of EGFR mutant NSCLC (31), for patients with common EGFR mutations (deletion of exon 19 and exon 21 L858R), the preferred option should be a 3rd-generation EGFR-TKIs.
Question 2: How do you choose treatment for comutant NSCLC with EGFR-sensitive mutations (19 Del or L858R)?
Expert opinion 1: Lorenzo Calvetti
At this time we do not choose different treatment when co-mutation (ex tp-53) are found at the molecular basal screening as role of chemotherapy in combination with TKIs are non defined. However in these patient a closer screening is practiced.
Expert opinion 2: Antonio Araujo
We choose, almost always, the 3rd-generation EGFR-TKI for first line treatment, when we have an EGFR-sensitive mutations NSCLC. If we have a comutant NSCLC, with mutations others than EGFR, it depends of the percentage of the allelic frequency of each mutation. We are guided by these frequency and try to choose the first treatment regarding the allelic frequency of each comutant alteration.
Expert opinion 3: Antonio Passaro
To date, the role of co-mutations in EGFR mutant disease is confirmed as a negative prognostic factor, in particular when focusing on p53. According to the international guideline, there are no specific indication to tailor the treatment of these patients, carrying co-alterations, considering the granularity of data evaluated in the randomized clinical trial. In these specific patients, the confirmed indication should be the use of 3rd-generation EGFR-TKIs.
Question 3: What treatment options are currently available for patients with EGFR-positive concurrent mutated leptomeningeal metastatic NSCLC?
Expert opinion 1: Lorenzo Calvetti
These patients have a poor prognosis and at this time treatment with the 3rd-generation EGFR-TKI is the available choice with potential effectiveness.
Expert opinion 2: Antonio Araujo
The 3rd-generation EGFR-TKI.
Expert opinion 3: Antonio Passaro
The management of patients with EGFR mutant NSCLC characterized by leptomeningeal disease remains a critical problem, considering that those patients have a very poor outcome and generally are excluded from the clinical trial. Considering the limited use of MRI at the time of diagnosis, very few patients are diagnosed with leptomeningeal metastasis at the time of diagnosis. For patients with metastatic disease characterized by leptomeningeal involvement, the use of osimertinib 160 mg daily as reported in the BLOOM study, showed a good anticancer activity, and has been accepted in many countries and a standard of care for this clinical condition. In the BLOOM study, many patients received also brain radiotherapy, despite its role in leptomeningeal metastasis remains unclear.
Conclusions
Under certain circumstances, co-mutations and leptomeningeal metastasis are poor prognostic factors for NSCLC patients. The CSF NGS detection and the Illumia high-throughput sequencing showed that the mutation type of leptomeningeal lesion was consistent with the primary lung lesions, identifying the similarity of tumor evolution between the primary and metastatic lesions. This is the first reported case to show that aumolertinib is highly effective at treating and has low toxicity in patients with EGFR and tumor suppressor gene mutations with leptomeningeal metastatic NSCLC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-841/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-841/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-841/coif). HZ is employed by Hansoh Health Technology Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Crinò L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v103-15. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Schuler M, Wu YL, Hirsh V, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol 2016;11:380-90. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Jordan EJ, Kim HR, Arcila ME, et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov 2017;7:596-609. [Crossref] [PubMed]
- Yu HA, Suzawa K, Jordan E, et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin Cancer Res 2018;24:3108-18. [Crossref] [PubMed]
- Kim Y, Lee B, Shim JH, et al. Concurrent Genetic Alterations Predict the Progression to Target Therapy in EGFR-Mutated Advanced NSCLC. J Thorac Oncol 2019;14:193-202. [Crossref] [PubMed]
- Kato S, Okamura R, Mareboina M, et al. Revisiting Epidermal Growth Factor Receptor (EGFR) Amplification as a Target for Anti-EGFR Therapy: Analysis of Cell-Free Circulating Tumor DNA in Patients With Advanced Malignancies. JCO Precis Oncol 2019;3:PO.18.00180.
- Ku GY, Haaland BA, de Lima Lopes G Jr. Gefitinib vs. chemotherapy as first-line therapy in advanced non-small cell lung cancer: meta-analysis of phase III trials. Lung Cancer 2011;74:469-73. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43-e55. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Yang JCH, Kim SW, Kim DW, et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol 2020;38:538-47. [Crossref] [PubMed]
- Yang JC, Camidge DR, Yang CT, et al. Safety, Efficacy, and Pharmacokinetics of Almonertinib (HS-10296) in Pretreated Patients With EGFR-Mutated Advanced NSCLC: A Multicenter, Open-label, Phase 1 Trial. J Thorac Oncol 2020;15:1907-18. [Crossref] [PubMed]
- Lu S, Dong X, Jian H, et al. Randomized phase III trial of aumolertinib (HS-10296, Au) versus gefitinib (G) as first-line treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) and EGFR exon 19 del or L858R mutations (EGFRm). J Clin Oncol 2021;39:9013. [Crossref]
- Passaro A, Attili I, Rappa A, et al. Genomic Characterization of Concurrent Alterations in Non-Small Cell Lung Cancer (NSCLC) Harboring Actionable Mutations. Cancers (Basel) 2021;13:2172. [Crossref] [PubMed]
- Zhang X, Wu Y, Hu Y, et al. EP08.02-039 An Effective Treatment for EGFR-mutated Lung Adenocarcinoma with Symptomatic Leptomeningeal Metastases Using Aumolertinib. J Thorac Oncol 2022;17:S415. [Crossref]
- Huang S, Li L, Yan N, et al. EP08.02-069 A Retrospective Study of Aumolertinib Monotherapy or Combination Therapy Treated EGFR-mutated NSCLC Patients with Leptomeningeal Metastases. J Thorac Oncol 2022;17:S433. [Crossref]
- Zhang Y, Zhang Y, Niu W, et al. Experimental Study of Almonertinib Crossing the Blood-Brain Barrier in EGFR-Mutant NSCLC Brain Metastasis and Spinal Cord Metastasis Models. Front Pharmacol 2021;12:750031. [Crossref] [PubMed]
- Qin K, Hou H, Liang Y, et al. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: a meta-analysis. BMC Cancer 2020;20:328. [Crossref] [PubMed]
- Yu R, Bai H, Li T, et al. TP53 mutations in circulating tumor DNA in advanced epidermal growth factor receptor-mutant lung adenocarcinoma patients treated with gefitinib. Transl Oncol 2021;14:101163. [Crossref] [PubMed]
- Passaro A, Malapelle U, Del Re M, et al. Understanding EGFR heterogeneity in lung cancer. ESMO Open 2020;5:e000919. [Crossref] [PubMed]
- Wang Y, Zhou Y, Hu Z. The Functions of Circulating Tumor Cells in Early Diagnosis and Surveillance During Cancer Advancement. J Transl Int Med 2017;5:135-8. [Crossref] [PubMed]
- Ruiz-Patiño A, Castro CD, Ricaurte LM, et al. EGFR Amplification and Sensitizing Mutations Correlate with Survival in Lung Adenocarcinoma Patients Treated with Erlotinib (MutP-CLICaP). Target Oncol 2018;13:621-9. [Crossref] [PubMed]
- Zhou Q, Zhang XC, Chen ZH, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:3316-21. [Crossref] [PubMed]
- Zheng MM, Li YS, Tu HY, et al. Genotyping of Cerebrospinal Fluid Associated With Osimertinib Response and Resistance for Leptomeningeal Metastases in EGFR-Mutated NSCLC. J Thorac Oncol 2021;16:250-8. [Crossref] [PubMed]
- Hanna NH, Robinson AG, Temin S, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol 2021;39:1040-91. Erratum in: J Clin Oncol 2021;39:2520. [Crossref] [PubMed]
- Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:339-57. [Crossref] [PubMed]
- Passaro A, Leighl N, Blackhall F, et al. ESMO expert consensus statements on the management of EGFR mutant non-small-cell lung cancer. Ann Oncol 2022;33:466-87. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


