
Prevalence, diagnosis, and treatment of chronic obstructive pulmonary disease in a hospitalized lung cancer population: a single center study
Highlight box
Key findings
• This study identified COPD as a common respiratory disorder frequently underdiagnosed and undertreated in hospitalized LC patients. The Department of Respiratory and Critical Care Medicine was found to have a significantly higher COPD diagnosis and inhalation treatment rate but a significantly lower screening rate than the Department of Thoracic Surgery. The mention of COPD in the discharge diagnosis may significantly sensitize more patients to inhalation treatment and increase COPD post-hospitalization awareness.
What is known and what is new?
• COPD management in LC patients is often overlooked.
• This study investigated the screening, prevalence, diagnosis, and treatment rate of COPD in hospitalized LC patients and analyzed the differences in COPD management between a surgical and non-surgical ward.
What is the implication, and what should change now?
• The study results emphasize the need for multidisciplinary collaboration to enhance COPD management and improve treatment outcomes in LC patients with COPD.
Introduction
The economic and medical burden of lung cancer (LC) and chronic obstructive pulmonary disease (COPD) is increasing dramatically worldwide and has become a significant public health problem (1,2). Recent epidemiological data indicate that the prevalence of COPD among Chinese adults aged 20 years and above is 8.6%, reaching up to 13.7% among those aged 40 years and above. The estimated number of COPD patients in China is around 100 million (3), and in 2020, COPD was projected to rank third in mortality and fifth in disease burden worldwide (4). LC is one of the leading causes of death in several countries (1). According to recent statistical data, LC accounted for 787,000 new cases and 631,000 deaths in 2015 in China, ranking first in incidence and mortality among malignant tumors in the country (5). In addition, COPD has been shown to be an important factor that may significantly increase the risk and severity of LC, especially in those with other accompanying factors such as smoking, airflow limitation, and others, but it could be significantly reduced if these high-risk patients undergo adequate and timely screening (6-8).
Airflow limitations, especially those resulting from cigarette smoke exposure, have been identified as significant risk factors for both COPD and LC (9,10). The pathological characteristic of COPD is chronic inflammation of the alveoli, caused by harmful substances in cigarette smoke or other environmental gases that stimulate the activation of various cytokines (11,12). Consequently, this chronic inflammation disrupts the normal alveolar structure, leading to emphysema, stimulating cell proliferation and gene mutation, and affecting the development of LC (11).
Clinical studies indicate that LC patients with COPD have a mortality rate of 4–33% (13). Two small sample size studies suggested that the prevalence of COPD in newly diagnosed LC patients can exceed 50% (14,15), which increases the risk of surgery and postoperative complications, leading to a worse overall prognosis than non-COPD patients (14,16,17). In addition, a meta-analysis of 14,171 LC patients, among whom 4,975 had coexisting COPD, showed that the coexistence of COPD in LC patients who had undergone surgical resection was associated with poor prognosis (18). Further, it is reported that COPD patients often have more respiratory symptoms, which decreases the overall quality of life as the severity of airway obstruction increases (14). Studies have found that approximately one-third of LC patients with COPD are not eligible for surgery, and the risk of surgical treatment for these patients is six times higher than for patients without COPD (4,14).
Given the high prevalence of COPD and the adverse prognostic impact of LC, improving and standardizing COPD management in LC patients is crucial because despite the availability of international treatment guidelines (19,20), COPD remains underdiagnosed and undertreated in the general population. In addition, as COPD is frequently viewed as a secondary health concern in comparison to LC, its diagnosis and treatment are often overlooked (21-23). However, current data on the status of COPD management in LC patients are limited, as previous studies primarily focused on LC patients undergoing thoracic surgery or those with early-stage non-small cell LC (22,24), thus paid less attention to the coexisting COPD condition. In addition, most of the existing literature did not elaborately assess and compare the screening, management and follow-up of COPD in LC patients treated in surgical and non-surgical wards.
Thus, to provide a deeper insight into the current status of COPD screening, diagnosis, treatment and follow-up in LC patients, we assessed the number of patients who underwent COPD screening, the rate of COPD treatment, drugs used and missed COPD diagnosis in their hospital discharge diagnosis between the Department of Respiratory and Critical Care Medicine and the Department of Thoracic Surgery. Collectively, our results revealed that COPD remains underdiagnosed and undertreated in the investigated LC populations, and the diagnosis and treatment rates of COPD in LC patients in the non-surgical ward were significantly higher than those in the surgical ward, thus urging the need for increased awareness and education among healthcare providers regarding the diagnosis and management of COPD in LC patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-267/rc).
Methods
Patient selection
We retrospectively analyzed the medical records of 3,578 patients with primary LC admitted to the Department of Thoracic Surgery and the Department of Respiratory and Critical Care Medicine of our hospital between January 2019 and December 2020. The study inclusion criteria were: (I) patients with primary bronchopulmonary carcinoma confirmed by histopathology or cytopathology; (II) the LC was diagnosed in accordance with the International Clinical Practice Guidelines in Oncology (25,26). The exclusion criteria were: (I) patients with malignant tumors that did not originate in the lung (e.g., primary metastases in the breast, bone, gastrointestinal tract, and genitourinary system, among others); (II) presence of precancerous lesions such as atypical squamous epithelial hyperplasia and atypical adenomatous hyperplasia; (III) benign lesions such as granulomatous hyperplasia or non-LC lesions; and (IV) primary diagnosis of other diseases, such as the heart (i.e., severe congestive heart failure), lungs (i.e., active pulmonary tuberculosis, uncontrolled and persistent asthma), and others that may affect patients’ lung function (i.e., abnormalities in large airways).
The LC cases were classified according to the “World Health Organization’s tissue classification of lung tumors” (27) and staged according to the eighth edition of the American Joint Committee on Cancer (28).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics review board of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2022-KL338-01), and individual consent for this retrospective analysis was waived.
Data collection
Patient data were obtained from our institutional electronic medical record system and the Department of Pulmonary Function Examination. Two authors independently reviewed the medical records of all subjects included in the study and extracted relevant data, such as gender, age, body mass index (BMI), smoking history, smoking pack-years (number of cigarettes per day × number of years of smoking), COPD-related treatment, previous respiratory diseases (i.e., chronic bronchitis, emphysema, bronchial asthma), histological type, clinical stage of LC, spirometry data, diagnosis at hospital discharge, and presence of other common comorbidities. Spirometry data, including actual vital capacity (VC) and percent predicted VC (VC%pred), actual forced expiratory volume in 1 s (FEV1) and percent predicted FEV1 (FEV1%pred), actual forced vital capacity (FVC) and percent predicted FVC (FVC%pred), as well as post-bronchodilator FEV1, FEV1%pred, FVC and FVC%pred, were retrospectively collected from the LC patients’ pulmonary function test (PFT) reports or medical records.
COPD diagnosis
To ensure an accurate COPD diagnosis, the COPD condition was determined for the investigated patients in this study only if they underwent spirometry at our hospital during this current hospitalization. Spirometry was performed according to American Thoracic Society and European Respiratory Society recommendations, with central quality assurance of spirometry tracings using a calibrated Master Screen pulmonary function test system (Jaeger, Co., Germany) performed by experienced technicians (29). COPD was defined as a ratio of post-bronchodilator (salbutamol, 200 µg) FEV1 to FVC of less than 0.7, according to the 2019 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (30). The degree of obstruction was staged in accordance with the predicted FEV1, whereby GOLD stage I (mild) referred to FEV1 ≥80% predicted, GOLD stage II (moderate) referred to FEV1 ≥50% but <80% of predicted, GOLD stage III (severe) referred to FEV1 ≥30% but <50% predicted, and GOLD stage IV (very severe) referred to FEV1 <30% of predicted.
In this study, the diagnostic rate of COPD was determined as the proportion of LC patients who underwent spirometry during hospitalization, were spirometry-defined COPD and had COPD mentioned in their discharge diagnosis. Treatment rate was determined as the percentage of patients with spirometry-defined COPD who received inhaled therapy starting from hospitalization following spirometry-defined COPD, such as a long-acting muscarinic antagonist (LAMA), a combined LAMA and long-acting β2 agonist (LABA), a combination of inhaled corticosteroids (ICS) and LABA, or triple therapy (ICS/LABA/LAMA). Further, COPD awareness was defined as the proportion of LC patients with spirometry-defined COPD who were aware of their underlying COPD condition at last follow-up (September 2022), irrespective of a COPD diagnosis documented in the discharge diagnosis.
Follow-up
The LC patients with COPD undergo long-term follow-up every 3 months in our hospital. In this study, we followed up all the included spirometry-defined COPD LC patients until September 2022 to obtain information about whether they were aware of their underlying COPD condition (referred to as COPD awareness in this study) and adherence to COPD inhalation therapy after discharge. The longest follow-up time was 46 months.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 21) software. Normally distributed data are presented as mean ± standard deviation, while non-normally distributed data are expressed as median and quartiles. Group comparisons were calculated using either an independent t-test (for parametric data) or the Mann-Whitney test (for non-parametric data). Categorical data were analyzed using the chi-square test (χ2) and Fisher’s exact tests. Unpaired t-tests were used to compare continuous variables. In addition, we determined the difference in COPD awareness between patients with and without a COPD discharge diagnosis. Variables that showed a significant association (P<0.05) in univariate analysis were included in multivariate analysis. A P value less than 0.05 was considered statistically significant.
Results
Baseline data and characteristics
This study cohort comprised 3,578 LC patients (2,184 men and 1,394 women) admitted to the Department of Respiratory and Critical Care Medicine and Thoracic Surgery between January 2019 and December 2020. Of them, 77.2% (2,762/3,578) underwent spirometry for COPD screening (Figure 1). Analysis of lung function data revealed that 25.0% (690/2,762) of patients were diagnosed with COPD by spirometry, and the overall COPD treatment rate was 45.2% (312/690). Of note, all the investigated LC patients had no past COPD history and had not received related inhalation therapy.
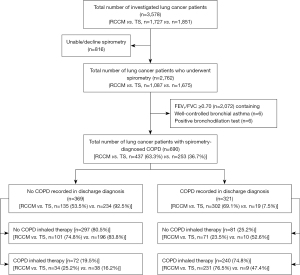
Table 1 presents a comparison of the baseline demographic and clinical characteristics of LC patients with and without COPD. LC patients with coexisting COPD were older and had a lower BMI than those from the LC alone group (P<0.001). Most LC patients with coexisting COPD were male, with a higher number of pack-years and a higher percentage of smokers. The clinical tumor stage of these patients was significantly more advanced than those with LC only (P<0.001). Moreover, LC patients with coexisting COPD were more likely to have coronary heart disease and cerebrovascular disease and were more prone to squamous cell carcinoma than those in the LC alone group (P<0.05).
Table 1
| Variables | Total (n=2,762) | LC (n=2,072) | LC + COPD (n=690) | t/Z/χ2 | P |
|---|---|---|---|---|---|
| Gender | 228.455 | <0.001 | |||
| Male | 1,673 (60.6) | 1,087 (52.5) | 586 (84.9) | ||
| Female | 1,089 (39.4) | 985 (47.5) | 104 (15.1) | ||
| Age (years) | 62.57±10.14 | 60.73±10.13 | 68.11±7.90 | 19.747 | <0.001 |
| BMI (kg/m²) | 24.12±3.37 | 24.32±3.27 | 23.51±3.61 | 5.240 | <0.001 |
| Smoking history | 970 (35.1) | 553 (26.7) | 417 (60.4) | 258.694 | <0.001 |
| Smoking pack-years | 40.00 (25.00, 60.00) | 33.00 (20.00, 50.00) | 40.00 (30.00, 60.00) | −5.747 | <0.001 |
| Histology | 244.748 | <0.001 | |||
| AC | 1,836 (66.5) | 1,541 (74.4) | 295 (42.8) | ||
| SCC | 513 (18.6) | 271 (13.1) | 242 (35.1) | ||
| SCLC | 244 (8.8) | 153 (7.4) | 91 (13.2) | ||
| Others | 169 (6.1) | 107 (5.2) | 62 (9.0) | ||
| Clinical stage | −10.461 | <0.001 | |||
| 0 | 23 (0.8) | 21 (1.0) | 2 (0.3) | ||
| I | 1,206 (43.7) | 1,024 (49.4) | 182 (26.4) | ||
| II | 329 (11.9) | 240 (11.6) | 89 (12.9) | ||
| III | 628 (22.7) | 400 (19.3) | 228 (33.0) | ||
| IV | 576 (20.9) | 387 (18.7) | 189 (27.4) | ||
| Comorbidity | |||||
| Hypertension | 494 (17.9) | 364 (17.6) | 130 (18.8) | 0.571 | 0.450 |
| CHD | 158 (5.7) | 104 (5.0) | 54 (7.8) | 7.561 | 0.006 |
| Cardiac failure | 12 (0.4) | 7 (0.3) | 5 (0.7) | 1.790 | 0.181 |
| Diabetes | 241 (8.7) | 181 (8.7) | 60 (8.7) | 0.001 | 0.974 |
| Cerebrovascular disease | 238 (8.6) | 149 (7.2) | 89 (12.9) | 21.413 | <0.001 |
| Other tumors | 81 (2.9) | 63 (3.0) | 18 (2.6) | 0.339 | 0.560 |
Data are expressed as median (interquartile range), mean ± standard deviation, or n (%). LC, lung cancer; COPD, chronic obstructive pulmonary disease; LC + COPD, lung cancer complicated with COPD; BMI, body mass index; AC, adenocarcinoma; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; CHD, coronary heart disease.
Pulmonary function screening in LC patients
Among the LC patients who underwent spirometry, we observed that 90.5% (1,675/1,851) of patients in the Department of Thoracic Surgery underwent spirometry, while the proportion was only 62.9% (1,087/1,727) in the Department of Respiratory and Critical Care Medicine, and the difference was significantly different (P<0.001) (Table 2).
Table 2
| Subject | Total (n=3,578) | RCCM (n=1,727) | TS (n=1,851) | χ2 | P |
|---|---|---|---|---|---|
| With PFT | 2,762 (77.2) | 1,087 (62.9) | 1,675 (90.5) | 385.187 | <0.001 |
| Without PFT | 816 (22.8) | 640 (37.1) | 176 (9.5) |
Data are expressed as n (%). LC, lung cancer; PFT, pulmonary function test; RCCM, Department of Respiratory and Critical Care Medicine; TS, Department of Thoracic Surgery.
The data of LC spirometry-defined COPD patients in the Department of Respiratory Medicine and Thoracic Surgery are shown in Table 3. The results showed that those from the Department of Respiratory and Critical Care Medicine were more likely to be older and have poorer FVC and FEV1 than those in the Department of Thoracic Surgery (P<0.001). The proportion of males, smoking history, small cell carcinoma, hypertension and diabetes in the non-surgical department was significantly higher than those in the Thoracic Surgery department (P<0.05). In addition, we found that the LC clinical stage and COPD GOLD stage among the patients in the Department of Respiratory and Critical Care Medicine were significantly more advanced than those in the Department of Thoracic Surgery (P<0.05).
Table 3
| Subject | Total (n=690) | RCCM (n=437) | TS (n=253) | t/Z/χ2 | P |
|---|---|---|---|---|---|
| Gender | 21.229 | <0.001 | |||
| Male | 586 (84.9) | 392 (89.7) | 194 (76.7) | ||
| Female | 104 (15.1) | 45 (10.3) | 59 (23.3) | ||
| Age (years) | 68.11±7.9 | 69.32±8.03 | 66.04±7.23 | 5.363 | <0.001 |
| BMI (kg/m²) | 23.50±3.61 | 23.19±3.65 | 24.03±3.48 | 2.960 | 0.003 |
| Smoking history | 417 (60.4) | 322 (73.7) | 95 (37.5) | 87.499 | <0.001 |
| Smoking pack-years | 40.00 (30.00, 60.00) | 40.00 (30.00, 60.00) | 40.00 (30.00, 67.50) | −0.340 | 0.734 |
| Histology | 57.386 | <0.001 | |||
| AC | 295 (42.8) | 151 (34.6) | 144 (56.9) | ||
| SCC | 242 (35.1) | 155 (35.5) | 87 (34.4) | ||
| SCLC | 91 (13.2) | 85 (19.5) | 6 (2.4) | ||
| Others | 62 (9.0) | 46 (10.5) | 16 (6.3) | ||
| Clinical stage | −15.896 | <0.001 | |||
| 0 | 2 (0.3) | 0 (0.0) | 2 (0.8) | ||
| I | 182 (26.4) | 38 (8.7) | 144 (56.9) | ||
| II | 89 (12.9) | 39 (8.9) | 50 (19.8) | ||
| III | 228 (33.0) | 180 (41.2) | 48 (19.0) | ||
| IV | 189 (27.4) | 180 (41.2) | 9 (3.6) | ||
| Comorbidity | |||||
| Hypertension | 130 (18.8) | 94 (21.5) | 36 (14.2) | 5.555 | 0.018 |
| CHD | 54 (7.8) | 36 (8.2) | 18 (7.1) | 0.280 | 0.596 |
| Cardiac failure | 5 (0.7) | 4 (0.9) | 1 (0.4) | 0.096 | 0.756 |
| Diabetes | 60 (8.7) | 51 (11.7) | 9 (3.6) | 13.284 | <0.001 |
| Cerebrovascular disease | 89 (12.9) | 63 (14.4) | 26 (10.3) | 2.444 | 0.118 |
| Other tumors | 18 (2.6) | 10 (2.3) | 8 (3.2) | 0.481 | 0.488 |
| GOLD stage | −5.104 | <0.001 | |||
| I | 212 (30.7) | 112 (25.6) | 100 (39.5) | ||
| II | 367 (53.2) | 233 (53.3) | 134 (53.0) | ||
| III | 98 (14.2) | 81 (18.5) | 17 (6.7) | ||
| IV | 13 (1.9) | 11 (2.5) | 2 (0.8) | ||
| Spirometry | |||||
| VC (L) | 2.76±0.76 | 2.66±0.77 | 2.91±0.71 | 4.223 | <0.001 |
| VC %pred | 83.42±19.07 | 80.01±18.96 | 89.33±17.83 | 6.359 | <0.001 |
| FVC (L) | 2.81±0.76 | 2.72±0.77 | 2.96±0.73 | 4.095 | <0.001 |
| FVC %pred | 87.53±19.15 | 84.08±18.88 | 93.48±18.16 | 6.386 | <0.001 |
| FEV1 (L) | 1.73±0.55 | 1.67±0.56 | 1.85±0.51 | 4.375 | <0.001 |
| FEV1 %pred | 69.49±19.12 | 66.5±19.42 | 74.63±17.46 | 5.654 | <0.001 |
Data are expressed as median (interquartile range), mean ± standard deviation, or n (%). LC, lung cancer; COPD, chronic obstructive pulmonary disease; LC + COPD, lung cancer complicated with COPD; BMI, body mass index; AC, adenocarcinoma; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; CHD, coronary heart disease; GOLD, global initiative for chronic obstructive lung disease; VC, vital capacity; VC %pred, percent predicted VC; FVC, forced vital capacity; FVC %pred, percent predicted FVC; FEV1, forced expiratory volume in 1 s. FEV1%pred, percent predicted FEV1; L, liters; RCCM, Department of Respiratory and Critical Care Medicine; TS, Department of Thoracic Surgery.
Documentation of COPD diagnosis in LC patients at discharge
Of the 2,762 LC patients who underwent spirometry, 25% (690/2,762) were confirmed to have COPD, demonstrating a prevalence rate of spirometry-defined COPD of 40.2% (437/1,087) in the Department of Respiratory and Critical Care Medicine and 15.1% (253/1,675) in the Department of Thoracic Surgery. Among the 690 spirometry-defined COPD patients, only 46.5% (n=321) had a discharge diagnosis of COPD, among whom 302 (69.1%) were from the Department of Respiratory and Critical Care Medicine, while the other 19 (7.5%) were from the Department of Thoracic Surgery (χ2, 244.370; P<0.001), suggesting that doctors in the non-surgical ward paid significantly more attention to the underlying COPD condition than those in the surgical ward.
Missed COPD diagnosis in LC patients at discharge
Although COPD was confirmed in 690 patients by spirometry, patients who did not have COPD recorded in their hospital discharge diagnosis (i.e., LC only as discharge diagnosis rather than LC and COPD) were considered as missed COPD diagnosis in this study. Thus, the overall missed COPD diagnosis in this study was 53.5% (369/690). Further, the missed COPD diagnosis rate in the Department of Respiratory and Critical Care Medicine was significantly lower than that in the Department of Thoracic Surgery (30.9% vs. 92.5%, χ2, 244.370, P<0.001). Additionally, the missed diagnosis rate of COPD GOLD II, III and IV stage was significantly lower than that of GOLD I stage, and the missed diagnosis rate of COPD GOLD III and IV stage was significantly lower than that of GOLD II stage (P<0.001) (Table 4).
Table 4
| GOLD classification of COPD severity | Total (n=690) | RCCM (n=437) | TS (n=253) | |||||
|---|---|---|---|---|---|---|---|---|
| Missed diagnosis | Diagnosed | Missed diagnosis | Diagnosed | Missed diagnosis | Diagnosed | |||
| I | 140 (66.0) | 72 (34.0) | 48 (42.9) | 64 (57.1) | 92 (92.0) | 8 (8.0) | ||
| II | 193 (52.6)a | 174 (47.4) | 68 (29.2) | 165 (70.8) | 125 (93.3) | 9 (6.7) | ||
| III | 34 (34.7)a,b | 64 (65.3) | 19 (23.5) | 62 (76.5) | 15 (88.2) | 2 (11.8) | ||
| IV | 2 (15.4)a,b | 11 (84.6) | 0 (0.0)a | 11 (100.0) | 2 (100.0) | 0 (0.0) | ||
| χ2 | 35.040 | 14.843 | 1.462 | |||||
| P | <0.001 | 0.002 | 0.700 | |||||
Data are expressed as n (%). a, compared with grade 1; b, compared with grade 2; both P<0.05. GOLD, global initiative for chronic obstructive lung disease; COPD, chronic obstructive pulmonary disease; RCCM, Department of Respiratory and Critical Care Medicine; TS, Department of Thoracic Surgery.
COPD inhalation treatment
As shown in Table 5, the rate of COPD inhalation treatment in patients at the Department of Respiratory and Critical Care Medicine during hospitalization was significantly higher than that in the Department of Thoracic Surgery [60.6% (265/437) vs. 18.6% (47/253); P<0.001]. In addition, the proportion of COPD inhalation treatment among LC patients with a discharge diagnosis of COPD was significantly higher than those undiagnosed at discharge (74.8% vs. 19.5%; P<0.001).
Table 5
| Subject | Total (n=690) | RCCM (n=437) | TS (n=253) | χ2 | P |
|---|---|---|---|---|---|
| Total | 114.451 | <0.001 | |||
| Inhaled | 312 (45.2) | 265 (60.6) | 47 (18.6) | ||
| No inhaled | 378 (54.8) | 172 (39.4) | 206 (81.4) | ||
| Discharge diagnosis of COPD | 8.035 | 0.005 | |||
| Inhaled | 240 (74.8) | 231 (76.5) | 9 (47.4) | ||
| No inhaled | 81 (25.2) | 71 (23.5) | 10 (52.6) | ||
| Discharge no diagnosis of COPD | 4.362 | 0.037 | |||
| Inhaled | 72 (19.5) | 34 (25.2) | 38 (16.2) | ||
| No inhaled | 297 (80.5) | 101 (74.8) | 196 (83.8) | ||
| χ2 | 211.575 | 102.888 | 9.294 | ||
| P | <0.001 | <0.001 | 0.002 | ||
Data are expressed as n (%). LC, lung cancer; COPD, chronic obstructive pulmonary disease; LC + COPD, lung cancer complicated with COPD; RCCM, Department of Respiratory and Critical Care Medicine; TS, Department of Thoracic Surgery.
Furthermore, 45.2% (312/690) of LC patients with coexisting COPD received inhalation treatment, among whom triple therapy (ICS/LABA/LAMA) was the most commonly used regimen (101/312, 32.3%), followed by LAMA alone (100/312, 32.1%) and ICS/LABA combination (74/312, 23.7%). Comparatively, dual bronchodilator therapy (LAMA/LABA) was the least commonly used (37/312, 11.9%).
Awareness of COPD and adherence to inhaled therapy
Follow-up was performed for all the 690 spirometry-defined COPD patients; however, only 300 were successfully contacted because 285 had died and 105 were lost to follow-up and their contacted patients’ relatives could not provide accurate follow-up information. The results showed that 26.3% (79/300) of the patients with COPD in discharge diagnosis were aware of their underlying COPD condition, and 12.3% (37/300) adhered to COPD inhalation therapy. Notably, all patients adhering to inhaled therapy were from the 79 patients with COPD in discharge diagnosis (Figure 2).

Factors influencing COPD awareness and adherence to inhaled therapy
Univariate analysis of LC patients who were aware or unaware of COPD is presented in Table 6. Patients who were aware of COPD were slightly older, and the proportion of patients with a smoking history and discharge diagnosis of COPD was higher than those unaware of COPD. The COPD GOLD stage in the patients who were aware of COPD was significantly more advanced than those in the unawareness group. Comparison of the VC, FEV1 and FEV1%pred between the two groups showed that the lung function index of the COPD-aware group was significantly lower than that in the COPD-unaware group (P<0.05). Multivariate analysis (Figure 3) indicated that discharge diagnosis of COPD could significantly increase the proportion of COPD awareness [odds ratio (OR), 4.858; 95% confidence interval (CI): 2.526–9.344; P<0.001].
Table 6
| Subject | Unaware COPD (n=221) | Aware COPD (n=79) | t/Z/χ2 | P |
|---|---|---|---|---|
| Gender | 3.508 | 0.061 | ||
| Male | 168 (76.0) | 68 (86.1) | ||
| Female | 53 (24.0) | 11 (13.9) | ||
| Age (years) | 66.14±7.45 | 68.32±7.10 | 2.251 | 0.025 |
| Smoking history | 99 (44.8) | 46 (58.2) | 4.204 | 0.040 |
| Smoking pack-years | 50.00 (30.00, 75.00) | 40.00 (28.75, 62.50) | −1.185 | 0.236 |
| Histology | 5.448 | 0.142 | ||
| AC | 125 (56.6) | 33 (41.8) | ||
| SCC | 69 (31.2) | 35 (44.3) | ||
| SCLC | 13 (5.9) | 5 (6.3) | ||
| Others | 14 (6.3) | 6 (7.6) | ||
| Clinical stage | −1.529 | 0.126 | ||
| 0 | 2 (0.9) | 0 (0.0) | ||
| I | 108 (48.9) | 33 (41.8) | ||
| II | 40 (18.1) | 15 (19.0) | ||
| III | 54 (24.4) | 20 (25.3) | ||
| IV | 17 (7.7) | 11 (13.9) | ||
| COPD diagnosis | 38.292 | <0.001 | ||
| Discharge diagnosis of COPD | 44 (19.9) | 45 (57.0) | ||
| Discharge no diagnosis of COPD | 177 (80.1) | 34 (43.0) | ||
| GOLD stage | −4.239 | <0.001 | ||
| I | 96 (43.4) | 20 (25.3) | ||
| II | 109 (49.3) | 36 (45.6) | ||
| III | 15 (6.8) | 21 (26.6) | ||
| IV | 1 (0.5) | 2 (2.5) | ||
| Spirometry | ||||
| VC (L) | 2.91±0.76 | 2.62±0.74 | 2.955 | 0.003 |
| FEV1 (L) | 1.87±0.53 | 1.58±0.54 | 4.188 | <0.001 |
| FEV1%pred | 75.76±17.84 | 63.73±20.02 | 4.976 | <0.001 |
Data are expressed as median (interquartile range), mean ± standard deviation, or n (%). LC, lung cancer; COPD, chronic obstructive pulmonary disease; AC, adenocarcinoma; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; GOLD, global initiative for chronic obstructive lung disease; VC, vital capacity; FEV1, forced expiratory volume in 1 s; FEV1%pred, percent predicted FEV1.

Discussion
The principal finding of our observational retrospective study is that COPD remains considerably underdiagnosed and undertreated in LC patients despite specific recommendations in treatment guidelines. The prevalence of spirometry-defined COPD in hospitalized LC patients was 25.0%, the overall COPD diagnostic rate (at hospital discharge) was low (46.5%), and the COPD treatment rate was 45.2%.
In our study, we observed that the prevalence of COPD was in line with some previous research and also notably lower than others, which reported a much wider prevalence range, varying from 36.7% to 62.9% (14,15,24,31). These differences in prevalence may be due to the small sample size, the inclusion of a high proportion of LC patients who were treated in a surgical ward (surgeons might have focused more on treating the more severe disease, such as LC), and limitations such as only assessing patients with early or late-stage tumors. Our study, which included 3,578 LC patients, comprising 1,851 patients in the Department of Thoracic Surgery and 1,727 patients in the Department of Respiratory and Critical Care Medicine, covered various tumor stages and pathological types, thus largely overcoming the limitations in the present literature.
According to a previous study, pulmonary function testing showed that 40% to 70% of newly diagnosed LC patients had varying degrees of COPD (32). However, it is noteworthy that a large proportion of COPD cases in LC patients (72% to 93%) remained undiagnosed because most patients are usually asymptomatic until their FEV1 drops significantly (23). In a retrospective study comprising 102 newly diagnosed LC patients, 37 had LC and COPD. It was observed that 36 of these patients had not been previously diagnosed with COPD, and 21 patients (25%) had not received standardized COPD treatment (33). A multicenter study comprising 602 LC patients reported that their reported prevalence of COPD was 51.5%, and the underdiagnosis rate was 71.6%, indicating that COPD is commonly underdiagnosed in LC patients (31). In our study, the overall underdiagnosis rate in the investigated LC population was 53.5%, which was consistent with data from other surveys (22,34,35).
COPD may be undiagnosed in LC populations due to the following reasons: (I) the COPD symptoms might be mistakenly regarded as cancer presentations (36); (II) the primary treatment priority in LC patients is to treat the underlying cancer condition, which may have a greater threat to the patient’s survival, leading to a lack of attention towards COPD management; (III) LC patients may have multiple coexisting conditions that can complicate the diagnosis and treatment of COPD; and (IV) some surgeons may not always be aware of the high prevalence of COPD in LC patients, and may not have the necessary knowledge and training to diagnose and treat COPD appropriately. Our study demonstrated that the discharge diagnosis of COPD could significantly increase the proportion of LC patients with COPD who are treated with inhaled drugs. Additionally, we observed that diagnosing and treating patients with mild to moderate COPD were more likely to be overlooked. These findings align with previous research, which has also reported a substantial number of COPD patients who remain undiagnosed and, therefore, not treated according to guidelines (19,20). The reasons for missed diagnosis of mild and moderate COPD patients could be due to the subtle nature of respiratory symptoms, such as chest tightness and wheezing, which may not be readily apparent (17). Another significant factor contributing to the neglect of COPD diagnosis is that LC is a highly malignant and life-threatening disease, and clinicians may prioritize diagnosing and treating LC over screening and treating underlying airway diseases.
In our study, we found that respiratory physicians had significantly higher rates of diagnosing and treating COPD with inhaled drugs compared to thoracic surgeons. However, the screening rate for COPD was significantly lower among thoracic surgeons. The primary reason for these differences is that the clinical focus and attention of thoracic surgeons are mainly on preoperative diagnosis, surgical indications, choice of surgical methods, surgical safety, postoperative pathology, wound healing, and rehabilitation. Therefore, their awareness and familiarity with COPD guidelines might be limited, as is the case with many non-respiratory physicians (32). The higher screening rate for COPD in the Department of Thoracic Surgery may be attributed to the preoperative routine lung function tests required for thoracic surgery. However, insufficient attention has been given to diagnosing (discharge diagnosis) and treating LC patients with COPD; thus, it is recommended that thoracic surgeons pay more attention to LC patients with COPD, and patients with LC should undergo a bronchodilation test in addition to routine pulmonary function measurement after admission. Once the results are available, respiratory physicians should be consulted for a clear diagnosis and the formulation of standard treatment plans. After discharge, follow-up visits should be conducted in patients from both departments.
Our study also found that squamous cell carcinoma was more prevalent among the LC patients with coexisting COPD compared to those with LC alone, which is consistent with the results of a foreign study (14). The main reason for this association is that COPD and LC share many common etiological or risk factors, such as long-term smoking, exposure to air pollution, engaging in harmful occupations (such as coal and silica dust exposure), and systemic inflammation (10). As previously mentioned, LC patients with coexisting COPD are typically older, with a higher proportion of men, and more than 60.4% have a smoking history. The degree of airway obstruction is also more severe in this group, consistent with previous reports (37,38). In our study, we compared the clinical characteristics of LC patients with coexisting COPD treated at a non-surgical and surgical ward and found that most patients in the Department of Respiratory and Critical Care Medicine had a more advanced clinical stage and a higher COPD grade, with a decrease in FEV1, FEV1%pred, VC and VC%pred, as well as decreased FVC%pred. The 38 patients with early LC were admitted to the Department of Respiratory and Critical Care Medicine due to poor lung function, resulting in a loss of surgical opportunities. Previous studies have shown that even in early COPD patients, the incidence of postoperative lung complications is higher than that in non-small cell lung cancer (NSCLC) patients with normal lung function (39). In addition, when NSCLC patients have moderate to severe airflow restriction and emphysema, the risk of postoperative pulmonary complications significantly increases after complete resection (40). Due to their poor physical function, they may be unable to tolerate radical surgical resection and may need to consider other local treatment methods (16,41).
Inhalation therapy is a common treatment option for LC patients with coexisting COPD. The rationale behind this therapy is to deliver medication directly to the lungs, allowing for maximum therapeutic benefit while minimizing potential systemic side effects and increasing treatment adherence, as well as being more practical and easier to use (42,43). Our results showed that triple therapy (ICS/LABA/LAMA) was the most commonly used, while dual bronchodilator therapy (LAMA/LABA) was the least commonly used. The potential reasons could be due to the severity of the disease as a large proportion of the treated patients had moderate to very severe COPD (GOLD stage II to IV), coexisting conditions with lung cancer, which may also be complicated with respiratory infections, and to a certain extent, cost of medications as some triple therapy regimens might be more cost-effective.
This present study also revealed several factors associated with a diagnosis of COPD in LC patients, including being male, older age, a history of smoking, recorded COPD in discharge diagnosis, a high COPD GOLD stage, and decreased VC, FEV1, and FEV1%pred. Furthermore, our multivariate analysis demonstrated that patients who received a discharge diagnosis of COPD were significantly more likely to receive guideline-consistent treatment. This finding is consistent with a previous survey conducted by Fudan University (Shanghai, China) and underscores the importance of timely and accurate diagnosis of COPD in improving patient outcomes (22). Based on our study results, we propose that the following steps could be taken in the future to improve the diagnosis and treatment of COPD in LC patients: (I) more emphasis should be placed on the routine screening for COPD, such as spirometry testing or other PFTs, in this population to identify early-stage COPD before it progresses; (II) improved communication between oncologists and pulmonologists could help ensure that COPD is accurately screened, diagnosed and treated appropriately in LC patients; (III) considering that patients with COPD may not always recognize their symptoms or seek medical attention for them, particularly if they are focused on their cancer diagnosis and treatment, which can lead to underdiagnosis; therefore increasing awareness and education about COPD symptoms and risk factors could help improve early detection and treatment of COPD, as well as treatment adherence in LC patients; and (IV) healthcare providers should take steps such as routine screening, increasing awareness and education about COPD, improving communication between departments and specialties, and providing access to specialized pulmonary care.
Our study has several limitations that must be considered when interpreting the results. First, it was a single-center and retrospective investigation, with data collection based on medical records. This could have introduced selection bias, and the findings may not be generalizable to other populations or healthcare settings. Second, the study included hospitalized patients admitted to different departments, leading to heterogeneity in patients’ characteristics. Specifically, about 51.7% and 60.6% of the subjects were from the Department of Thoracic Surgery in the stage of lung function screening analysis and analysis of prevalence, respectively. Patients admitted to the surgical ward may have better lung function, which could explain the low prevalence of COPD in the LC subjects in our study. Third, we only included patients with spirometry, which could have led to an underestimation of the prevalence of COPD in our study. Fourth, since the patients did not undergo diffusing capacity of lung for carbon monoxide (DLCO) testing, this might have affected the evaluation of LC combined with emphysema.
Conclusions
Our study highlights the feasibility of screening for and treating COPD in LC patients in both the Department of Respiratory and Critical Care Medicine and the Department of Thoracic Surgery. We confirmed that COPD remains underdiagnosed and under-treated in this patient population. Thus, respiratory physicians and thoracic surgeons should collaborate to develop airway disease management programs for LC patients to improve their treatment outcomes, quality of life and prognosis by more accurate and timely diagnosing COPD in LC patients and implementing appropriate treatment.
Acknowledgments
We thank Shengli Li, a senior statistician at the Clinical Research Institute of Xuzhou Medical University, for participating in the study design and analysis of the results of this paper.
Funding: This work was supported by the Six Talent Peaks Project in Jiangsu Province, China (No. WSN-081, China); Xuzhou City Bureau of Science and Technology Project (Nos. KC21237, and KC18058, China); Jiangsu Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. SJCX22_1265, China); Xuzhou Medical Key Talents Project (No. XWRCHT20220063, China); and The Social Development Projects of Key R&D Programs in Xuzhou city (No. KC22097).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-267/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-267/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-267/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-267/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics review board of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2022-KL338-01), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151-210. [Crossref] [PubMed]
- Wang C, Xu JY, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706-17.
- Ajimizu H, Ozasa H, Sato S, et al. Survival impact of treatment for chronic obstructive pulmonary disease in patients with advanced non-small-cell lung cancer. Sci Rep 2021;11:23677. [Crossref] [PubMed]
- Zhang SW, Sun KX, Zheng RS, et al. Cancer incidence and mortality in China, 2015. JNCC 2021;1:2-11.
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Kaaks R, Christodoulou E, Motsch E, et al. Lung function impairment in the German Lung Cancer Screening Intervention Study (LUSI): prevalence, symptoms, and associations with lung cancer risk, tumor histology and all-cause mortality. Transl Lung Cancer Res 2022;11:1896-911. [Crossref] [PubMed]
- Young RP, Duan F, Chiles C, et al. Airflow Limitation and Histology Shift in the National Lung Screening Trial. The NLST-ACRIN Cohort Substudy. Am J Respir Crit Care Med 2015;192:1060-7. [Crossref] [PubMed]
- Balata H, Harvey J, Barber PV, et al. Spirometry performed as part of the Manchester community-based lung cancer screening programme detects a high prevalence of airflow obstruction in individuals without a prior diagnosis of COPD. Thorax 2020;75:655-60. [Crossref] [PubMed]
- Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121-7. [Crossref] [PubMed]
- Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr Opin Pulm Med 2009;15:303-7. [Crossref] [PubMed]
- Zeskind JE, Lenburg ME, Spira A. Translating the COPD transcriptome: insights into pathogenesis and tools for clinical management. Proc Am Thorac Soc 2008;5:834-41. [Crossref] [PubMed]
- Turner MC, Chen Y, Krewski D, et al. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007;176:285-90. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. [Crossref] [PubMed]
- Loganathan RS, Stover DE, Shi W, et al. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest 2006;129:1305-12. [Crossref] [PubMed]
- Wei S, Chen F, Liu R, et al. Outcomes of lobectomy on pulmonary function for early stage non-small cell lung cancer (NSCLC) patients with chronic obstructive pulmonary disease (COPD). Thorac Cancer 2020;11:1784-9. [Crossref] [PubMed]
- Yi YS, Ban WH, Sohng KY. Effect of COPD on symptoms, quality of life and prognosis in patients with advanced non-small cell lung cancer. BMC Cancer 2018;18:1053. [Crossref] [PubMed]
- Xu W, Zhu J, Li L, et al. The Prognostic Role of Chronic Obstructive Pulmonary Disease for Lung Cancer After Pulmonary Resection. J Surg Res 2022;275:137-48. [Crossref] [PubMed]
- Sekine Y, Katsura H, Koh E, et al. Early detection of COPD is important for lung cancer surveillance. Eur Respir J 2012;39:1230-40. [Crossref] [PubMed]
- Çolak Y, Afzal S, Nordestgaard BG, et al. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med 2017;5:426-34. [Crossref] [PubMed]
- Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med 2018;6:421-30. [Crossref] [PubMed]
- Zhang J, Zhou JB, Lin XF, et al. Prevalence of undiagnosed and undertreated chronic obstructive pulmonary disease in lung cancer population. Respirology 2013;18:297-302. [Crossref] [PubMed]
- Bednarek M, Maciejewski J, Wozniak M, et al. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax 2008;63:402-7. [Crossref] [PubMed]
- Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014;145:346-53. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:497-530. [Crossref] [PubMed]
- Ganti AKP, Loo BW, Bassetti M, et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:1441-64. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019;200:e70-88. [Crossref] [PubMed]
- Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164. [Crossref] [PubMed]
- Mouronte-Roibás C, Leiro-Fernández V, Ruano-Raviña A, et al. Chronic Obstructive Pulmonary Disease in Lung Cancer Patients: Prevalence, Underdiagnosis, and Clinical Characterization. Respiration 2018;95:414-21. [Crossref] [PubMed]
- Spyratos D, Papadaki E, Lampaki S, et al. Chronic obstructive pulmonary disease in patients with lung cancer: prevalence, impact and management challenges. Lung Cancer (Auckl) 2017;8:101-7. [Crossref] [PubMed]
- Kobayashi S, Suzuki S, Niikawa H, et al. Preoperative use of inhaled tiotropium in lung cancer patients with untreated COPD. Respirology 2009;14:675-9. [Crossref] [PubMed]
- Sekine Y, Fujisawa T, Suzuki K, et al. Detection of chronic obstructive pulmonary disease in community-based annual lung cancer screening: Chiba Chronic Obstructive Pulmonary Disease Lung Cancer Screening Study Group. Respirology 2014;19:98-104. [Crossref] [PubMed]
- Arnedillo Muñoz A. The underdiagnosis of Chronic Obstructive Pulmonary disease in women. Another pending task? Arch Bronconeumol 2013;49:221-2. [Crossref] [PubMed]
- Bastin AJ, Starling L, Ahmed R, et al. High prevalence of undiagnosed and severe chronic obstructive pulmonary disease at first hospital admission with acute exacerbation. Chron Respir Dis 2010;7:91-7. [Crossref] [PubMed]
- Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019;394:407-18. [Crossref] [PubMed]
- Hashimoto N, Matsuzaki A, Okada Y, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med 2014;14:14. [Crossref] [PubMed]
- Kim ES, Kim YT, Kang CH, et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early-stage COPD. Int J Chron Obstruct Pulmon Dis 2016;11:1317-26. [Crossref] [PubMed]
- Shin S, Park HY, Kim H, et al. Joint effect of airflow limitation and emphysema on postoperative outcomes in early-stage nonsmall cell lung cancer. Eur Respir J 2016;48:1743-50. [Crossref] [PubMed]
- Leduc C, Antoni D, Charloux A, et al. Comorbidities in the management of patients with lung cancer. Eur Respir J 2017;49:1601721. [Crossref] [PubMed]
- Jo YS. Long-Term Outcome of Chronic Obstructive Pulmonary Disease: A Review. Tuberc Respir Dis (Seoul) 2022;85:289-301. [Crossref] [PubMed]
- Jo H, Park S, Kim NE, et al. Impact of COPD Treatment on Survival in Patients with Advanced Non-Small Cell Lung Cancer. J Clin Med 2022;11:2391. [Crossref] [PubMed]


