
The interplay of physical and cognitive function in rehabilitation of interstitial lung disease patients: a narrative review
Introduction
Interstitial lung disease (ILD) is an umbrella term for diseases with abnormal diffuse parenchymal lung changes. ILDs can be due to identifiable causes, such as occupational or environmental inhalation exposures, familial, connective tissue diseases, and drugs. They can also arise from unknown causes, known as idiopathic interstitial pneumonias. The clinical presentation and prevalence of these phenotypes are heterogenous (1). The clinical course of the different ILD ranges from completely reversible to self-limiting to progressive and can be fatal despite optimal management. Globally, ILDs have a reported incidence ranging from 1 to 31.5 per 100,000 person-years and prevalence ranging from 6.3 to 71 per 100,000 people (2).
Antifibrotic therapies for ILD have improved, which in turn have offset lung function decline and extended life expectancy (3-5). Complex medication regimens with immunosuppressive medications are common in ILD patients with different aetiologies resulting in a high frequency of potential drug–disease interactions (6). However, these treatments together with longevity have presented an increasing number of adverse effects from polypharmacy (7,8). Although antifibrotic therapies aim to slow disease progression, to date, reduction in symptoms or improvement in activities of daily living (ADL) and health-related quality of life (HRQL) have not been shown (7).
Aging results in progressive physiologic alterations of several bodily systems that can compound changes due to chronic respiratory diseases such as ILD (9). Although 60% to 70% of idiopathic pulmonary fibrosis (IPF) patients die as a consequence of an exacerbation, other causes of death can result from comorbidities related to aging, lifestyle, and disease progression (i.e., lung cancer, cardiovascular diseases, and pulmonary hypertension) (10). Across several studies, frailty is highly prevalent in ILD patients (4,11-13). An increasing number of reports have documented sarcopenia (loss of muscle mass and function) in ILD (13-15), which may be more evident in aging populations (16) and importantly, a risk factor for pending frailty (17,18). Frailty and sarcopenia are associated with increased adverse outcomes including falls, functional declines, and mortality (19-21). Hence, maintaining muscle mass and muscle strength are key elements to preventing muscle dysfunction in ILD (12) and related comorbidities (22,23).
Dyspnea, a highly prevalent symptom in ILD patients has been attributed to ventilatory limitation resulting from decreased lung compliance and gas exchange impairment (24). Dyspnea is considered to contribute to decreased ADL, HRQL, exercise capacity, and physical inactivity in ILD. However, dyspnea may not only limit physical activity but also unduly load cognitive demands. Increased ventilatory demands associated with dyspnea require conscious efforts and cortical activation (25), which may limit cognitive capacity for other tasks. Through similar processes, dyspnea may limit ILD patients via its affective sensation and by limiting the available cognitive capacity (i.e., concentration, memory, and executive function) for well-coordinated purposeful movement (26).
Due to the increasing median age of ILD patients, several new considerations for rehabilitation arise as a result of aging, polypharmacy, and comorbidities (4). Aging-related syndromes such as sarcopenia and frailty are recognized as contributors to physical impairments in ILD, and daily activities may be further limited by cognitive interference and impairments. This review aims to discuss the multifaceted issues related to physical and cognitive function that limit physical activities in ILD patients and provide potential new directions for pulmonary rehabilitation. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-209/rc).
Methods
The search strategy was developed with the assistance of a reference librarian (AO-C) (Table 1) (27). Ovid MEDLINE and EBSCO CINAHL Ultimate databases were searched from inception to October 19th 2022 using search terms based on concepts of: interstitial lung diseases, frailty, muscular atrophy, skeletal muscle dysfunction, cognitive dysfunction, sleep quality, sleep disorders, anxiety disorders, or depressive disorders. The search was limited to human studies and full-text articles in English or Japanese (Appendix 1). After eligible texts were screened, additional references were included from references cited in the screened articles.
Table 1
| Items | Specification |
|---|---|
| Date of search | 19 October 2022 |
| Databases and other sources searched | Ovid Medline, CINAHL Ultimate (EBSCOhost) |
| Search terms used | exp lung diseases, interstitial/ |
| Frailty/ | |
| exp muscular atrophy/ | |
| (Skeletal muscle* adj2 dysfunction*).mp. | |
| Cognition disorders/ or exp cognitive dysfunction/ | |
| Sleep/ or sleep quality/ | |
| exp sleep wake disorders/ | |
| exp anxiety disorders/ | |
| exp depressive disorder/ | |
| exp anxiety/ | |
| Depression/ | |
| Timeframe | 1946–2022 |
| Inclusion and exclusion criteria | English and Japanese language |
| Selection process | MH, TT, RK, AOC, DR, and WDR conducted the selection, and obtained consensus |
Main findings—physical and cognitive limitations in ILD patients and related evaluations
The main findings of this review describe the multifaceted issues related to physical and cognitive function in ILD patients (Figure 1). Examination of frailty in ILD provides an underlying construct to further describe physical and cognitive limitations. Moreover, how the exercise training in pulmonary rehabilitation can be complemented through a cognitive or motor control lens is proposed. The main findings will describe: (I) frailty in ILD; (II) skeletal muscle dysfunction in ILD; (III) cognitive limitations in ILD; (IV) exercise training in pulmonary rehabilitation for ILD patients.
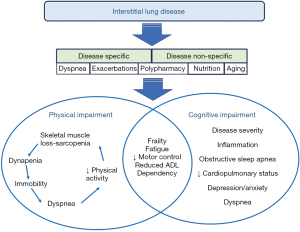
Frailty in ILD
Previous studies reported the prevalence of frailty in ILD patients to range from approximately 24% to 55% (Table 2). However, the evidence of frailty in ILD patients is not well-described. Detailed mechanisms contributing to frailty are not completely understood and the optimal assessment tool for frailty in ILD patients remains to be defined. Moreover, ILD patients with frailty have decreased activity levels, and hence, maintenance of physical activity is essential to mitigate frailty. This section will define frailty, describe the three most common frailty models utilized in the chronic lung disease literature, and highlight the clinical implications of frailty in ILD.
Table 2
| Author, year, country | Age, years | Sex (M:F) | Diagnosis | Prevalence of frailty, % | Clinical outcomes |
|---|---|---|---|---|---|
| Fried frailty phenotype | |||||
| Montgomery et al. (28), 2022, Australia | 55 | 49%:51% | Patients listed for lung transplant; ILD (n=130) | 44 | The addition of cognition and depression to assessment of physical function increased number classified as frail, but these measures did not change the strength of association with lung transplant waitlist mortality |
| Farooqi et al. (18), 2021, Canada | 68 | 55%:45% | ILD (n=463): IPF 183, CTD-ILD 79, HP 27, sarcoidosis 22, other 152 | 26 | Frailty was independently associated with an increased risk of death |
| Montgomery et al. (29), 2020, Australia | 57 | 71%:29% | ILD (n=100): IPF 68, HP 11, NSIP 3, CTD-ILD 7, other 11 | 24 | Frailty was associated with anemia, hypoalbuminemia, and the need for supplemental oxygen |
| Sheth et al. (30), 2019, USA | 76 | 58%:42% | IPF (n=48) | 48 | Factors related to frailty were aging, lower FVC, DLco, 6MWD, severe fatigue and dyspnea, and increased comorbidities |
| Rozenberg et al. (31), 2018, Canada | 59 | 58%:42% | ILD (n=34) | 26 | Frailty had moderate correlations with 6MWD, short physical performance battery, and activities of daily living |
| Singer et al. (32), 2015, USA | 58† | 51%:49%† | Patients listed for lung transplant; ILD (n=208) | 29 | Frailty is independently associated with greater disability and an increased risk of delisting or death pre-transplant |
| Cumulative deficit scales | |||||
| Guler et al. (33), 2020, Canada | M: 67, F: 63 | 43%:57% | Fibrotic ILDs (n=540): IPF 100 | 50 | Functional ageing is associated with adverse health outcomes (quality of life, hospitalizations and survival) |
| Milne et al. (4), 2017, Canada | 69 | 54%:46% | ILD (n=129): IPF 41 | 50 | Dyspnea severity is independently associated with frailty and a more important determinant of frailty than pulmonary function |
| Guler et al. (34), 2017, Canada | 61 | 20%:80% | Systemic sclerosis associated ILD (n=86) and CTD-ILD (n=167) | 55 | Dyspnea is strongly associated with frailty |
†, data for the entire cohort, not specifically the ILD subgroup. No studies were found that applied the Clinical Frailty Scale. M, males; F, females; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; HP, hypersensitivity pneumonitis; CTD-ILD, connective tissue disease - associated interstitial lung disease; NSIP, nonspecific interstitial pneumonia; FVC, forced vital capacity; DLco, diffusing capacity of lung for carbon monoxide; 6MWD, six-minute walking distance.
Definition of frailty
The concept of frailty is a disorder of multiple interrelated physiological systems and is considered to be distinct from the functional losses associated with physiological aging and comorbidities (35,36). A consensus group consisting of six international societies defined physical frailty as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death” (37). Despite the work by consensus groups globally, a gold standard definition of frailty remains to be defined, especially in the ILD population (37-39). To date, no specific treatment for frailty has been proposed, other than modifying a sedentary lifestyle and nutritional factors (35,40). However, it is considered that early intervention has the potential to mitigate frailty, improve HRQL and reduce health care costs (37).
Frailty assessments have shown that both physical and cumulative deficits contribute to this syndrome (41). The main models of frailty are: (I) the physical frailty model, also indicated as phenotypic or syndromic frailty, and (II) the cumulative deficit frailty model, which captures the deficits in physical and psychosocial elements in an individual. Frailty assessments are meant to capture inter-related measures that are known to be predictive of clinical outcomes, through assessments of physical function, sarcopenia, cognitive and psychological assessments, nutritional state, and immunologic alterations (42).
Frailty scales
A number of frailty scales have been developed to identify varying degrees of physical, psychological, or social function, with 67 frailty scales applied to community-dwelling populations based on a 2016 report (43). Three scales appear to be used most often in chronic lung disease and may be suitable to evaluate ILD patients. The Fried Frailty Phenotype (FFP) index (44) is the most common frailty index utilized to date and proposes five components (weakness; slowness; unintentional weight loss; exhaustion and low physical activity). Patients who have three or more criteria are classified as frail. In contrast, the cumulative deficits model evaluates frailty based on an accumulation of health deficits (symptoms, laboratory measures, disabilities, and comorbidities), and captures physical, psychological, and social function (45,46). Increased frailty based on accumulation of deficits has been shown to be associated with adverse clinical outcomes in a diverse group of patient populations including community dwelling older adults, solid organ transplant populations, and chronic disease states (47-50). A third scale, the clinical frailty scale evaluates the overall level of frailty and fitness (51) and consists of specific domains: comorbidity, function, and cognition. This scale provides a score ranging from 1 (very fit) to 9 (terminally ill) (51) and a higher score has been associated with worse clinical outcomes in community dwelling adults and individuals with chronic obstructive pulmonary disease (COPD) (i.e., increased mortality and admission to an institution) (52). The clinical frailty scale has not been applied to-date in ILD patients but may offer a complementary measure of frailty in ILD patients in the clinical setting. To-date, only the Fried and cumulative deficits scales have been utilized in ILD patients (Table 2), and the most sensitive and responsive measure in ILD remains to be determined (33).
Frailty in ILD patients and its clinical implications
Several studies reported evidence of frailty in ILD patients (Table 2). The prevalence of frailty ranged from 24% to 48% when the Fried scale was applied to patients in three different countries (Australia, Canada, and USA) (Table 2) (18,28-32,53). The prevalence was higher in the three studies that utilized the cumulative deficits index ranging from 50 to 55% (4,34,54).
Across several ILD studies using the Fried and cumulative deficits scales (Table 2), frailty was commonly associated with increased ILD disease severity (lung function, supplemental oxygen, and six-minute walking distance (6MWD) (29-32). Frailty may predict medication side effects (33) and has been shown to be associated with a significantly higher prevalence of depressive symptoms in ILD patients (55). Furthermore, arthropathy was common in connective tissue associated ILD and may have a profound impact on functional mobility and physical activity levels. Thus, patients with connective tissue disease-associated ILD were more likely to be frail than non-frail (56). Several studies utilizing the cumulative frailty index demonstrated that dyspnea was a stronger determinant of frailty than measures of lung function (4,34). Importantly, frailty was associated with an increased risk of hospitalizations, worse HRQL and higher risk of delisting while awaiting lung transplantation. In addition, frail patients were shown to have three times higher risk of mortality (29,32,54). Another report corroborated this finding and showed that frailty was predictive of mortality, independent of age, sex, lung function, and underlying ILD diagnosis (18).
Summary of frailty
Detailed mechanisms contributing to frailty remain to be determined but it is considered to be a common risk factor for adverse outcomes in ILD patients. Frailty domains such as weight loss and exhaustion could be influenced by medications (steroid or anti-fibrotic use) and their interactions (18). Frailty appears to be associated with worse clinical status, exercise intolerance, skeletal muscle dysfunction, and decreased HRQL in ILD. Further studies are needed to determine the optimal assessment tools and to develop multifaceted strategies to prevent and counter frailty in ILD patients (34,54).
Skeletal muscle limitations in ILD
Multiple factors have been shown to contribute to skeletal muscle dysfunction in ILD patients. Most investigations have examined limb muscles (57,58), however, respiratory muscle dysfunction has been considered because it can also impact the progression to respiratory failure (59). This section will describe issues of limb muscle dysfunction related to loss of muscle mass and function, along with several sarcopenia definitions. Risk factors for skeletal muscle dysfunction such as corticosteroids, physical inactivity, aging, and hypoxemia will be briefly discussed. The literature on respiratory muscle dysfunction will also be synthesized.
Limb muscle dysfunction and its associated risk factors
Multiple factors contribute to skeletal muscle loss in chronic lung disease, including but not limited to disease progression, physical inactivity, and corticosteroid use (60). Skeletal muscle dysfunction in ILD patients has received increased attention more recently and has important implications on daily function (58,61,62). In addition to skeletal muscle dysfunction, other contributing factors limiting daily function include: dyspnea, hypoxemia, poor sleep, comorbidities, and psychological stressors (63), which can in turn exacerbate skeletal muscle dysfunction. Although anti-fibrotic therapy may slow lung function loss, IPF patients receiving this therapy appear to have ongoing skeletal muscle loss, where skeletal muscle mass has been shown to be an important prognostic marker of survival (12). Moreover, ILDs other than IPF are treated with systemic corticosteroids and immunosuppressive therapies (64,65) that can contribute to skeletal muscle dysfunction through various side effects. This is considered to occur due to increased muscle proteolysis and decreased protein synthesis (66,67) which contributes to muscle atrophy, decreased muscle fiber size and corresponding skeletal muscle weakness (68-70). This can be increasingly exacerbated by long-term corticosteroid treatment in ILD patients (57). Corticosteroid treatment has been associated with skeletal muscle weakness, even in those with mild dyspnea (71), highlighting the importance of early intervention in ILD through exercise and physical activity to counter skeletal muscle dysfunction.
Skeletal muscle dysfunction is often considered a part of the common diagnostic criteria for frailty (72) through interrelated factors such as muscle weakness, physical inactivity and weight loss, which are often associated with muscle wasting. In addition, the common symptoms of fatigue and dyspnea in ILD can lead to a perpetuating downward spiral of physical inactivity and can accentuate deconditioning. Increased fatigue has an important impact on ADL and physical activity levels, independent of age and disease severity, and frailty encompasses other elements beyond skeletal muscle and physical function such as psychosocial function.
Physical activity in ILD patients
Physical activity is one of the most important factors in mitigating frailty and skeletal muscle dysfunction. Wallaert et al. (73) showed that IPF patients walked 65% fewer daily steps compared to the healthy sedentary control group. Additionally, ILD patients with greater impairments in physiological function had lower physical activity levels and demonstrated greater sedentary behaviors (74). In lung transplant candidates with severe ILD, quadriceps strength demonstrated a moderate correlation with total daily step count, whereas no significant correlation with lung function was observed (75).
Physical inactivity may contribute to skeletal muscle deconditioning and a cycle of decreased physical fitness and exercise intolerance (76,77). In particular, daily steps count in ILD patients showed that fewer than 3,300 daily steps was associated with an increased risk of mortality (73,78,79). However, other studies observed no associations between baseline daily steps count or its association with 12-month survival (80,81). Thus, unlike the association of physical inactivity with skeletal muscle dysfunction in ILD, the association between daily step count and prognosis in ILD patients requires further characterization.
Sarcopenia in ILD
Sarcopenia has been defined as a systemic, progressive skeletal muscle decline that involves the loss of muscle mass and function and is associated with adverse outcomes including falls, functional decline, frailty, and mortality in community dwelling populations (19,82). The definitions for sarcopenia have been variable with both the 2019 European Working Group on Sarcopenia in Older People 2 (EWGSOP2) (82) and the Sarcopenia Definitions and Outcomes Consortium (SDOC) (83) utilizing low muscle strength as a primary criterion. However, EWGSOP2 also added the criterion of low muscle mass whereas SDOC also requires low gait speed. In Asia, the Asian Working Group for Sarcopenia (AWGS) 2019 consensus (84) is often utilized, which requires low muscle mass and one of either low strength or physical performance.
Only a few studies have provided evidence of sarcopenia in ILD patients (Table 3). According to EWGSOP2 definition (82), sarcopenia was identified in 19 (22.9%) IPF patients, in a cohort of consecutive patients followed in 9 hospitals across Italy (14). Using the AWGS 2019 criteria (84), sarcopenia was identified in 32.1% ILD patients (13) and 39.3% in IPF patients (15) living in Japan. Given the variability in diagnostic criteria for sarcopenia, comparisons between studies are often challenging among cohorts. The following sections highlight studies that assessed skeletal muscle mass, strength or function, which are key elements comprising sarcopenia.
Table 3
| Author, year, country | Age, sex (M:F) | Diagnosis, n | Definition criteria | Prevalence of sarcopenia | GAP score | BMI, kg/m2 | %FVC, % | Muscle mass | Strength/function | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modality | Reduction in size | Muscle group/function | Comment | |||||||||
| Hanada et al. (13), 2022, Japan | 71 [67–77] years, 65%:35% | ILD, 78 | AWGS 2019 | 32.1% | 3 [2–4] | 23 [20–28] | 85 [63–93] | SMI: 7 [5–7] cm; calf circumference: 33 [30–37] cm | No significant difference compared to non-sarcopenia | QF: 23 [16–33] kgf; HF: 23 [19–33] kgf | No significant difference compared to non-sarcopenia | |
| Fujita et al. (15), 2022, Japan | 73.1±7.7 years, 88%:12% | IPF, 56 | AWGS 2019 | 39.3% | 4 [3–5] | 20.7±2.8 | 74.6±14.8 | ASMI: M 6.2 [6.0–6.5] cm; F 5.1 [5.0–5.2] cm | Significantly lower in sarcopenic males compared to non-sarcopenia males | HF: M 26.1 [23.0–28.5] kg, F 15.8 [13.8–16.4] kg; gait speed: 0.8±0.2 m/s | HF significantly lower in males compared to non-sarcopenic male group; no significant difference compared to non-sarcopenic group in gait speed | |
| Wickerson et al. (85), 2020, Canada | Mild ILD: 60±9 years, 60%:40%; severe ILD: 65±5 years, 62%:38% | Mild ILD (n=10): IPF 3, other 7; severe ILD (n=13): IPF 4, other 9 | NA | NA | NA | Mild ILD, 27±4; severe ILD, 26±3 | Mild ILD, 81±17; severe ILD, 59±20 | NA | NA | Knee-extensor peak torque: mild ILD 137±44 N∙m, severe ILD 104±32 N∙m; elbow-flexor peak torque: mild ILD 57±18 N∙m, severe ILD 40±18 N∙m | No significant difference compared to healthy group (n=13) as well as between mild and severe ILD in knee-extensor or elbow-flexor peak torque; no significant difference between mild and severe ILD in knee-extensor or elbow-flexor peak torque | |
| Guler et al. (86), 2019, Canada | M: 69±10 years, F: 66±9 years; 64%:36% |
ILD (n=115): IPF 40, other: 75 | NA | NA | NA | M: 28±4; F: 28±6 | M: 77±17; F: 72±22 | SMI: M 7.9±0.9 kg/m2, F 6.2±1.0 kg/m2; ALM: M 24.0±3.7 kg, F 16.2±2.6 kg; body fat: M 29.1%±5.2%, F 39.8%±6.5% | Significantly lower muscle mass and higher fat mass in individuals with more impaired pulmonary function | HF: M 40.2±9.6 kg, F 25.6±5.7 kg; 4MGS: M 1.33±0.3 m/s, F 1.25±0.3 m/s | Males with ILD had a weaker HF compared to an age-matched healthy Canadian, although HF was not lower than expected in females with ILD; 4MGS was similar to the general population | |
| Mendes et al. (58), 2015, Canada | 61±8 years; 73%:27% | IPF: 23; other: 3 | NA | NA | NA | 27±3 | 49±13 | Rectus femoris CSA, 7.6±2.1 cm; gastrocnemius and soleus layer thickness, 2.8±0.6 cm; biceps layer thickness, 2.6±0.4 cm | Significantly smaller muscle size than community adults |
Knee extension peak torque, 119±35 N·m; Ankle plantarflexion peak torque, 37±19 N·m; Biceps brachii peak torque, 39±19 N·m |
Leg muscle strength is lower than community adults | |
| Mendoza et al. (87), 2014, Chili | 64.4±7.7 years; 92%:8% | IPF: 15; unclassifiable ILD: 10 | NA | NA | NA | 28.6±4.7 | 78.7±14.0 | FFM, 59.5±10.3 kg; %Fat: 30.5±5.4 | NS | TwQ: 8.0±2.4 kg | QF is lower than local community participants. | |
| Watanabe et al. (88), 2013, Japan | M: 62 [36–69] years, F: 59 [34–75] years; 30%:70% | f-NSIP: 30 | NA | NA | NA | NA | %VC, M: 87.5 [57.1–110.6], F: 71.7 [41.7–100.0] | NA | NA | QF: M 148.5 [77.5–236] N·m, F 74.5 [45–110] N·m; HF: M 39.3 [32.5–49.5] kg, F 22.3 [10–36.5] kg | Significantly decreased QF in 16 subjects; both QF and HF were significantly higher in male than in female | |
| Wickerson et al. (75), 2013, Canada | 62 [53–65] years | IPF: 12; other: 12 | NA | NA | NA | 25.5±3.5 | 48.9±14 | NA | NA | QT, 120±36 N·m | QT was moderately correlated with daily steps | |
| Kozu et al. (76), 2011, Japan | 67.5±7.8 years | IPF: 45 | NA | NA | NA | 21.2±3.3 | 80.1±17.3 | NA | NA | QF, 19.1±10.1 kg; HF, 23.2±9.1 kg | No significant difference compared to COPD group | |
| Nishiyama et al. (89), 2005, Japan | 64±9 years, 85%:15% | IPF: 41 | NA | NA | NA | NA | %VC, 76.6±16.8 | NA | NA | QF: 87±28 N; HF: 32±19 N | QF was significantly related to VO2 max | |
Data are presented as mean ± standard deviation, median [interquartile range], or numbers (%). M, male; F, female; GAP score, Gender, age, and lung physiology score; BMI, body mass index; ILD, interstitial lung disease; AWGS 2019, the Asian Working Group for Sarcopenia 2019 consensus; SMI, skeletal muscle index; ASMI, appendicular skeletal muscle index; QF, quadriceps force; HF, hand grip force; IPF, idiopathic pulmonary fibrosis; ALM, appendicular lean mass; 4MGS, 4 meter gait speed test; CSA, cross-sectional area; FFM, fat-free mass; TwQ, quadriceps twitch force in response to magnetic femoral nerve stimulation; COPD, chronic obstructive pulmonary disease; f-NSIP, fibrotic- non-specific interstitial pneumonia; QT, quadriceps torque; FVC, forced vital capacity; NSIP, non-specific interstitial pneumonia; N, number of patients; VC, vital capacity; VO2, oxygen consumption.
Assessment of skeletal muscle mass
Reports utilizing the sarcopenia criteria in ILD have been limited (82,84). Skeletal muscle mass in ILD has often been quantified using the cross-sectional area of thoraco-abdominal muscles measured from computed tomography (CT) (90). Studies have shown that decreased cross-sectional areas of erector spinae muscles (ESMCSA) (91,92) and pectoralis muscles (PM CSA) (62,93) measured with CT images were associated with lower skeletal muscle mass index, decreased quadriceps strength, increased ILD severity (11,93-95) and mortality (93). Future evaluation of low muscle mass may be informative through more accessible modalities such as body composition with dual-energy X-ray absorptiometry (DXA) (86) or bioelectrical impedance analysis (BIA) (96).
Assessment of skeletal muscle strength and function
Skeletal muscle strength, specifically quadriceps strength, has been shown to be reduced in ILD in several studies, as shown in Table 3 (58,87,88). Mendoza et al. demonstrated with non-volitional tests that quadriceps strength and endurance were significantly lower in fibrotic ILD patients than community healthy controls (87). Furthermore, quadriceps force was observed to be lower in IPF patients compared to COPD prior to rehabilitation, which may help explain part of the variable response with pulmonary rehabilitation between the two disease states (76). Quadriceps strength has been shown to be an important determinant of exercise capacity (75,87,89), including post-rehabilitation (76).
The exact contribution of disease severity and lung function on muscle strength remains unclear, as some studies have suggested a positive association (88,89), whereas others have demonstrated no correlation (75). There have been no studies to our knowledge that have demonstrated the contribution of skeletal muscle strength or function on ILD survival. Thus, further exploration of skeletal muscle function with disease severity and clinical outcomes remains to be investigated.
Respiratory muscle function
Respiratory muscles in ILD patients have been investigated in several studies with smaller sample sizes that show disparate outcomes. During the early phases of ILD, in spite of increased work of breathing, it is considered that the respiratory muscles are generally preserved. Unlike COPD, the chest wall and diaphragm curvature are altered to a lesser extent which appears to allow diaphragm dependency during tidal ventilation (61). Although preservation of maximal inspiratory pressures (MIP) has been reported in some investigations (61,97), several decrements have been shown including: reduced MIP (98), a greater neural drive (97,98), and reduced non-volitional diaphragm force (97). Further, diaphragm thickening fraction and mobility, visualized using ultrasound, were lower in ILD patients and these impairments were associated with disease severity as determined by forced vital capacity (FVC) percent predicted (99). Moreover, as IPF progresses to advanced stages, hypercapnia may be indicative of ventilatory failure (i.e., respiratory muscles are unable to meet the ventilatory demands) (100).
ILD associated with systemic inflammatory conditions may present with additive atrophic or myopathic changes that compound respiratory muscle impairment. A 3-year study on a cohort of 36 patients with systemic sclerosis-associated ILD showed progressive atrophy of chest wall musculature (i.e., latissimus dorsi muscle, erector spinae muscle, serratus anterior muscle, inferior trapezius muscle, and inferior pectoralis major muscle) (101). Hence, respiratory muscle atrophy in some ILD may be an independent contributor to FVC decline given their major role in forced expiration (102). Taken together, it was recommended that ILD progression should be evaluated by comprehensive assessments that include evaluations of respiratory muscle mass and function rather than FVC alone (101). Sarcopenia related respiratory muscle dysfunction may play a key role in the development of respiratory failure (61,103).
Summary of limb and respiratory muscle dysfunction
The relationship between sarcopenia and adverse events has been reported across several disease states (104-106). Sarcopenia is directly linked to physical inactivity but its impact on clinical outcomes has not well-described in ILD patients (107). Irrespective of the definition used across studies, sarcopenia appears prevalent with common risk factors including corticosteroid therapy, physical inactivity, increased bed rest from exacerbations, and hypoxemia. Sarcopenia can have important effects on overall well-being and functional capacity in ILD patients, which may bring out limitations in overall physical conditioning and cognitive function.
Cognitive limitations in ILD patients
The constraints of cognition limiting physical activities in ILD patients need to be considered because limb muscle movement is initiated and refined by cortical activation. Moreover, respiratory muscle function during elevated ventilation also requires cortical activation (108). Due to the demands of physical function on cortical activation, physical activities can be hampered by cognitive impairment. Even with normal cognitive capacity, movement could be further hindered by poor motor control and interference by other tasks that require cognition. This section will describe the limited evidence of cognitive impairment in ILD patients and how cognitive interference and poor motor control may further diminish physical activities in ILD patients.
Risk factors and prevalence
ILD patients have several risk factors (i.e., hypoxemia, inflammation, aging) associated with cognitive impairment, however, its prevalence is not well documented (109). A restrictive ventilatory pattern was correlated with worse cognitive performance in the Atherosclerosis Risk in Communities Study that examined a sample of 10,975 men and women (110,111). Smaller samples of patients showed evidence of decreased cognitive performance in those with severe IPF compared to those with more mild disease and healthier controls (112). Montreal Cognitive Assessment (MoCA) scores were lower in IPF patients compared to control subjects but not compared to COPD patients (109). Cognitive impairment was evident in more than one-third of sarcoidosis patients, regardless of disease severity (113) but was even more common in those with neurosarcoidosis, recruited from the Dutch Neurosarcoidosis Registry (114). While the prevalence is not well defined in ILD patients, neither are the underlying mechanisms, but several potential contributors have been suggested.
Potential contributing factors to cognitive impairment
Causes of cognitive impairments in ILD are likely multifactorial and may be similar to those described in COPD (115-117), however, no neurobiological model of underlying causes has been proposed. Several factors have been identified as potential contributors to cognitive interference or impairment including: disease severity, cardiopulmonary status, inflammation, obstructive sleep apnea (OSA), depression, dyspnea, and fatigue. Patients with more severe IPF demonstrated greater cognitive impairment (112). Inflammation has been acknowledged as a causative agent in sarcoidosis given the improvement in cognition and fatigue after anti-TNF-α therapy (113). Worse cardiopulmonary indices of 6MWD, post-exercise heart rate, and oxygen saturation before and after exercise were predictors of worse performance on cognitive tasks in 51 patients with ILD (118). IPF patients with more severe OSA had greater cognitive impairment (109). Although depression can influence cognition and was found in a small cohort of those with severe IPF, most participants scored below the clinical threshold for clinical intervention (112). Dyspnea showed a strong association with depression (119). Fatigue, reported to affect up to 95% of ILD patients, has been considered to be partly consequential to cognitive impairment (120) but the converse has not been described. Taken together, the interrelationships among disease severity, inflammation, cardiopulmonary status, OSA, depression, fatigue, and dyspnea as moderators or mediators of cognitive impairment in ILD patients require further investigation.
Does cognitive interference limit physical function?
The ability to assess the contribution of cognition on physical performance in clinical settings and research has been undertaken using dual-task paradigms. These paradigms challenge cognitive capacity because the simultaneous performance of two different tasks can call upon similar resources or exceed capacity. This can lead to errors or decreased performance of the given tasks, termed dual task or cognitive interference (121). Dual-task interference has been shown in older adults (122,123), neurological disorders (i.e., Parkinson’s disease, stroke) (124-126), and COPD patients (121,122,127,128). Cognitive tasks used in the dual task paradigms applied in COPD patients included backwards spelling (121) and counting backwards (122,127,128). During dual tasking, decrements of the physical tasks were shown for gait speed (121,127), timed-up-and-go test (128), and balance (122). Given the evidence of dual-task interference in older adults and other disorders, the effect of cognitive interference on physical function will be important to explore in ILD patients.
Dyspnea’s role in cognitive interference
Dyspnea, a predominant symptom in ILD responsible for diminished ADL, HRQL, and exercise capacity (24), may also play a major role in cognitive interference with physical function. Physical impairments in ILD have been primarily attributed to skeletal muscle dysfunction, disease severity, and dyspnea. However, it remains unclear whether dyspnea can impose more than just an unpleasant sensation on neurocognitive processing. Increased ventilatory demands activate cortical regions and are considered to be more conscious than quiet breathing (108). With a finite cortical capacity, greater sensations of dyspnea or increased cognitive demands could potentially interfere with cognition and purposeful movement in ILD patients, similar to what has been demonstrated in healthy people and COPD patients. Induced dyspnea by inspiratory threshold or resistive loading impaired facial recognition (129), reduced accuracy of a Stroop color word test (130), and decreased timed up-and-go performance (131) in healthy adults. Further, indoor walking showed decreasing prefrontal neural activity, a sign of automaticity, in older and younger adults but not in COPD patients (123,132). In a similar light, dyspnea may not only affect how ILD patients feel during physical exertion but may also limit or interfere with the available cognitive capacity required for well-controlled, coordinated movement during daily physical activities.
Cognition and activities of daily living
ILD appears to have a progressive, profound effect on ADL. Individuals with interstitial lung abnormalities are much less likely to be independent in activities of daily living [adjusted odds ratio 0.70 (0.55 to 0.90)] (133). The Glittre-ADL, a functional ADL test that evaluates trunk, arm, and leg movements showed worse scores in those with more severe ILD (134). Advanced IPF can even limit self-care such as showering and simple household chores (134,135). Dyspnea, mood, fatigue, and depression appear to be contributing and/or aggravating factors (134,135). Although limitations of ADL in ILD patients have not been explicitly attributed to cognitive interference or impairment, this requires further study.
Routine daily activities often require carrying out two or more tasks simultaneously, known as dual tasking or multi-tasking (i.e., talking while walking or avoiding physical obstacles while walking). Automaticity can improve from single and dual-task training as shown in healthy adults and disorders such as stroke and amputees (136). There appears to be a transfer effect from cognitive training to physical tasks, such as with the timed-up-and-go test in older adults (136,137). Hand dexterity can also be improved through rehabilitation training, as shown in patients with neurological and rheumatoid conditions (138,139). Further, physical and cognitive training appears to induce distinct changes related to reaction time in dual-task performance (140). In fact, exercise training and physical activity (e.g., dance) can result in structural and functional changes in the hippocampus/parahippocampus area, the cerebellum, and the occipitotemporal cortex in adults older than 60 years (141,142).
Summary of cognitive limitations in ILD
Although cognitive impairment appears to be evident in ILD, the prevalence and the neurobiological model of underlying causes require further investigation. Regardless, the factors that contribute to cognitive interference during ADL (i.e., dyspnea) need to be defined. Lastly, many ADL require efficient coordinated movement such that exercise training during pulmonary rehabilitation could be advanced through a “motor control” lens that considers cognitive demands in addition to the current emphasis on aerobic and resistance training that primarily focus on peripheral muscle and cardiovascular adaptation.
Exercise training in pulmonary rehabilitation in ILD
Whole body exercise
Several studies provide evidence to support the benefits of pulmonary rehabilitation as a non-pharmacological therapy in ILD patients (76,143-146). It is an important therapeutic strategy because of its demonstrated, improved short-term outcomes for ILD patients irrespective of age, however, sustained benefits are less certain (147). Most studies report durations of 8 to 12 weeks but a couple of studies followed patients for 26 or 48 weeks (147). Recently, the effect of pulmonary rehabilitation in ILD has been comprehensively reported in an updated Cochrane Review (144). This review summarized evidence from 21 studies, of which data from sixteen studies were synthesized in meta-analyses (356 participants undertook pulmonary rehabilitation and 319 were control participants). Improvements in functional exercise capacity, dyspnea and HRQL were significant. In particular, the 6MWD was a sensitive outcome and exceeded its clinical minimum important difference with a mean improvement of 40 m (95% CI: 33–47) in 585 ILD participants. Holland et al. (56) emphasized that this updated Cochrane Review reported benefits of pulmonary rehabilitation that persisted as long as 6 to 12 months.
In general, the basic components of these programs are aerobic exercise training (i.e., walking, cycling) and resistance training (148-150). Systemic reviews have shown that pulmonary rehabilitation in ILD patients demonstrates significant improvements in exercise capacity, dyspnea and HRQL (144,149). Several previous reports have shown that exercise including resistance training actually improve sarcopenia in healthy older adults. Exercise programs have the potential to support muscle function in older people with sarcopenia (151,152). The evidence for peripheral muscle strength improvement in ILD has only been investigated in several studies, but there have been notable improvements in quadriceps strength with pulmonary rehabilitation (153,154).
Strategies for individual patient-tailored programs or reduction of exercise-induced hypoxemia and dyspnea are required to optimize training intensity. Because exercise-induced hypoxemia is common and may be severe in ILD patients, this issue needs to be addressed through the introduction of oxygen therapy and other strategies such as interval training to offset hypoxemia and recovery of oxygenation with rest. The intensity may need to be modified due to the inability of some ILD patients to perform the optimal prescribed load, especially in those who are frail or sarcopenic. One approach is to target an increase in total physical activity levels given that this is an important predictor of morbidity and mortality in patients with chronic respiratory diseases such as ILD (73,74,155).
As a countermeasure for ILD patients who exhibit significant desaturation, oxygen therapy during exercise is often used (156). The efficacy of supplemental oxygen in patients with IPF who have exercise-induced hypoxemia demonstrates that supplemental oxygen during exercise can improve endurance time, desaturation and subjective symptoms (157). Hence, the American Thoracic Society/European Respiratory Society (ATS/ERS) guideline statement regarding pulmonary rehabilitation recommends supplemental oxygen during exercise training for ILD patients (150,158).
Respiratory muscle training
Regardless of whether decrements in MIP are consistently shown, work of breathing is increased in ILD due to decreased lung compliance and this is associated with dyspnea on exertion (159). Of interest, dyspnea has been associated with decreased MIP in some patients (160), suggesting a nonspecific association with ILD (161). Evidence that inspiratory muscle training (IMT) can improve dyspnea and mitigate functional aspects associated with ILD progression is limited by the paucity of well-designed studies. One randomized controlled double-blind study showed that 15 sarcoidosis patients, who received IMT for 6 weeks, improved functional and maximal exercise capacity, increased MIP, and improved fatigue and dyspnea (162). Notably, peripheral muscle strength did not improve (162). A systematic scoping review, albeit a small number of studies, cautiously reported the benefit of IMT on exercise capacity, dyspnea, and inspiratory muscle function in ILD patients (163). This scoping review was limited by the small number of available reports and by their designs. It included studies that examined IMT combined with pulmonary rehabilitation, IMT applied to both patients with obstructive and/or restrictive disease and case reports of IMT alone (59). Patients reported improved breathlessness, activities of daily living and mobility after IMT (164).
Given the well-established benefit of IMT on decreasing dyspnea sensation in other populations, IMT is worthy of further investigation in ILD patients.
Potential future directions in pulmonary rehabilitation
Pulmonary rehabilitation regimens have demonstrated that ILD patients improve skeletal muscle strength and endurance in response to training (144). Motor control, defined as the ability to perform purposeful movement, can be limited during physical activity and exercise even in those without discernable cognitive impairment. Although this is well described in healthy adults (165), there is limited recognition of this perspective in acute and chronic respiratory conditions (166-168). Future approaches to rehabilitation could explore the integration of motor learning, dual tasking, and improving automaticity as they pertain to physical ADL (136,169).
Training strategies, previously applied to other populations could potentially improve coordination, dual-tasking, and multifaceted physical ADL in ILD patients. Even in those without cognitive impairment, physical performance may be limited by motor control, which may be affected by factors that limit cognitive capacity such as dyspnea, affect, fatigue, sleep, pain, and motivation (109,120,170-172). Ongoing evaluation of the cognitive influence on physical activity may also be important to identify limitations that require simplification during physical activities (i.e., avoid or reduce cognitive demands during walking). Considering the benefit of exercise and physical activity in older adults, and the exacerbating influence of inactivity (173,174), motor control relevant to daily activities requires further study in ILD patients. Generally, pulmonary rehabilitation improves comorbid depression and anxiety in chronic respiratory patients (147). However, the effectiveness of pulmonary rehabilitation for dementia has not been explicitly investigated. Moreover, often times clinical manifestations associated with dementia are exclusion criteria (i.e., the ability to attend sessions on a regular basis). Although pulmonary rehabilitation has the potential to improve cognition or diminish cognitive interference, its impact on dementia remains unclear.
In addition, future refinement of exercise training for ILD patients should consider modifications of training parameters (i.e., frequency, intensity, type time) and choice of equipment. Interval training offers an alternative endurance training for ILD patients who may not be able perform the continuous prescribed load. A feasibility study of oxygen dependent ILD patients demonstrated that 20 min of interval exercise (30 s bouts at 100% of peak work) was preferred to continuous exercise (50% of peak work) and induced less end-exercise Borg fatigue and lower heart rate response (175). Interval training can provide the total training time by repetitive bouts rather than continuous exercise, while reducing dyspnea and fatigue (156). However, further research regarding interval training for ILD patients needs to establish the merit of this approach.
Clinical implications
The current review provides some clinical considerations in the ILD population given the increased age, comorbidities and polypharmacy experienced by this population (5-7). Irrespective of the frailty index utilized, the concept of frailty in ILD has been shown to be associated with increased risk of hospitalizations and elevated risk of readmissions. Given that ILD exacerbations and hospitalizations are a significant source of morbidity and mortality (28,30,32,34,54-57), identification of frailty parameters that are potentially modifiable such as weight loss, skeletal muscle dysfunction, and physical activity should be important considerations in the management of ILD patients (56,57). In addition, the concept of cognitive impairment and interference is gaining increased recognition in the COPD literature (176), with a few studies now highlighting cognitive limitations in ILD. Thus, modifiable risk factors that can limit cognition, such as hypoxemia and dyspnea, need to be addressed by use of supplemental oxygen, increasing fitness, and utilization of energy conservation strategies. Consideration of a “motor control” lens in pulmonary rehabilitation that factors the contribution of dyspnea and motor learning on cognitive processing and multi-tasking, may prove to be a promising strategy in improving ADL and HRQL.
Conclusions
In recent years, treatments for ILD patients have improved with an evolving demographic of older patients and greater emphasis on frailty and physical function. Despite the high prevalence of frailty and skeletal muscle dysfunction in ILD, the underlying neurobiologic mechanisms require further definition. Cognitive impairment is evident in ILD, however, its implications for interference with physical daily activities is not well defined. The benefits of pulmonary rehabilitation’s primary focus on strength and aerobic conditioning could be complemented by different strategies such as interval training and the integration of motor learning. This could potentially help improve transfer of rehabilitation strategies to physical daily activities and HRQL.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-209/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-209/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-209/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet 2022;400:769-86. [Crossref] [PubMed]
- Kaul B, Cottin V, Collard HR, et al. Variability in Global Prevalence of Interstitial Lung Disease. Front Med (Lausanne) 2021;8:751181. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- Milne KM, Kwan JM, Guler S, et al. Frailty is common and strongly associated with dyspnoea severity in fibrotic interstitial lung disease. Respirology 2017;22:728-34. [Crossref] [PubMed]
- Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res 2019;20:205. [Crossref] [PubMed]
- Khor YH, Glaspole I, Goh NSL. Therapeutic burden in interstitial lung disease: Lessons to learn. Respirology 2019;24:566-71. [Crossref] [PubMed]
- Rozenberg D, Sitzer N, Porter S, et al. Idiopathic Pulmonary Fibrosis: A Review of Disease, Pharmacological, and Nonpharmacological Strategies With a Focus on Symptoms, Function, and Health-Related Quality of Life. J Pain Symptom Manage 2020;59:1362-78. [Crossref] [PubMed]
- Jo HE, Randhawa S, Corte TJ, et al. Idiopathic Pulmonary Fibrosis and the Elderly: Diagnosis and Management Considerations. Drugs Aging 2016;33:321-34. [Crossref] [PubMed]
- Rojas M, Mora AL, Kapetanaki M, et al. Aging and Lung Disease. Clinical Impact and Cellular and Molecular Pathways. Ann Am Thorac Soc 2015;12:S222-7. [Crossref] [PubMed]
- Caminati A, Lonati C, Cassandro R, et al. Comorbidities in idiopathic pulmonary fibrosis: an underestimated issue. Eur Respir Rev 2019;28:190044. [Crossref] [PubMed]
- Ebihara K, Iwanami Y, Yamasaki K, et al. Appendicular Skeletal Muscle Mass Correlates with Patient-Reported Outcomes and Physical Performance in Patients with Idiopathic Pulmonary Fibrosis. Tohoku J Exp Med 2021;253:61-8. [Crossref] [PubMed]
- Suzuki Y, Aono Y, Kono M, et al. Cause of mortality and sarcopenia in patients with idiopathic pulmonary fibrosis receiving antifibrotic therapy. Respirology 2021;26:171-9. [Crossref] [PubMed]
- Hanada M, Sakamoto N, Ishimoto H, et al. A comparative study of the sarcopenia screening in older patients with interstitial lung disease. BMC Pulm Med 2022;22:45. [Crossref] [PubMed]
- Faverio P, Fumagalli A, Conti S, et al. Sarcopenia in idiopathic pulmonary fibrosis: a prospective study exploring prevalence, associated factors and diagnostic approach. Respir Res 2022;23:228. [Crossref] [PubMed]
- Fujita K, Ohkubo H, Nakano A, et al. Frequency and impact on clinical outcomes of sarcopenia in patients with idiopathic pulmonary fibrosis. Chron Respir Dis 2022;19:14799731221117298. [Crossref] [PubMed]
- Saketkoo LA, Obi ON, Patterson KC, et al. Ageing with Interstitial lung disease: preserving health and well being. Curr Opin Pulm Med 2022;28:321-36. [Crossref] [PubMed]
- Mori H, Tokuda Y. Differences and overlap between sarcopenia and physical frailty in older community-dwelling Japanese. Asia Pac J Clin Nutr 2019;28:157-65. [Crossref] [PubMed]
- Farooqi MAM, O'Hoski S, Goodwin S, et al. Prevalence and prognostic impact of physical frailty in interstitial lung disease: A prospective cohort study. Respirology 2021;26:683-9. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636-46. [Crossref] [PubMed]
- Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 2015;16:247-52. [Crossref] [PubMed]
- Nolan CM, Maddocks M, Maher TM, et al. Gait speed and prognosis in patients with idiopathic pulmonary fibrosis: a prospective cohort study. Eur Respir J 2019;53:1801186. [Crossref] [PubMed]
- Marklund S, Bui KL, Nyberg A. Measuring and monitoring skeletal muscle function in COPD: current perspectives. Int J Chron Obstruct Pulmon Dis 2019;14:1825-38. [Crossref] [PubMed]
- Tyrovolas S, Panagiotakos D, Georgousopoulou E, et al. Skeletal muscle mass in relation to 10 year cardiovascular disease incidence among middle aged and older adults: the ATTICA study. J Epidemiol Community Health 2020;74:26-31. [Crossref] [PubMed]
- Bonini M, Fiorenzano G. Exertional dyspnoea in interstitial lung diseases: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev 2017;26:160099. [Crossref] [PubMed]
- Raux M, Straus C, Redolfi S, et al. Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J Physiol 2007;578:569-78. [Crossref] [PubMed]
- Brunette AM, Holm KE, Wamboldt FS, et al. Subjective cognitive complaints and neuropsychological performance in former smokers with and without chronic obstructive pulmonary disease. J Clin Exp Neuropsychol 2018;40:411-22. [Crossref] [PubMed]
- Searching the literature: A guide to comprehensive searching in the Health Sciences. [cited 2023 Jun 26]. Available online: https://guides.library.utoronto.ca/comprehensivesearching
- Montgomery E, Newton PJ, Chang S, et al. Frailty Measures in Patients Listed for Lung Transplantation. Transplantation 2022;106:1084-92. [Crossref] [PubMed]
- Montgomery E, Macdonald PS, Newton PJ, et al. Frailty as a Predictor of Mortality in Patients With Interstitial Lung Disease Referred for Lung Transplantation. Transplantation 2020;104:864-72. [Crossref] [PubMed]
- Sheth JS, Xia M, Murray S, et al. Frailty and geriatric conditions in older patients with idiopathic pulmonary fibrosis. Respir Med 2019;148:6-12. [Crossref] [PubMed]
- Rozenberg D, Mathur S, Wickerson L, et al. Frailty and clinical benefits with lung transplantation. J Heart Lung Transplant 2018;37:1245-53. [Crossref] [PubMed]
- Singer JP, Diamond JM, Gries CJ, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. Am J Respir Crit Care Med 2015;192:1325-34. [Crossref] [PubMed]
- Guler SA, Kwan JM, Leung JM, et al. Functional ageing in fibrotic interstitial lung disease: the impact of frailty on adverse health outcomes. Eur Respir J 2020;55:1900647. [Crossref] [PubMed]
- Guler SA, Kwan JM, Winstone TA, et al. Severity and features of frailty in systemic sclerosis-associated interstitial lung disease. Respir Med 2017;129:1-7. [Crossref] [PubMed]
- Aguilaniu B. Can a better understanding of frailty improve the quality of life of patients with fibrotic interstitial lung diseases? Eur Respir J 2020;55:1902255. [Crossref] [PubMed]
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752-62. [Crossref] [PubMed]
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392-7. [Crossref] [PubMed]
- Kwak D, Thompson LV. Frailty: Past, present, and future? Sports Med Health Sci 2021;3:1-10. [Crossref] [PubMed]
- Kojima G. Frailty Defined by FRAIL Scale as a Predictor of Mortality: A Systematic Review and Meta-analysis. J Am Med Dir Assoc 2018;19:480-3. [Crossref] [PubMed]
- Mudge AM, Hubbard RE. Frailty: mind the gap. Age Ageing 2018;47:508-11. [Crossref] [PubMed]
- Varughese R, Rozenberg D, Singer LG. An update on frailty in lung transplantation. Curr Opin Organ Transplant 2020;25:274-9. [Crossref] [PubMed]
- Schaenman JM, Diamond JM, Greenland JR, et al. Frailty and aging-associated syndromes in lung transplant candidates and recipients. Am J Transplant 2021;21:2018-24. [Crossref] [PubMed]
- Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53-61. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722-7. [Crossref] [PubMed]
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27:17-26. [Crossref] [PubMed]
- Mitnitski A, Bao L, Rockwood K. Going from bad to worse: a stochastic model of transitions in deficit accumulation, in relation to mortality. Mech Ageing Dev 2006;127:490-3. [Crossref] [PubMed]
- Varughese RA, Theou O, Li Y, et al. Cumulative Deficits Frailty Index Predicts Outcomes for Solid Organ Transplant Candidates. Transplant Direct 2021;7:e677. [Crossref] [PubMed]
- Bhanji RA, Narayanan P, Moynagh MR, et al. Differing Impact of Sarcopenia and Frailty in Nonalcoholic Steatohepatitis and Alcoholic Liver Disease. Liver Transpl 2019;25:14-24. [Crossref] [PubMed]
- Wilson ME, Vakil AP, Kandel P, et al. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplant 2016;35:173-8. [Crossref] [PubMed]
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489-95. [Crossref] [PubMed]
- Zhang D, Tang W, Dou LY, et al. Four different frailty models predict health outcomes in older patients with stable chronic obstructive pulmonary disease. BMC Geriatr 2022;22:57. [Crossref] [PubMed]
- Singer JP, Diamond JM, Anderson MR, et al. Frailty phenotypes and mortality after lung transplantation: A prospective cohort study. Am J Transplant 2018;18:1995-2004. [Crossref] [PubMed]
- Guler SA, Ryerson CJ. Frailty in patients with interstitial lung disease. Curr Opin Pulm Med 2020;26:449-56. [Crossref] [PubMed]
- Tremblay Labrecque PF, Dion G, Saey D. Functional clinical impairments and frailty in interstitial lung disease patients. ERJ Open Res 2022;8:00144-2022. [Crossref] [PubMed]
- Holland AE. Physiotherapy management of interstitial lung disease. J Physiother 2022;68:158-64. [Crossref] [PubMed]
- Hanada M, Sakamoto N, Ishimatsu Y, et al. Effect of long-term treatment with corticosteroids on skeletal muscle strength, functional exercise capacity and health status in patients with interstitial lung disease. Respirology 2016;21:1088-93. [Crossref] [PubMed]
- Mendes P, Wickerson L, Helm D, et al. Skeletal muscle atrophy in advanced interstitial lung disease. Respirology 2015;20:953-9. [Crossref] [PubMed]
- Hoffman M. Inspiratory muscle training in interstitial lung disease: a systematic scoping review. J Bras Pneumol 2021;47:e20210089. [Crossref] [PubMed]
- Vogiatzis I, Zakynthinos G, Andrianopoulos V. Mechanisms of physical activity limitation in chronic lung diseases. Pulm Med 2012;2012:634761.
- Panagiotou M, Polychronopoulos V, Strange C. Respiratory and lower limb muscle function in interstitial lung disease. Chron Respir Dis 2016;13:162-72. [Crossref] [PubMed]
- Moon SW, Choi JS, Lee SH, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res 2019;20:35. [Crossref] [PubMed]
- Wijsenbeek MS, Holland AE, Swigris JJ, et al. Comprehensive Supportive Care for Patients with Fibrosing Interstitial Lung Disease. Am J Respir Crit Care Med 2019;200:152-9. [Crossref] [PubMed]
- Kim R, Meyer KC. Therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis 2008;2:319-38. [Crossref] [PubMed]
- Vacchi C, Sebastiani M, Cassone G, et al. Therapeutic Options for the Treatment of Interstitial Lung Disease Related to Connective Tissue Diseases. A Narrative Review. J Clin Med 2020;9:407. [Crossref] [PubMed]
- Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 2008;197:1-10. [Crossref] [PubMed]
- Schakman O, Kalista S, Barbé C, et al. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 2013;45:2163-72. [Crossref] [PubMed]
- Dekhuijzen PN, Decramer M. Steroid-induced myopathy and its significance to respiratory disease: a known disease rediscovered. Eur Respir J 1992;5:997-1003.
- Fournier M, Huang ZS, Li H, et al. Insulin-like growth factor I prevents corticosteroid-induced diaphragm muscle atrophy in emphysematous hamsters. Am J Physiol Regul Integr Comp Physiol 2003;285:R34-43. [Crossref] [PubMed]
- Gupta A, Gupta Y. Glucocorticoid-induced myopathy: Pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab 2013;17:913-6. [Crossref] [PubMed]
- Hanada M, Ishimatsu Y, Sakamoto N, et al. Corticosteroids are associated with reduced skeletal muscle function in interstitial lung disease patients with mild dyspnea. Respir Med 2020;174:106184. [Crossref] [PubMed]
- Bone AE, Hepgul N, Kon S, et al. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis 2017;14:85-99. [Crossref] [PubMed]
- Wallaert B, Monge E, Le Rouzic O, et al. Physical activity in daily life of patients with fibrotic idiopathic interstitial pneumonia. Chest 2013;144:1652-8. [Crossref] [PubMed]
- Nishiyama O, Yamazaki R, Sano H, et al. Physical activity in daily life in patients with idiopathic pulmonary fibrosis. Respir Investig 2018;56:57-63. [Crossref] [PubMed]
- Wickerson L, Mathur S, Helm D, et al. Physical activity profile of lung transplant candidates with interstitial lung disease. J Cardiopulm Rehabil Prev 2013;33:106-12. [Crossref] [PubMed]
- Kozu R, Senjyu H, Jenkins SC, et al. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration 2011;81:196-205. [Crossref] [PubMed]
- Holland AE. Exercise limitation in interstitial lung disease - mechanisms, significance and therapeutic options. Chron Respir Dis 2010;7:101-11. [Crossref] [PubMed]
- Bahmer T, Kirsten AM, Waschki B, et al. Prognosis and longitudinal changes of physical activity in idiopathic pulmonary fibrosis. BMC Pulm Med 2017;17:104. [Crossref] [PubMed]
- Shingai K, Matsuda T, Kondoh Y, et al. Cutoff Points for Step Count to Predict 1-year All-Cause Mortality in Patients with Idiopathic Pulmonary Fibrosis. Respiration 2021;100:1151-7. [Crossref] [PubMed]
- Prasad JD, Paul E, Holland AE, et al. Physical activity decline is disproportionate to decline in pulmonary physiology in IPF. Respirology 2021;26:1152-9. [Crossref] [PubMed]
- Badenes-Bonet D, Rodó-Pin A, Castillo-Villegas D, et al. Predictors and changes of physical activity in idiopathic pulmonary fibrosis. BMC Pulm Med 2022;22:340. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. [Crossref] [PubMed]
- Bhasin S, Travison TG, Manini TM, et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc 2020;68:1410-8. [Crossref] [PubMed]
- Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-307.e2. [Crossref] [PubMed]
- Wickerson L, Mathur S, Brooks D, et al. Skeletal muscle oxygenation and regional blood volume during incremental limb loading in interstitial lung disease. ERJ Open Res. 2020;6:00083-2019. [Crossref] [PubMed]
- Guler SA, Hur SA, Lear SA, et al. Body composition, muscle function, and physical performance in fibrotic interstitial lung disease: a prospective cohort study. Respir Res 2019;20:56. [Crossref] [PubMed]
- Mendoza L, Gogali A, Shrikrishna D, et al. Quadriceps strength and endurance in fibrotic idiopathic interstitial pneumonia. Respirology 2014;19:138-43. [Crossref] [PubMed]
- Watanabe F, Taniguchi H, Sakamoto K, et al. Quadriceps weakness contributes to exercise capacity in nonspecific interstitial pneumonia. Respir Med 2013;107:622-8. [Crossref] [PubMed]
- Nishiyama O, Taniguchi H, Kondoh Y, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest 2005;127:2028-33. [Crossref] [PubMed]
- McClellan T, Allen BC, Kappus M, et al. Repeatability of Computerized Tomography-Based Anthropomorphic Measurements of Frailty in Patients With Pulmonary Fibrosis Undergoing Lung Transplantation. Curr Probl Diagn Radiol 2017;46:300-4. [Crossref] [PubMed]
- Awano N, Inomata M, Kuse N, et al. Quantitative computed tomography measures of skeletal muscle mass in patients with idiopathic pulmonary fibrosis according to a multidisciplinary discussion diagnosis: A retrospective nationwide study in Japan. Respir Investig 2020;58:91-101. [Crossref] [PubMed]
- Suzuki Y, Yoshimura K, Enomoto Y, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci Rep 2018;8:14074. [Crossref] [PubMed]
- Molgat-Seon Y, Guler SA, Peters CM, et al. Pectoralis muscle area and its association with indices of disease severity in interstitial lung disease. Respir Med 2021;186:106539. [Crossref] [PubMed]
- Pietro KM, Ricardo G, Rui GPND, et al. Relationship of pectoralis muscle area and skeletal muscle strength with exercise tolerance and dyspnea in interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis 2017;34:200-8. [Crossref] [PubMed]
- Rozenberg D, Martelli V, Vieira L, et al. Utilization of non-invasive imaging tools for assessment of peripheral skeletal muscle size and composition in chronic lung disease: A systematic review. Respir Med 2017;131:125-34. [Crossref] [PubMed]
- Rinaldi S, Gilliland J, O'Connor C, et al. Fat-Free Mass Index Controlled for Age and Sex and Malnutrition Are Predictors of Survival in Interstitial Lung Disease. Respiration 2021;100:379-86. [Crossref] [PubMed]
- Walterspacher S, Schlager D, Walker DJ, et al. Respiratory muscle function in interstitial lung disease. Eur Respir J 2013;42:211-9. [Crossref] [PubMed]
- Gorini M, Spinelli A, Ginanni R, et al. Neural respiratory drive and neuromuscular coupling during CO2 rebreathing in patients with chronic interstitial lung disease. Chest 1989;96:824-30. [Crossref] [PubMed]
- Santana PV, Prina E, Albuquerque AL, et al. Identifying decreased diaphragmatic mobility and diaphragm thickening in interstitial lung disease: the utility of ultrasound imaging. J Bras Pneumol 2016;42:88-94. [Crossref] [PubMed]
- Janowiak P, Szymanowska-Narloch A, Siemińska A. IPF Respiratory Symptoms Management - Current Evidence. Front Med (Lausanne) 2022;9:917973. [Crossref] [PubMed]
- Nawata T, Shirai Y, Suzuki M, et al. Chest wall muscle atrophy as a contributory factor for forced vital capacity decline in systemic sclerosis-associated interstitial lung disease. Rheumatology (Oxford) 2021;60:250-5. [Crossref] [PubMed]
- Sieck GC, Ferreira LF, Reid MB, et al. Mechanical properties of respiratory muscles. Compr Physiol 2013;3:1553-67. [Crossref] [PubMed]
- Hamada R, Oshima Y, Yoshioka Y, et al. Comparison of international and Japanese predictive equations for maximal respiratory mouth pressures. Respir Investig 2022;60:847-51. [Crossref] [PubMed]
- Xia L, Zhao R, Wan Q, et al. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies. Cancer Med 2020;9:7964-78. [Crossref] [PubMed]
- Sasaki KI, Fukumoto Y. Sarcopenia as a comorbidity of cardiovascular disease. J Cardiol 2022;79:596-604. [Crossref] [PubMed]
- Kim SE, Kim DJ. Sarcopenia as a prognostic indicator of liver cirrhosis. J Cachexia Sarcopenia Muscle 2022;13:8-10. [Crossref] [PubMed]
- Patterson KC, Shah RJ, Porteous MK, et al. Interstitial Lung Disease in the Elderly. Chest 2017;151:838-44. [Crossref] [PubMed]
- Raux M, Straus C, Redolfi S, et al. Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J Physiol 2007;578:569-78. [Crossref] [PubMed]
- Tudorache V, Traila D, Marc M, et al. Impact of moderate to severe obstructive sleep apnea on the cognition in idiopathic pulmonary fibrosis. PLoS One 2019;14:e0211455. [Crossref] [PubMed]
- Tudorache E, Marc M, Traila D, et al. Cognitive Impairment in Chronic Lung Diseases. In: Akarsu S. An Overview and Management of Multiple Chronic Conditions. London: IntechOpen, 2020.
- Pathan SS, Gottesman RF, Mosley TH, et al. Association of lung function with cognitive decline and dementia: the Atherosclerosis Risk in Communities (ARIC) Study. Eur J Neurol 2011;18:888-98. [Crossref] [PubMed]
- Bors M, Tomic R, Perlman DM, et al. Cognitive function in idiopathic pulmonary fibrosis. Chron Respir Dis 2015;12:365-72. [Crossref] [PubMed]
- Elfferich MD, Nelemans PJ, Ponds RW, et al. Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration 2010;80:212-9. [Crossref] [PubMed]
- Voortman M, De Vries J, Hendriks CMR, et al. Everyday cognitive failure in patients suffering from neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2019;36:2-10. [Crossref] [PubMed]
- Yin M, Wang H, Hu X, et al. Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm Med 2019;19:203. [Crossref] [PubMed]
- Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis 2010;5:263-9. [Crossref] [PubMed]
- Lee JM, Grabb MC, Zipfel GJ, et al. Brain tissue responses to ischemia. J Clin Invest 2000;106:723-31. [Crossref] [PubMed]
- Giannouli V, Markopoulou A, Kiosseoglou G, et al. Neuropsychological functioning in patients with interstitial lung disease. Appl Neuropsychol Adult 2022;29:1290-5. [Crossref] [PubMed]
- Ryerson CJ, Berkeley J, Carrieri-Kohlman VL, et al. Depression and functional status are strongly associated with dyspnea in interstitial lung disease. Chest 2011;139:609-16. [Crossref] [PubMed]
- Kahlmann V, Moor CC, Wijsenbeek MS. Managing Fatigue in Patients With Interstitial Lung Disease. Chest 2020;158:2026-33. [Crossref] [PubMed]
- Hassan SA, Campos MA, Kasawara KT, et al. Changes in Oxyhemoglobin Concentration in the Prefrontal Cortex during Cognitive-Motor Dual Tasks in People with Chronic Obstructive Pulmonary Disease. COPD 2020;17:289-96. [Crossref] [PubMed]
- Van Hove O, Cebolla AM, Andrianopoulos V, et al. The influence of cognitive load on static balance in chronic obstructive pulmonary disease patients. Clin Respir J 2021;15:351-7. [Crossref] [PubMed]
- Hassan SA, Bonetti LV, Kasawara KT, et al. Decreased automaticity contributes to dual task decrements in older compared to younger adults. Eur J Appl Physiol 2022;122:965-74. [Crossref] [PubMed]
- Al-Yahya E, Dawes H, Smith L, et al. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev 2011;35:715-28. [Crossref] [PubMed]
- Christofoletti G, Andrade LP, Beinotti F, et al. Cognition and dual-task performance in older adults with Parkinson's and Alzheimer's disease. Int J Gen Med 2014;7:383-8. [Crossref] [PubMed]
- Yang YR, Chen YC, Lee CS, et al. Dual-task-related gait changes in individuals with stroke. Gait Posture 2007;25:185-90. [Crossref] [PubMed]
- Heraud N, Alexandre F, Gueugnon M, et al. Impact of Chronic Obstructive Pulmonary Disease on Cognitive and Motor Performances in Dual-Task Walking. COPD 2018;15:277-82. [Crossref] [PubMed]
- Morlino P, Balbi B, Guglielmetti S, et al. Gait abnormalities of COPD are not directly related to respiratory function. Gait Posture 2017;58:352-7. [Crossref] [PubMed]
- Vinckier F, Morélot-Panzini C, Similowski T. Dyspnoea modifies the recognition of fearful expressions by healthy humans. Eur Respir J 2018;51:1702253. [Crossref] [PubMed]
- Sucec J, Herzog M, Van Diest I, et al. The impairing effect of dyspnea on response inhibition. Int J Psychophysiol 2018;133:41-9. [Crossref] [PubMed]
- Nierat MC, Demiri S, Dupuis-Lozeron E, et al. When Breathing Interferes with Cognition: Experimental Inspiratory Loading Alters Timed Up-and-Go Test in Normal Humans. PLoS One 2016;11:e0151625. [Crossref] [PubMed]
- Hassan SA, Bonetti LV, Kasawara KT, et al. Loss of Neural Automaticity Contributes to Slower Walking in COPD Patients. Cells 2022;11:1606. [Crossref] [PubMed]
- Axelsson GT, Putman RK, Araki T, et al. Interstitial lung abnormalities and self-reported health and functional status. Thorax 2018;73:884-6. [Crossref] [PubMed]
- Alexandre HF, Cani KC, Araújo J, et al. Reliability and validity of the Glittre-ADL test to assess the functional status of patients with interstitial lung disease. Chron Respir Dis 2021;18:14799731211012962. [Crossref] [PubMed]
- Swigris JJ, Brown KK, Abdulqawi R, et al. Patients' perceptions and patient-reported outcomes in progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180075. [Crossref] [PubMed]
- Paul SS, Ada L, Canning CG. Automaticity of walking – implications for physiotherapy practice. Physical Therapy Reviews 2005;10:15-23.
- Pothier K, Vrinceanu T, Intzandt B, et al. A comparison of physical exercise and cognitive training interventions to improve determinants of functional mobility in healthy older adults. Exp Gerontol 2021;149:111331. [Crossref] [PubMed]
- Sankar K, Michael Christudhas JC. Influence of aging, disease, exercise, and injury on human hand movements: A systematic review. Proc Inst Mech Eng H 2021;235:1221-56. [Crossref] [PubMed]
- Hammond A, Prior Y. The effectiveness of home hand exercise programmes in rheumatoid arthritis: a systematic review. Br Med Bull 2016;119:49-62. [Crossref] [PubMed]
- Vrinceanu T, Blanchette CA, Intzandt B, et al. A Comparison of the Effect of Physical Activity and Cognitive Training on Dual-Task Performance in Older Adults. J Gerontol B Psychol Sci Soc Sci 2022;77:1069-79. [Crossref] [PubMed]
- Rektorova I, Klobusiakova P, Balazova Z, et al. Brain structure changes in nondemented seniors after six-month dance-exercise intervention. Acta Neurol Scand 2020;141:90-7. [Crossref] [PubMed]
- Ji L, Steffens DC, Wang L. Effects of physical exercise on the aging brain across imaging modalities: A meta-analysis of neuroimaging studies in randomized controlled trials. Int J Geriatr Psychiatry 2021;36:1148-57. [Crossref] [PubMed]
- Holland AE, Hill CJ, Glaspole I, et al. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med 2012;106:429-35. [Crossref] [PubMed]
- Dowman L, Hill CJ, May A, et al. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2021;2:CD006322. [Crossref] [PubMed]
- Dowman LM, McDonald CF, Hill CJ, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax 2017;72:610-9. [Crossref] [PubMed]
- Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008;13:394-9. [Crossref] [PubMed]
- Yohannes AM, Casaburi R, Dryden S, et al. The effectiveness of pulmonary rehabilitation on chronic obstructive pulmonary disease patients with concurrent presence of comorbid depression and anxiety. Respir Med 2022;197:106850. [Crossref] [PubMed]
- Bolton CE, Blakey JD, Morgan MD, et al. The British Thoracic Society guideline on pulmonary rehabilitation in adults: your opinion is noted. Thorax 2014;69:388-9. [Crossref] [PubMed]
- Hanada M, Kasawara KT, Mathur S, et al. Aerobic and breathing exercises improve dyspnea, exercise capacity and quality of life in idiopathic pulmonary fibrosis patients: systematic review and meta-analysis. J Thorac Dis 2020;12:1041-55. [Crossref] [PubMed]
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. [Crossref] [PubMed]
- Chen N, He X, Feng Y, et al. Effects of resistance training in healthy older people with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Eur Rev Aging Phys Act 2021;18:23. [Crossref] [PubMed]
- Papadopoulou SK, Papadimitriou K, Voulgaridou G, et al. Exercise and Nutrition Impact on Osteoporosis and Sarcopenia-The Incidence of Osteosarcopenia: A Narrative Review. Nutrients 2021;13:4499. [Crossref] [PubMed]
- Naz I, Ozalevli S, Ozkan S, et al. Efficacy of a Structured Exercise Program for Improving Functional Capacity and Quality of Life in Patients With Stage 3 and 4 Sarcoidosis: A randomized controlled trial. J Cardiopulm Rehabil Prev 2018;38:124-30. [Crossref] [PubMed]
- Perez-Bogerd S, Wuyts W, Barbier V, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res 2018;19:182. [Crossref] [PubMed]
- Vainshelboim B, Kramer MR, Izhakian S, et al. Physical Activity and Exertional Desaturation Are Associated with Mortality in Idiopathic Pulmonary Fibrosis. J Clin Med 2016;5:73. [Crossref] [PubMed]
- Nakazawa A, Cox NS, Holland AE. Current best practice in rehabilitation in interstitial lung disease. Ther Adv Respir Dis 2017;11:115-28. [Crossref] [PubMed]
- Arizono S, Furukawa T, Taniguchi H, et al. Supplemental oxygen improves exercise capacity in IPF patients with exertional desaturation. Respirology 2020;25:1152-9. [Crossref] [PubMed]
- Mendes RG, Castello-Simões V, Trimer R, et al. Exercise-Based Pulmonary Rehabilitation for Interstitial Lung Diseases: A Review of Components, Prescription, Efficacy, and Safety. Front Rehabil Sci 2021;2:744102. [Crossref] [PubMed]
- Fitting JW, Frascarolo P, Jéquier E, et al. Resting energy expenditure in interstitial lung disease. Am Rev Respir Dis 1990;142:631-5. [Crossref] [PubMed]
- Spiesshoefer J, Henke C, Kabitz HJ, et al. Respiratory Muscle and Lung Function in Lung Allograft Recipients: Association with Exercise Intolerance. Respiration 2020;99:398-408. [Crossref] [PubMed]
- Faisal A, Alghamdi BJ, Ciavaglia CE, et al. Common Mechanisms of Dyspnea in Chronic Interstitial and Obstructive Lung Disorders. Am J Respir Crit Care Med 2016;193:299-309. [Crossref] [PubMed]
- Karadallı MN, Boşnak-Güçlü M, Camcıoğlu B, et al. Effects of Inspiratory Muscle Training in Subjects With Sarcoidosis: A Randomized Controlled Clinical Trial. Respir Care 2016;61:483-94. [Crossref] [PubMed]
- Hoffman M. Inspiratory muscle training in interstitial lung disease: a systematic scoping review. J Bras Pneumol 2021;47:e20210089. [Crossref] [PubMed]
- Hoffman M, Assis MG, Augusto VM, et al. The effects of inspiratory muscle training based on the perceptions of patients with advanced lung disease: a qualitative study. Braz J Phys Ther 2018;22:215-21. [Crossref] [PubMed]
- Hübner L, Voelcker-Rehage C. Does physical activity benefit motor performance and learning of upper extremity tasks in older adults? - A systematic review. Eur Rev Aging Phys Act 2017;14:15. [Crossref] [PubMed]
- Smith JM, Lee AC, Zeleznik H, et al. Home and Community-Based Physical Therapist Management of Adults With Post-Intensive Care Syndrome. Phys Ther 2020;100:1062-73. [Crossref] [PubMed]
- Mantilla CB, Sieck GC. Neuromotor control in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2013;114:1246-52. [Crossref] [PubMed]
- Beauchamp MK, Brooks D, Goldstein RS. Deficits in postural control in individuals with COPD - emerging evidence for an important secondary impairment. Multidiscip Respir Med 2010;5:417-21. [Crossref] [PubMed]
- Clark DJ. Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci 2015;9:246. [Crossref] [PubMed]
- Cleutjens FA, Janssen DJ, Ponds RW, et al. COgnitive-pulmonary disease. Biomed Res Int 2014;2014:697825. [Crossref] [PubMed]
- Mermigkis C, Stagaki E, Amfilochiou A, et al. Sleep quality and associated daytime consequences in patients with idiopathic pulmonary fibrosis. Med Princ Pract 2009;18:10-5. [Crossref] [PubMed]
- Hur SA, Guler SA, Khalil N, et al. Impact of Psychological Deficits and Pain on Physical Activity of Patients with Interstitial Lung Disease. Lung 2019;197:415-25. [Crossref] [PubMed]
- Klimova B, Dostalova R. The Impact of Physical Activities on Cognitive Performance among Healthy Older Individuals. Brain Sci 2020;10:377. [Crossref] [PubMed]
- Pickersgill JW, Turco CV, Ramdeo K, et al. The Combined Influences of Exercise, Diet and Sleep on Neuroplasticity. Front Psychol 2022;13:831819. [Crossref] [PubMed]
- Wickerson L, Brooks D, Granton J, et al. Interval aerobic exercise in individuals with advanced interstitial lung disease: a feasibility study. Physiother Theory Pract 2021;37:1034-42. [Crossref] [PubMed]
- Rassam P, Pazzianotto-Forti EM, Matsumura U, et al. Impact of cognitive capacity on physical performance in chronic obstructive pulmonary disease patients: A scoping review. Chron Respir Dis 2023;20:14799731231163874. [Crossref] [PubMed]


