
Risk stratification of postoperative pulmonary complications in elderly patients undergoing lung cancer resection: a propensity score-matched study
Highlight box
Key findings
• Higher ASA classification and open thoracotomy are associated with PPCs after pulmonary resection for lung cancer among elderly patients.
What is known and what is new?
• Risk factors for PPCs can generally be divided into patient-related, clinical-related, and procedure-related factors. Limited evidence exists on the predictive risk factors for PPCs among elderly people undergoing thoracic surgery.
• We used propensity score matching to reduce confounding biases to identify the risk factors associated with PPCs. Both PSM and binary logistic regression showed that ASA class > II and open thoracotomy approach for surgery were independent risk factors for developing PPCs in the elderly population.
What is the implication, and what should change now?
• Higher ASA classification and open thoracotomy were perioperative risk factors associated with PPCs following lung cancer resection. These factors may be important in proper patient selection and operative planning, and interventions like nutritional supplementation and prehabilitation may be important considerations in preventing PPCs.
Introduction
Lung cancer is a leading cause of cancer-related mortality worldwide, with an estimated 2.21 million new cases and 1.80 million deaths occurring globally in 2020 (1). Lung cancer mainly affects the elderly, with a median age at diagnosis of 71 years (2). In China, lung cancer accounts for about 30% of all cancer deaths (3) and is expected to increase by approximately 40% by 2030 (4). With the rapid expansion of the aging population in China, the incidence of lung cancer among the elderly is also increasing dramatically, leading to a significant disease burden (5). Treatment and care pathways for lung cancer in the elderly population remain a major public health concern in China.
Surgery remains the standard treatment for patients with resectable lung cancer and can be safely performed in elderly patients (6). However, surgery also impairs postoperative respiratory function and may lead to postoperative pulmonary complications (PPCs) (7). With improved surgical techniques, anesthesia, and perioperative care, enhanced recovery after surgery (ERAS) protocols have shown improved outcomes in thoracic surgery (8). However, PPCs are still common among patients undergoing lung cancer surgery, with reported incidence rates ranging from 10% to 50%, depending on the definition of PPCs, the assessment of clinical criteria, and the surgical procedure (9). The most reported pulmonary complications include atelectasis, pneumonia, and acute respiratory failure requiring prolonged mechanical ventilation or re-intubation in within 48 hours (10). PPCs are major contributors to mortality and morbidity following lung resection, accounting for up to 84% of all deaths (10). In addition, PPCs are associated with increased medical expenses due to prolonged length of stay and the need for intensive care (10). Thus, performing preoperative risk stratification to identify and optimize modifiable risk factors will be crucial in preventing the occurrence of PPCs.
Risk stratification can be defined as the process of determining a patient’s probability of experiencing a particular complication based on their clinical or laboratory data, and can help guide preventative interventions accordingly (11). A wide range of risk factors associated with PPCs have been identified, and can be generally divided into patient-, clinical-, and procedure-related factors (8,11). Notably, elderly patients are potentially at increased risk of PPCs and often have more age-related comorbidities, impaired immunity, and malnutrition, which may contribute to postoperative complications (12-14). However, limited evidence exists regarding predictive risk factors for PPCs among elderly patients undergoing thoracic surgery. Thus, this study aimed to evaluate potential clinical predictors of PPCs in elderly lung cancer patients undergoing pulmonary resection using a propensity score (PS)-matched cohort of patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-923/rc).
Methods
Patients and study design
A retrospective cohort study was conducted among lung cancer patients who underwent pulmonary resection for lung cancer at the Third Affiliated Hospital of Kunming Medical University, China between January 2016 and December 2019. The patient inclusion criteria were as follows: (I) aged more than 65 years and physical status Eastern Cooperative Oncology Group (ECOG) score of 0–1; (II) patients who underwent pulmonary resection for lung cancer. The exclusion criteria were as follows: (I) patients who underwent emergency surgery; (II) patients who had undergone reoperation within 30 days; (III) patients with insufficient clinical data or who were lost to follow-up. Among a total of 609 patients who underwent resection for lung cancer, 456 were eligible and included in our study, including 314 without PPCs and 142 with PPCs. The propensity score matching (PSM) produced 135 matched pairs. Figure 1 shows the flowchart describing patient enrollment and PSM. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Third Affiliated Hospital of Kunming Medical University (No. KYLX2022016), and the requirement for patient consent was waived due to the retrospective nature of this study.

Data collection
Clinical information was extracted from patient medical records and included the following 5 types: (I) patient information, including age, sex, body mass index (BMI), and smoking status; (II) comorbidities, including hypertension, diabetes, coronary artery disease, cerebrovascular disease, tuberculosis, obstructive and restrictive lung disease, and acute respiratory infection; (III) treatment history, including lung resection, and neoadjuvant chemotherapy; (IV) preoperative characteristics, including pre-albumin (Pre-ALB), forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC, diffusing capacity of the lungs for carbon monoxide (DLCO), and American Society of Anesthesiologists (ASA) classification; (V) operative characteristics, including tumor staging, laterality of resection, surgical approach, resection type, histological type, surgery duration, perioperative bleeding, perioperative fluid infusion, and perioperative blood transfusion.
Surgical treatment
Patients in both groups adhered to an ERAS protocol for perioperative care, which is a patient-centered and evidence-based multimodal approach to care for surgical patients (15). All patients underwent pulmonary resection for lung cancer, the type of resection include lobectomy, sub-lobectomy (pulmonary segment resection, pulmonary wedge resection, double lobectomy, sleeve resection, pneumonectomy). the patients choose VATS approach base on the relationship between tumors and pulmonary hilus or the pulmonary hilar vessels was encapsulated by tumor or not. Preoperative care included patient education, lung function training, and nutritional support. Intraoperative care included maintaining normothermia. Postoperative care included multimodality analgesia, oral nutritional support if food intake was less than 60% of normal, as well as physical therapy when appropriate.
PPCs assessment
PPCs included respiratory failure, pneumonia, pulmonary embolism, pleural effusion. Notably, surgical site infection, empyema, chylothorax, subcutaneous emphysema, rupture of pulmonary bulla, and prolonged air leaks were considered surgical complications and thus were not included as PPCs in this study. The diagnosis of PPCs was based on clinical symptoms [such as a productive cough, fever, oxygen saturation (SpO2) below 90%, and dyspnea] and findings on postoperative chest X-rays or computed tomography (CT) scans (such as pleural effusion, atelectasis, pulmonary consolidation, and infiltrates). According to the presence or absence of PPCs after surgery, the patients were divided into 2 groups: the PPC group and the non-PPC group.
PSM and statistical analysis
PSM is a statistical matching technique that attempts to estimate the treatment effect by accounting for covariates that predict receiving the treatment (16). It has been widely used in observational studies to reduce confounding biases by balancing all observed relevant covariates between the comparative groups thus minimizing the potential bias (17).
SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. PSM analysis was performed to reduce confounding for covariates: with a tolerance of 0.05. PSM is a superior statistical method of adjusting for potential baseline variables, as they were considered the most likely possible confounders, and included gender, age, BMI, smoking pack years, comorbidities (hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disease, tuberculosis, obstructive lung disease, restrictive lung disease, history of previous lung resection, acute respiratory infection, and neoadjuvant chemotherapy history), Pre-ALB, FVC, FEV1, FEV1/FVC, DLCO, history of chemotherapy, chronic respiratory disease, and acute respiratory infection history. In order to avoid pairing dissimilar individuals in this observational study, 1:1 nearest-neighbor matching was used, and a restriction condition was affixed, that is, the difference between the PS to be at most 0.1. Independent 2-sample t-test for continuous variables and the chi-squared test or continuity correlation for categorical variables were conducted for group comparisons. Preoperative data and operative data were compared between the PPC group and the non-PPCs group to evaluate the risk factors for PPCs. Factors with statistical differences were then included in the binary logistic regression to identify the potential independent risk factors for developing PPCs. A P value <0.05 was considered statistically significant.
Results
Propensity score matching
A total of 609 elderly patients with suspected lung cancer intended to undergo pulmonary resection during the study period. Among them, 153 cases were excluded due to incomplete information in 95 patients (62.1%), non-cancerous disease in 54 patients (35.3%), and surgical refusal in 4 patients (2.6%).
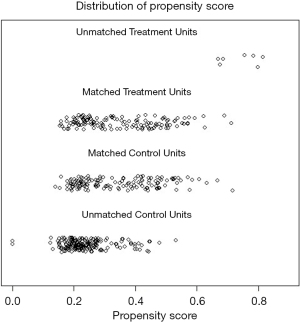
PSM was performed to reduce confounding. there are no differences were observed in parameters between the PPC group and non-PPC groups. The success of matching can be seen in the dot-plot of individual PSs in Figure 2 and the standardized differences before and after matching in Figure 3.

Patient characteristics
From 2016 to 2019, we performed a retrospective analysis of 456 lung cancer patients including 282 males (61.8%) and 174 females (38.2%), with an average age of 69.11±3.68 years. PPC developed in 142 (31.1%) patients, including 99 (21.7%) with pneumonia, 39 (8.6%) with pleural effusion, 22 (4.8%) with pneumothorax, 12 (2.6%) with respiratory failure, 13 (2.9%) with atelectasis, and 2 (0.4%) with pulmonary embolism.
Comparison of baseline characteristics between the PPC group and the non-PPC group before and after PSM are shown in Table 1. Before PSM, the 2 groups showed significant differences in gender (P=0.02), smoking pack years (P=0.02), and chronic obstructive pulmonary disease (COPD) (P=0.003). In order to reduce the potential for confounding, we established the new cohort using PSM with a ratio of 1:1, which produced 135 matched pairs. This analysis eliminated differences in all the observed baseline characteristics in the primary cohort (P>0.05).
Table 1
| Characteristics | Primary cohort | PSM cohort | |||||
|---|---|---|---|---|---|---|---|
| Non-PPC (N=314) | PPC (N=142) | P value | Non-PPC (N=135) | PPC (N=135) | P value | ||
| Gender | 0.02 | 0.51 | |||||
| Male | 183 (58.3) | 99 (69.7) | 88 (65.2) | 93 (68.9) | |||
| Female | 131 (41.7) | 43 (30.3) | 47 (34.8) | 42 (31.1) | |||
| Age (years) | 69.11±3.68 | 69.15±3.67 | 0.92 | 69.30±3.67 | 69.16±3.71 | 0.75 | |
| Smoking (pack-years) | 14.42±21.72 | 20.09±24.58 | 0.02 | 19.16±23.84 | 19.41±24.48 | 0.93 | |
| BMI (kg/m2) | 22.69±2.77 | 22.31±3.07 | 0.05 | 22.03±2.48 | 22.35±3.07 | 0.07 | |
| Comorbidities | |||||||
| Hypertension | 108 (34.4) | 41 (28.9) | 0.24 | 39 (28.9) | 40 (29.6) | 0.89 | |
| DM | 31 (9.9) | 9 (6.3) | 0.21 | 8 (5.9) | 9 (6.7) | 0.80 | |
| Coronary artery disease | 13 (4.1) | 11 (7.7) | 0.87 | 9 (6.7) | 10 (7.4) | 0.81 | |
| Cerebrovascular disease | 10 (3.2) | 6 (4.2) | 0.96 | 5 (3.7) | 6 (4.4) | 0.75 | |
| Pulmonary tuberculosis | 14 (4.5) | 8 (5.6) | 0.21 | 5 (3.7) | 8 (5.9) | 0.39 | |
| COPD | 3 (1.0) | 17 (12.0) | 0.003 | 1 (0.7) | 5 (3.7) | 0.18 | |
| Restrictive lung disease | 4 (1.3) | 1 (0.7) | 0.78 | 1 (0.7) | 1 (0.7) | 1.00 | |
| Lung resection history | 0 (0.0) | 3 (2.1) | 0.51 | 3 (2.2) | 3 (2.2) | 1.00 | |
| Neoadjuvant chemotherapy history | 17 (5.4) | 12 (8.5) | 0.21 | 11 (8.1) | 11 (8.1) | 1.00 | |
| Acute respiratory infection history | 68 (21.7) | 58 (40.8) | 0.21 | 11 (8.1) | 11 (8.1) | 1.00 | |
Data are presented as mean ± standard deviation or n (%). PSM, propensity score matching; PPC, postoperative pulmonary complication; BMI, body mass index; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Preoperative clinical characteristics
The preoperative clinical characteristics of the PPCs group and the non-PPCs group before and after PSM are listed in Table 2. Before PSM, the 2 groups showed significant differences in Pre-ALB (P=0.04), FVC (P=0.001), FEV1 (P=0.005), and FEV1/FVC (P=0.014). After PSM, the distribution of preoperative clinical characteristics in the 2 groups was balanced. No differences were observed in the Pre-ALB, FVC, FEV1, and FEV1/FVC between the non-PPC and PPC groups.
Table 2
| Characteristics | Primary cohort | PSM cohort | |||||
|---|---|---|---|---|---|---|---|
| Non-PPC (n=314) | PPC (n=142) | P value | Non-PPC (n=135) | PPC (n=135) | P value | ||
| Pre-ALB (g/L) | 0.04 | 0.69 | |||||
| <45 | 90 (28.7) | 54 (38.0) | 44 (32.6) | 48 (35.6) | |||
| ≥45 | 224 (71.3) | 88 (62.0) | 91 (67.4) | 87 (64.4) | |||
| FVC | 0.001 | 0.21 | |||||
| <80% | 95 (30.3) | 66 (46.5) | 49 (36.3) | 59 (43.7) | |||
| ≥80% | 219 (69.7) | 76 (53.5) | 86 (63.7) | 76 (56.3) | |||
| FEV1 | 0.005 | 0.58 | |||||
| <80% | 64 (20.4) | 46 (32.4) | 35 (25.9) | 39 (28.9) | |||
| ≥80% | 250 (79.6) | 96 (67.6) | 100 (74.1) | 96 (71.1) | |||
| FEV1/FVC | 0.014 | 0.41 | |||||
| <80% | 66 (21.0) | 45 (31.7) | 35 (25.9) | 41 (30.4) | |||
| ≥80% | 248 (79.0) | 97 (68.3) | 100 (74.1) | 94 (69.6) | |||
| DLCO | 0.07 | 0.65 | |||||
| <80% | 51 (16.2) | 33 (23.2) | 27 (20.0) | 31 (23.0) | |||
| ≥80% | 263 (83.8) | 109 (76.8) | 108 (80.0) | 104 (77.0) | |||
Data are presented as n (%). PSM, propensity score matching; PPC, postoperative pulmonary complication; Pre-ALB, pre-albumin; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; DLCO, diffusing capacity of the lungs for carbon monoxide.
Operative clinical risk factors for PPCs
Comparison of risk factors between the PPC group and the non-PPC group before and after PSM are listed in Table 3. Before PSM, the 2 groups showed significant differences in pathologic stage (P=0.02), surgical approach (P=0.03), surgery duration (P=0.02), perioperative bleeding (P=0.02), perioperative blood transfusion (P=0.04), and ASA classification (P=0.01). After PSM, the ASA classification remained significant, with more cases of ASA class > II in the PPC group than in the non-PPC group (1.6% vs. 7.7%, P=0.036). Similar findings were observed in the surgical approach in that there were more cases of open thoracotomy in the PPC group than in the non-PPCs group (36.3% vs. 25.9%, P=0.045).
Table 3
| Characteristics | Primary cohort | PSM cohort | |||||
|---|---|---|---|---|---|---|---|
| Non-PPC (n=314) | PPC (n=142) | P value | Non-PPC (n=135) | PPC (n=135) | P value | ||
| Pathologic stage | 0.02 | 0.07 | |||||
| I | 184 (58.6) | 64 (45.1) | 81 (60.0) | 63 (46.7) | |||
| II | 73 (23.2) | 40 (28.2) | 31 (23.0) | 37 (27.4) | |||
| III | 57 (18.2) | 38 (26.8) | 23 (17.0) | 35 (25.9) | |||
| Side of resection | 0.79 | 0.9 | |||||
| Right lobe | 182 (58.0) | 83 (58.5) | 79 (58.5) | 80 (59.3) | |||
| Left lobe | 132 (42.0) | 59 (41.5) | 56 (41.5) | 55 (40.7) | |||
| Surgical approach | 0.03 | 0.045 | |||||
| Open thoracotomy | 80 (25.5) | 54 (38.0) | 35 (25.9) | 49 (36.3) | |||
| VATS | 234 (74.5) | 88 (62.0) | 100 (74.1) | 86 (63.7) | |||
| Resection type | 0.04 | 0.22 | |||||
| More than lobectomy | 22 (7.0) | 20 (14.1) | 11 (8.1) | 20 (14.8) | |||
| Lobectomy | 236 (75.2) | 95 (66.9) | 96 (71.1) | 88 (65.2) | |||
| Sublobar resection | 56 (17.8) | 27 (19.0) | 28 (20.7) | 27 (20.0) | |||
| Histology | 0.46 | 0.99 | |||||
| Adenocarcinoma | 227 (72.3) | 96 (67.6) | 93 (68.9) | 92 (68.1) | |||
| Squamous-cell carcinoma | 81 (25.8) | 44 (30.9) | 40 (29.6) | 41 (30.4) | |||
| Others | 6 (1.9) | 2 (1.4) | 2 (1.5) | 2 (1.5) | |||
| Surgical duration (min) | 170.00±71.40 | 186.90±71.52 | 0.02 | 171.89±74.49 | 187.74±71.91 | 0.07 | |
| Perioperative bleeding (mL) | 140.94±145.12 | 196.83±267.52 | 0.02 | 158.70±167.27 | 190.37±268.87 | 0.24 | |
| Perioperative fluid infusion (mL) | 1,629.30±442.29 | 1,716.90±502.22 | 0.06 | 1,634.44±462.97 | 1,701.11±484.17 | 0.24 | |
| Perioperative blood transfusion | 8 (2.5) | 9 (6.3) | 0.04 | 4 (3.0) | 8 (5.9) | 0.23 | |
| ASA classification | 0.01 | 0.036 | |||||
| ≤ II | 309 (98.4) | 131 (92.3) | 133 (98.5) | 125 (92.6) | |||
| > II | 5 (1.6) | 11 (7.7) | 2 (1.5) | 10 (7.4) | |||
Data are presented as mean ± standard deviation or n (%). PPC, postoperative pulmonary complications; PSM, propensity score matching; VATS, video-assisted thoracoscopic surgery; ASA, American Society of Anesthesiologists.
Regression analysis
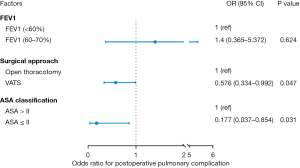
In order to further identify independent risk factors for PPCs in this study, we performed a binary logistic regression analysis to control for all potential confounders. As shown in Figure 4, ASA class ≤ II and surgical approach [video-assisted thoracoscopic surgery (VATS)] were protective factors for PPC.
Discussion
Lung cancer is common in the aging population, and elderly patients are at increased risk of PPCs due to impaired physiology, decreased metabolic rate, lower baseline health status, poor surgical response, and slower recovery speed. In this study, we performed a retrospective observational study evaluating a cohort of elderly lung cancer patients who underwent lung resection and compared the preoperative and operative characteristics between the PPC and non-PPC groups using PSM, followed by a binary logistic regression to identify predictors of PPCs. We observed an incidence rate of 31.1% for PPCs among elderly lung cancer patients undergoing lung resection surgery, with pneumonia being the most common PPC (21.7%). Both PSM and binary logistic regression showed that ASA class > II and an open thoracotomy approach were independent risk factors for developing PPCs.
The incidence of PPCs in our study was consistent with other studies (18,19) and fell in the range of 10–50% (20). The reported incidence of PPCs following lung resection varies across studies mainly due to a divergence in definition, diagnostic criteria, and assessment of PPCs as well as variation in research design, surgical approaches, and patients’ baseline health status among various studies, which all contribute to differences in post-surgical clinical outcomes including PPCs. A standard definition for PPCs will be needed in the future for better cross-study comparisons.
The finding that pneumonia was the most common form of PPC in our study was consistent with the literature showing pneumonia as the most common complication following pulmonary resection in the elderly (10). This may be explained by the fact that our study sample comprised a high percentage of patients with a history of acute respiratory infection in the past month, which is consistent with previous study (8). This finding suggests that clinicians and researchers should pay special attention to elderly patients’ potential risk of developing pneumonia after lung resection and take necessary measures to prevent pneumonia (21).
Our study demonstrated that ASA class > II was an independent risk factor predicting the occurrence of PPCs among elderly patients following lung resection, which was consistent with previous studies showing similar results (22-24). Higher ASA physical status (ASA class > II) has been well demonstrated to be associated with PPCs following thoracic surgeries in various studies (10,25). Miura et al. (22) conducted a small retrospective study of octogenarians undergoing therapeutic resection for lung cancer and found that a higher ASA score was correlated with an increased incidence of postsurgical morbidity and mortality. The positive association between ASA score and PPC incidence may be explained by the increased rates of pneumonia, unplanned intubation, and prolonged ventilator support among patients with higher ASA scores (26). This finding indicates that the ASA score is an important and useful indicator for the assessment of PPC risk and burden.
We observed a significantly higher incidence of PPCs following open thoracotomy compared to VATS, a result in line with previous findings (27-29). In Cao et al.’s (28) meta-analysis of 4 PSM studies involving 3,634 patients, the VATS group had a significantly lower incidence of pneumonia than the open thoracotomy group. Laursen et al.’s PSM study also showed VATS was associated with fewer post-surgical minor and major complications and lower morbidity (29). Although open thoracotomy provides excellent exposure and access to vital structures for lung cancer resection, it has been reported to result in distorted compliance of the chest wall due to impaired respiratory pathology (30), increased postoperative pain (31), post-surgical complications, and prolonged recovery time (27). In addition, VATS patients have fewer physical limitations in their postoperative rehabilitation period and are more likely to engage in postoperative rehabilitation activities such as deep breathing and effective coughing, which all help prevent the occurrence of PPCs. Our findings provide further evidence of the advantages of VATS over an open thoracotomy in reducing PPCs and improving patient outcomes (32).
In this study, FEV1 was not predictive of PPCs after PSM, although univariate analysis showed significant differences in patients’ FEV1 between the PPC group and non-PPC group before PSM. These results were consistent with Berry et al.’s (33) study of 340 VATS lobectomy patients showing that FEV1 was not a significant independent risk factor for the 12% of patients who developed PPCs. Preoperative lung function has been a conventionally studied factor for predicting pulmonary morbidity and mortality after pulmonary resection. However, in the past 2 decades, there have been refinements in preoperative risk stratification and cancer staging, combined with advances in surgical and anesthetic approaches, which have allowed for more patients with impaired lung function to undergo therapeutic surgical resection (33,34). Our findings may be related to the implementation of preoperative preventive measures based on an ERAS protocol, which may help prevent PPCs among patients with a poor FEV1.
Several limitations of this study should be noted. First, this study was conducted at a single hospital and the cohort may not be representative of patients from other hospitals or other areas. Second, although we performed PSM to eliminate selection biases, it should be acknowledged that PSM has its own limitations as it requires full data for all covariates used leading us to exclude 153 patients, which may have affected our results. Additionally, there was inevitably some variability in the surgical techniques and skills, which may have affected incidence of the PPCs among patients and needs to be further studied.
Conclusions
Our retrospective propensity-matched study demonstrates that open thoracotomy and ASA class > II were associated with a higher risk of PPCs among elderly patients undergoing lung cancer resection. Proper patient selection and operative planning may be important considerations in preventing PPCs, and precautions should be taken when operating on elderly patients undergoing an open thoracotomy with an ASA class > II.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Nos. 82260508 and 81860325) and The Joint Special Funds for the Department of Science and Technology of Yunnan Province-Kunming Medical University (Nos. 202201AY070001-135, 202201AY070001-156, and 202101AY070001-178).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-923/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-923/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-923/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-923/coif). JL is a robotic proctor for Intuitive Surgical and site principal investigator for clinical trials funded by Genentech and Novartis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Third Affiliated Hospital of Kunming Medical University (No. KYLX2022016), and the requirement for patient consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Cancer. Date: February 2022. [cited 2022 11 November]. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer
- Howlader N, Noone AM, Krapcho M, et al., SEER cancer statistics review, 1975-2017. Bethesda, MD: National Cancer Institute; 2020.
- Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center 2022;2:1-9. [Crossref]
- Martín-Sánchez JC, Lunet N, González-Marrón A, et al. Projections in Breast and Lung Cancer Mortality among Women: A Bayesian Analysis of 52 Countries Worldwide. Cancer Res 2018;78:4436-42. [Crossref] [PubMed]
- Wang L, Tang Y, Roshanmehr F, et al. The Health Status Transition and Medical Expenditure Evaluation of Elderly Population in China. Int J Environ Res Public Health 2021;18:6907. [Crossref] [PubMed]
- Kamel MK, Port JL. Oncologic considerations in the elderly. Curr Opin Anaesthesiol 2018;31:6-10. [Crossref] [PubMed]
- Li XF, Jin L, Yang JM, et al. Effect of ventilation mode on postoperative pulmonary complications following lung resection surgery: a randomised controlled trial. Anaesthesia 2022;77:1219-27. [Crossref] [PubMed]
- Sabaté S, Mazo V, Canet J. Predicting postoperative pulmonary complications: implications for outcomes and costs. Curr Opin Anaesthesiol 2014;27:201-9. [Crossref] [PubMed]
- Jain A, Philip B, Begum M, et al. Risk Stratification for Lung Cancer Patients. Cureus 2022;14:e30643. [PubMed]
- Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [Crossref] [PubMed]
- Rauniyar R, Li L, Adboulaye HA, et al. Risk stratification of postoperative pulmonary complications (PPCs) in elderly patients after lung cancer resection. International Journal of Science Inventions Today 2021;10:16-29.
- Okada S, Shimada J, Kato D, et al. Long-Term Prognostic Impact of Severe Postoperative Complications After Lung Cancer Surgery. Ann Surg Oncol 2019;26:230-7. [Crossref] [PubMed]
- Okada S, Shimomura M, Ishihara S, et al. Clinical significance of postoperative pulmonary complications in elderly patients with lung cancer. Interact Cardiovasc Thorac Surg 2022;35:ivac153. [Crossref] [PubMed]
- Yutaka Y, Sonobe M, Kawaguchi A, et al. Prognostic impact of preoperative comorbidities in geriatric patients with early-stage lung cancer: Significance of sublobar resection as a compromise procedure. Lung Cancer 2018;125:192-7. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Rosenbaum PR, Donald DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983;70:41-55. [Crossref]
- Wang J. To use or not to use propensity score matching? Pharm Stat 2021;20:15-24. [Crossref] [PubMed]
- Shiono S, Abiko M, Sato T. Postoperative complications in elderly patients after lung cancer surgery. Interact Cardiovasc Thorac Surg 2013;16:819-23. [Crossref] [PubMed]
- Agostini PJ, Lugg ST, Adams K, et al. Risk factors and short-term outcomes of postoperative pulmonary complications after VATS lobectomy. J Cardiothorac Surg 2018;13:28. [Crossref] [PubMed]
- Yepes-Temiño MJ, Monedero P, Pérez-Valdivieso JR, et al. Risk prediction model for respiratory complications after lung resection: An observational multicentre study. Eur J Anaesthesiol 2016;33:326-33. [Crossref] [PubMed]
- Yamamichi T, Ichinose J, Iwamoto N, et al. Correlation Between Smoking Status and Short-term Outcome of Thoracoscopic Surgery for Lung Cancer. Ann Thorac Surg 2022;113:459-65. [Crossref] [PubMed]
- Miura N, Kohno M, Ito K, et al. Lung cancer surgery in patients aged 80 years or older: an analysis of risk factors, morbidity, and mortality. Gen Thorac Cardiovasc Surg 2015;63:401-5. [Crossref] [PubMed]
- Han B, Li Q, Chen X. Frailty and postoperative complications in older Chinese adults undergoing major thoracic and abdominal surgery. Clin Interv Aging 2019;14:947-57. [Crossref] [PubMed]
- Ramachandran SK, Nafiu OO, Ghaferi A, et al. Independent predictors and outcomes of unanticipated early postoperative tracheal intubation after nonemergent, noncardiac surgery. Anesthesiology 2011;115:44-53. [Crossref] [PubMed]
- Marret E, Miled F, Bazelly B, et al. Risk and protective factors for major complications after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2010;10:936-9. [Crossref] [PubMed]
- Lakshminarasimhachar A, Smetana GW. Preoperative Evaluation: Estimation of Pulmonary Risk. Anesthesiol Clin 2016;34:71-88. [Crossref] [PubMed]
- Agostini P, Lugg ST, Adams K, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy†. Interact Cardiovasc Thorac Surg 2017;24:931-7. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. [Crossref] [PubMed]
- Tukanova K, Papi E, Jamel S, et al. Assessment of chest wall movement following thoracotomy: a systematic review. J Thorac Dis 2020;12:1031-40. [Crossref] [PubMed]
- Hernandez-Vaquero D, Vigil-Escalera C, Pérez-Méndez I, et al. Survival After Thoracoscopic Surgery or Open Lobectomy: Systematic Review and Meta-Analysis. Ann Thorac Surg 2021;111:302-13. [Crossref] [PubMed]
- Hireche K, Canaud L, Lounes Y, et al. Thoracoscopic Versus Open Lobectomy After Induction Therapy for Nonsmall Cell Lung Cancer: New Study Results and Meta-analysis. J Surg Res 2022;276:416-32. [Crossref] [PubMed]
- Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51; discussion 1051-2. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Evolution of surgical outcomes for nonsmall cell lung cancer: time trends in 1465 consecutive patients undergoing complete resection. Ann Thorac Surg 2004;77:1926-30; discussion 1931. [Crossref] [PubMed]


